Back to Journals » International Journal of General Medicine » Volume 17
Diagnostic Value of Carotid Plaque Assessment with AIS Based on Quantitative Parameters of Dual-Layer Detector Spectral CT
Authors Meng X, Li F, Wu W, Wu J
Received 22 November 2023
Accepted for publication 15 March 2024
Published 30 March 2024 Volume 2024:17 Pages 1263—1272
DOI https://doi.org/10.2147/IJGM.S448852
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Xiaoying Meng,1,2 Fei Li,2 Wenpei Wu,3 Juncang Wu1,2
1Department of Neurology, Hefei Hospital Affiliated to Anhui Medical University (Hefei Second People’s Hospital), Hefei, Anhui, 230011, People’s Republic of China; 2The Fifth Clinical School of Medicine, Anhui Medical University, Hefei, Anhui, 230032, People’s Republic of China; 3Department of Neurology, Hefei Second People’s Hospital Affiliated to Bengbu Medical College, Hefei, Anhui, 230011, People’s Republic of China
Correspondence: Juncang Wu, Department of Neurology, Hefei Hospital Affiliated to Anhui Medical University (Hefei Second People’s Hospital), Hefei, Anhui, 230011, People’s Republic of China, Email [email protected]
Purpose: To investigate the quantitative assessment of carotid plaque by each parameter of dual-layer detector spectral CT and its diagnostic value in patients with acute cerebral infarction.
Patients and Methods: Eighty-three patients with carotid atherosclerotic plaques who underwent spectral CT scanning were retrospectively included. Forty-two patients with acute ischaemic stroke (AIS) were included in the study group, and 41 patients without AIS were included in the control group. We compared the detection of carotid plaques in the two groups and the differences in the spectral quantitative parameters of the plaques in the two groups, and their diagnostic efficacy was obtained.
Results: The detection rate of carotid plaques in the AIS group was higher than that in the non-AIS group (p< 0.05); the carotid plaques in the AIS group mainly consisted of non-calcified plaques, while those in the non-AIS group mainly consisted of calcified plaques. The effective atomic number (Zeff), slope of the energy spectrum curve (λH), electron density (ED), and iodine-no-water value of the carotid plaques in the AIS group were lower than those in the non-AIS (p< 0.05). For the differentiation of the carotid plaques in the AIS group from those in the non-AIS group, the area under the curve (AUC) of Zeff amounted to 0.637 (cut-off value: 11.865; sensitivity: 72.5%; specificity: 56.2%), the AUC of λH amounted to 0.628 (cut-off value: 19.56; sensitivity: 76.3%; specificity: 51.6%), and that for ED amounted to 0.624 (cut-off value: 110.45; sensitivity: 60.0%; specificity: 64.1%), AUC of iodine-no-water value amounted to 0.645 (cut-off value: 9.125; sensitivity: 61.3%; specificity: 65.6%).
Conclusion: In summary, the quantitative parameters of dual-layer detector spectral CT can be used to assess plaque stability and have certain value in the diagnosis of AIS. The quantitative parameters can effectively differentiate carotid plaques in AIS and non-AIS patients.
Keywords: ischemic stroke, carotid plaque, spectral CT, effective atomic number, iodine density values, anhydrous iodine values, electron density
Introduction
According to the Global Burden of Disease 2019, stroke is the second leading cause of death and the third leading combined cause of death and disability globally.1 Stroke can be categorized into ischemic and hemorrhagic stroke. In 2010, the global incidence of ischemic and hemorrhagic stroke was 11.6 million and 5.3 million, respectively, which increased to 13.7 million worldwide by 2016, and ischemic strokes caused 2.7 million deaths,2 Between 1990 and 2019, stroke incidence increased by a total of 70%, mortality by 43%, and disability-adjusted life years (DALYs) by 143%; in 2019, the number of DALYs due to stroke was much greater in men (77 million) than in women (66 million) worldwide, and age-standardized mortality rates were higher in men than in women 96.4 per 100,000 vs 73.5 per 100,000), but point estimates of new and prevalent strokes are lower in men than in women.3 Acute ischemic stroke (AIS) is the most common type of stroke, with high morbidity, disability mortality, recurrence, and economic burden. It has always been a major health and life threatening disease for middle-aged and elderly people worldwide. The main risk factors for AIS include hypertension, diabetes mellitus, atherosclerosis, etc,1 of which atherosclerosis is the most important cause of ischemic stroke,4 and as far as more and more studies have shown, the occurrence of AIS is not only related to the degree of vessel stenosis but also occurs in many patients with mild stenosis or even no stenosis, and 30% of ischemic strokes are associated with carotid atherosclerotic plaques.5 The prevalence of increased carotid intima-media thickness was approximately 27.6% in the 30–79 year age range between 2000 and 2020, with a 57.49% increase in the number of people with the condition; the prevalence of carotid plaque was the prevalence of carotid plaque was about 21.1%, an increase of 58.97%; carotid stenosis was about 1.5%, an increase of 59.13%. The prevalence of carotid plaque was about 21.1%, an increase of 58.97%; carotid stenosis was about 1.5%, an increase of 59.13%.6 Erosion and rupture of carotid atherosclerotic plaques can lead to thrombus formation, which follows the blood flow to the distal intracranial arteries,7 which is an embolic event, and then leads to the development of AIS. This shows that carotid plaques are closely related to ischemic stroke, and proper recognition and early identification of carotid plaques are crucial for the development of cerebrovascular events. Therefore, imaging to identify and evaluate carotid plaque is of great value for the diagnosis, treatment, and prognosis of AIS.
Digital subtraction angiography (DSA) has long been recognized as the gold standard for detecting carotid atherosclerosis, which can clearly show the degree of carotid artery stenosis and plaque, but it is an invasive examination that is clinically time-consuming, expensive, and inconvenient. High-resolution magnetic resonance imaging (HRMRI), with its high spatial and tissue resolution, is currently the noninvasive “gold standard” for carotid plaque detection, but it has many contraindications (metal implants in the body or claustrophobia), imaging speed is sluggish and costly.8,9 Most importantly, AIS has a rapid onset and is generally nocturnal, however performing an emergency and night MRI is unlikely in most hospitals. Computed tomography angiography (CTA), with its high spatial resolution and fast image acquisition time, can relatively accurately assess the vessel wall size, the load, morphological characteristics, and vulnerability of high-risk plaque.10 Dual-layer detector spectral CT is an emerging technology developed in recent years. The dual-layer detector realizes “simultaneous, homogenous, and isotropic” data acquisition without the need to set up dual-energy scanning in advance. Conventional and spectral images can be obtained in a single scanning, including multiple quantitative parameters of the energy spectrum, which can be used for quantitative analysis.11–13 Quantitative parameters of spectral CT have been used in a variety of clinical studies, but there are few reports on the evaluation and further diagnostic value of carotid plaque in AIS. Therefore, in this study, we used the IQon energy-spectrum CT machine from Philips, the Netherlands, to quantitatively evaluate carotid plaques in patients with AIS by multiple parameters and to provide more quantitative bases for the diagnosis of AIS.
Materials and Methods
Study Subjects
Eighty-three patients with carotid atherosclerotic plaque or stenosis admitted to the Second People’s Hospital of Hefei City from March 2022 to April 2023 were retrospectively collected. All patients underwent IQon energy-spectrum CT scanning, of which 42 patients who developed acute ischemic stroke (AIS) were included in the study group, and the other 41 patients without AIS were included in the control group. Inclusion criteria: ① All patients underwent one-stop CT plain scanning and CTA scanning; ② IQon energy-spectrum CT images were clear without obvious motion artifacts; ③ Compliance with the diagnostic criteria for AIS set by the Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke 2014; ④ Age ≥18 years old. Exclusion criteria: ① Patients with brain tumor, patients with cognitive dysfunction, patients with severe craniocerebral injury, and patients with severe intracranial infection; ② Incomplete imaging data used for analysis; ③ Incomplete clinical data; and ④ The cause of AIS is cardiogenic embolic.
Methods of Examination
Energy spectrum CT scanning was performed by the IQon energy spectrum CT machine of Philips, Netherlands. The patient lay supine on the examination table with the head placed in the head frame of the examination bed. The scanning direction was from the foot side to the head side, and the scanning range was from the aortic arch to the cerebral Willians ring. Scanning conditions: collimator width, 64 × 0.625 mm; rotational speed, 0.27 s/revolution; tube voltage, 120 kV; and tube current with automatic modulation technique. Then, 100 mL of iodixanol (containing 320 mg/mL of iodine) contrast agent was injected into the right median elbow vein at a flow rate of 5.0 mL/s using a double-barrel high-pressure syringe. Immediately after the injection, 40 mL of saline was injected at the same flow rate. Spectral CT images were obtained, spectral reconstruction was performed to obtain spectral base images (SBI), and all spectral analysis data were sent to a dedicated spectral workstation for analysis. Using the Philips SpDS post-processing workstation, a region of interest (ROI) of 2–5 mm2 was set at the largest level of the carotid atherosclerotic plaque, and measurements were taken three times, and the average value was taken. The parameters for spectral analysis included: effective atomic number (Zeff), the slope of the spectral Hounsfield unit curve (λH), iodine concentration (IC), iodine no water, and electron density (ED). All measurements were assessed independently and blinded by two radiologists with more than 5 years of experience in diagnostic neuroimaging.
Plaque Type Evaluation
Plaques were categorized into calcified plaques (≥130HU with calcified area ≥50%), non-calcified plaques (<60HU with no calcification component) and mixed plaques (60–129 HU with less than 50% calcified area and containing fibro-lipidic components) based on the CT imaging of the plaques and the CT value obtained on the 70KeV single energy image.
Statistical Methods
SPSS26.0 was used for statistical analysis. Continuous variables conforming to normal distribution were expressed as mean ± standard deviation (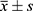 ), and comparisons between groups were made by t-test for two independent samples. Continuous variables that did not conform to normal distribution were expressed as median or quartiles, and comparisons between groups were made by the Mann–Whitney U-test for two independent samples. Categorical variable data were expressed as frequency (percentage) [n(%)], and comparisons of data between groups were made by the chi-square test (χ2). Receiver operating characteristic (ROC) curve was used to analyze the value of quantitative parameters of carotid plaque in the clinical diagnosis of AIS. P ≤0.05 was considered statistically significant.
), and comparisons between groups were made by t-test for two independent samples. Continuous variables that did not conform to normal distribution were expressed as median or quartiles, and comparisons between groups were made by the Mann–Whitney U-test for two independent samples. Categorical variable data were expressed as frequency (percentage) [n(%)], and comparisons of data between groups were made by the chi-square test (χ2). Receiver operating characteristic (ROC) curve was used to analyze the value of quantitative parameters of carotid plaque in the clinical diagnosis of AIS. P ≤0.05 was considered statistically significant.
Results
A total of 83 patients with carotid atherosclerotic plaques or stenosis were included in this study, including 41 in the AIS group and 42 in the non-AIS group. All of them underwent head CT scanning and arterial imaging with dual-layer detector spectral CT. The demographics of the enrolled patients and their basic clinical data are summarized in Table 1.
 |
Table 1 Demographic and Clinical Information on the Study Population |
Demographics and Basic Clinical Data of the Study Subjects
There were 41 patients in the AIS group and 42 patients in the non-AIS group. There was no statistically significant difference between the two groups in terms of age, gender and vascular risk factors (hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, atrial fibrillation, history of smoking, and history of alcohol consumption) and Baseline laboratory tests (Triglyceride, Total cholesterol, Low density lipoprotein, High density lipoprotein, Homocysteine, Fasting blood sugar, White blood cell, BMI (p > 0.05, Table 1)).
Carotid Atherosclerotic Plaque Examination in Two Groups
As shown in Table 2, the detection rate of atherosclerotic plaque in the AIS group was higher than that in the control group, and the difference was statistically significant (p < 0.05).
 |
Table 2 Comparison of Carotid Atherosclerotic Plaque Detection Between the Two Groups [n (%)] |
Comparison of Plaque Types Detected in the Two Groups
As shown in Table 3, a total of 80 plaques were detected in patients in the AIS group and 64 plaques in the non-AIS group. The detection rate of non-calcified plaques in the AIS group was higher than that in the non-AIS group (p < 0.05), and the detection rate of calcified plaques was lower than that in the non-AIS group (p < 0.05), with statistically significant differences; the detection rate of mixed plaques in the AIS group was higher than that in the non-AIS group (p > 0.05), with no statistically significant differences.
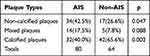 |
Table 3 Comparison of Carotid Atherosclerotic Plaque Types Between the Two Groups [n (%)] |
Comparison of Plaque Parameters Between the Two Groups
As shown in Table 4, among the energy spectral parameters of the plaques in the two groups of patients, the differences in effective atomic number, slope of the energy spectral curve, electron density, and anhydrous iodine value were statistically significant (p < 0.05); whereas the difference in iodine density value was not statistically significant (p > 0.05).
 |
Table 4 Comparison of Carotid Plaque Energy Spectrum Parameters Between AIS and Non-AIS Groups (M, IQR) |
Diagnostic Value of Each Energy Spectrum Parameter of Plaques for AIS
The ROC curve (Figure 1) and Table 5 show the diagnostic efficacy of each energy spectrum parameter for AIS; among these parameters, the iodine no water value had an area under curve (AUC) of 0.645, and the specificity and sensitivity for the diagnosis of AIS were 0.656 and 0.613. Based on the parameters obtained cut-off values, we obtained respective true-false-positive (TP/FP) and true-false-negative (TN/FN) values for the diagnosis of carotid plaques in this study [Figures 2–5, AIS group on the left (1) and non-AIS group on the right (2)].
 |
Table 5 Optimal Diagnostic Thresholds and Diagnostic Efficacy of ROC Curve Analysis of Energy Spectrum Parameters for AIS |
 |
Figure 1 ROC curves of carotid plaques leading to the development of AIS as assessed by quantitative parameters (λH, Zeff, ED, and Iodine no water) of spectral CT. |
 |
Figure 2 True-false-positive and true-false-negative results for the diagnosis of the subjects of this study using the cut-off values of the energy spectrum curves(λH). |
 |
Figure 3 True-false-positive and true-false-negative results for the diagnosis of the subjects of this study using the cut-off values of iodine no water. |
 |
Figure 4 True-false-positive and true-false-negative results for the diagnosis of the subjects of this study using the cut-off values of effective atomic number (Zeff). |
 |
Figure 5 True-false-positive and true-false-negative results for the diagnosis of the subjects of this study using the cut-off values of electron density(ED). |
Discussion
Acute ischemic stroke is the second leading cause of disability and death globally,14 bringing a heavy burden to the whole society. Carotid atherosclerosis is one of the major causes of ischemic stroke, and rupture of atherosclerotic plaques can lead to thrombosis, with embolization of thrombus into distal intracranial arteries15 leading to ischemic events. Proper identification and evaluation of carotid atherosclerotic plaques is extremely important for the diagnosis, treatment, and prevention of AIS. Carotid CTA is a reliable and relatively noninvasive method of assessing carotid plaque.16 As a double-detector spectral CT, IQon energy spectrum CT is a new dual-energy CT imaging technology, which requires no prior selection of scanning mode and does not increase the radiation dose of patients. Spectral images and data can be obtained in a single scanning, which can be used for retrospective analysis.17–19
The development of AIS is closely related to unstable plaques, whose rupture, and embolus dislodgement leading to vessel occlusion are the main pathogenic mechanisms. Clinically, plaques are often classified into calcified plaques, non-calcified plaques, and mixed plaques.20 Calcified plaques are stable, not easy to rupture, and more secure, also known as hard plaques, while non-calcified plaques have a high lipid content and a thin fibrous cap on the surface which may rupture and form thrombi, resulting in cardiovascular and cerebral vascular events, so they are also known as soft plaques or vulnerable plaques.21,22 In this study, we found that the carotid atherosclerotic plaques in the AIS group were mainly non-calcified (soft plaques), whereas the carotid atherosclerotic plaques in the non-AIS group were predominantly calcified (hard plaques); whereas, the use of energy–spectrum CT allows for further quantitative analysis of the parameters of the two plaques in both groups, such as the effective atomic number (Eff-Z), the slope of the energy–spectrum curves, the electron density, the iodine density, and so on. The effective atomic number corresponds to different elements according to the different attenuation coefficients of X-ray absorption of different substances, and the atomic number of the element that is the atomic number of the substance. The energy spectrum curve is the change of the absorption coefficient of a certain substance with the change of energy, which can be used to distinguish between different substances.23 Using the IQon energy spectroscopy CT technique, by exploiting the large difference in atomic number between the two elements iodine and water, it is possible to obtain the absorption attenuation difference, which is more intuitively reflecting the attenuation image of different substances.24,25 Relevant studies have shown that different plaques have different energy spectral profiles and effective atomic numbers, which are mainly manifested as calcified plaques > fibrous plaques > thrombus-like plaques > fatty plaques,26 therefore, the higher parameter quantification suggests that the more calcified components in the plaques, the more stable the plaques are. In this study, the non-AIS group was mainly dominated by soft plaques, and the AIS group was dominated by hard plaques, and the slopes of the energy spectral curves, effective atomic numbers, electron densities, and anhydrous iodine values of the former were higher than those of the latter, and the differences were statistically significant (P < 0.05), while the differences in the iodine density values of the plaques in the two groups were not statistically significant (P > 0.05). According to the results of the study and previous studies, it can be suggested that the quantitative energy spectral parameters of the slope of the energy spectrum curve, effective atomic number, electron density, and iodine no water value can be used to assess plaque stability; the higher the quantification of the parameters, the higher the degree of calcification, the higher the stability of the plaque, and the lower the risk of developing AIS. In this study, the ROC curve was further applied to show the critical values and diagnostic efficacy of each energy spectral parameter, as shown in Table 5, indicating that when the slope of the energy spectral curve of a plaque, the effective atomic number, the electron density, and the iodine no water value are less than 19.56, 11.865, 110.45, and 9.125, respectively, the plaque is considered to be at a higher risk of causing AIS, and the specificity, sensitivity, and AUC can be seen in Table 5. Among these parameters, the iodine no water value had an area under curve (AUC) of 0.645, and the specificity and sensitivity for the diagnosis of AIS were 0.656 and 0.613.
The occurrence of stroke is not only related to the location, size, and morphology of the plaque, but also to the internal properties of the plaque. Compared with conventional CT, which only provides a single parameter, spectral CT provides us with more parameters reflecting the properties of the plaque, and in this study, we hope to find out which of these parameters are closely related to stroke.
We found that the effective atomic number, slope of the energy spectrum, electron density, and anhydrous iodine value of carotid plaques on spectral CT were associated with stroke, and the next step will be to further investigate the use of these results to predict the risk of stroke onset and recurrence, the effect on the effectiveness of drug therapy, and the relationship with the risk of surgery. The next step will be to further study these results to predict the risk of stroke recurrence and recurrence, the effect of drug treatment, and the relationship with surgical risk. Meanwhile, dynamic monitoring of these parameters within the plaque can also help to evaluate the effectiveness of intervention in patients with high-risk carotid plaques.
As the world has been watching, stroke prevention itself is extremely important, and screening for stroke has long been a key goal for neurologists. What we need to do before an AIS event occurs is to screen the high-risk group. First of all, for those who go to the hospital on their own, the clinician will ask for a careful medical history, and if there is a history of stroke or TIA, age over 40 with atrial fibrillation or heart valve disease, hypertension, dyslipidemia, diabetes mellitus, obesity, or a family history of stroke, then further screening is extremely necessary. It is well known that in clinical practice, vascular ultrasound is usually the first choice for screening for vascular disease in the neck; we can then collect their data and recommend further spectral CTA for patients with abnormalities; for patients with a history of stroke or cerebrovascular disease, we can recommend direct spectral CTA. On the other hand, the integration of medicine and prevention is also extremely important. This is a wider screening for the community population, after the questionnaire survey and simple and convenient carotid ultrasound examination, data collection and management can be carried out to screen out the high-risk group of stroke and carotid vascular abnormality, to contact and follow up, and to carry out further spectral CT examination for them.
Due to the specificity of iodine CT, it is less suitable for those with severe renal insufficiency, heart failure, and allergy to iodine. However, screening with CTA is necessary for patients with risk factors for stroke. For example, patients with smoking, hyperlipidemia, hypertension, diabetes, obesity, and family history of stroke. We believe that screening is necessary for asymptomatic patients with high-risk factors or atypical symptoms (occasional dizziness, headache, memory loss, and other long-term chronic ischemic symptoms), middle-aged and elderly people with a family history of stroke or cerebrovascular disease (who are in good health), patients with stenosis and plaques suggested by clinical carotid ultrasound and who can be further clarified by CTA to determine the degree of stenosis and the composition of plaques and patients who have undergone CEA or CAS in the past for follow-up.
In summary, more exploration and research is needed regarding the quantitative parameters of spectral CT for the assessment of high-risk carotid plaques.
Limitations
There are some limitations of this study. First, the number of cases was small and from one clinical center. Secondly, this study was retrospective, and there were some biases in the selection. In addition, the laboratory indicators in this study were not comprehensive enough and were not further explored for potentially influential laboratory indicators. Finally, this study did not use thresholds to externally validate the prediction results.
Conclusion
In summary, the quantitative parameters of dual-layer detector spectral CT can be used to assess plaque stability and have a certain value in the diagnosis of AIS. The quantitative parameters can effectively differentiate carotid plaques in AIS and non-AIS patients.
Ethics Approval and Informed Consent
The studies involving human participants were reviewed and approved by the Clinical Trial Ethics Committee of the Second People’s Hospital of Hefei (2023-Scientific Research-006). Written informed consent was obtained from all participants and in accordance with the principle of the Helsinki Declaration.
Acknowledgments
We are grateful to the study participants and the staff of the Department of Neurology of the Second People’s Hospital of Hefei for their cooperation in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Hefei Key Common Technology R&D Project (GJ2022SM07).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. doi:10.1016/S1474-4422(21)00252-0
2. Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(20 Suppl 2):S6–S16. doi:10.1212/WNL.0000000000012781
3. Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022 [published correction appears in Int J Stroke. 2022 Apr;17(4):478]. Int J Stroke. 2022;17(1):18–29. doi:10.1177/17474930211065917
4. Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: a Report From the American Heart Association [published correction appears in Circulation. 2018 Mar 20;137(12):e493]. Circulation. 2018;137(12):e67–e492. doi:10.1161/CIR.0000000000000558
5. Magge R, Lau BC, Soares BP, et al. Clinical risk factors and CT imaging features of carotid atherosclerotic plaques as predictors of new incident carotid ischemic stroke: a retrospective cohort study. AJNR Am J Neuroradiol. 2013;34(2):402–409. doi:10.3174/ajnr.A3228
6. Song P, Fang Z, Wang H, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. 2020;8(5):e721–e729. doi:10.1016/S2214-109X(20)3011
7. van Dam-Nolen DHK, Truijman MTB, van der Kolk AG, et al. Carotid plaque characteristics predict recurrent ischemic stroke and TIA: the PARISK (Plaque At RISK) Study. JACC Cardiovasc Imaging. 2022;15(10):1715–1726. doi:10.1016/j.jcmg.2022.04.003
8. Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106(11):1368–1373. doi:10.1161/01.cir.0000028591.44554.f9
9. Zhang L, Li X, Lyu Q, Shi G. Imaging diagnosis and research progress of carotid plaque vulnerability. J Clin Ultrasound. 2022;50(7):905–912. doi:10.1002/jcu.23266
10. Syed MB, Fletcher AJ, Forsythe RO, et al. Emerging techniques in atherosclerosis imaging. Br J Radiol. 2019;92:20180309. doi:10.1259/bjr.20180309
11. Sueta D, Utsunomiya D, Izumiya Y, et al. Novel assessment of retrospective on-demand analysis of venous thromboembolism by dual-layer spectral-detector CT. J Cardiol Cases. 2018;18(3):88–91. doi:10.1016/j.jccase.2018.05.003
12. Große Hokamp N, Maintz D, Shapira N, Chang H, Noël PB. Technical background of a novel detector-based approach to dual-energy computed tomography. Diagn Interv Radiol. 2020;26(1):68–71. doi:10.5152/dir.2019.19136
13. Wang X, Liu D, Zeng X, et al. Dual-energy CT quantitative parameters for the differentiation of benign from malignant lesions and the prediction of histopathological and molecular subtypes in breast cancer. Quant Imag Med Surg. 2021;11:1946–1957. doi:10.21037/qims-20-825
14. Tao L, ShiChuan W, DeTai Z, Lihua H. Evaluation of lipoprotein-associated phospholipase A2, serum amyloid A, and fibrinogen as diagnostic biomarkers for patients with acute cerebral infarction. J Clin Lab Anal. 2020;34:e23084. doi:10.1002/jcla.23084
15. Kopczak A, Schindler A, Sepp D, et al. Complicated Carotid Artery Plaques and Risk of Recurrent Ischemic Stroke or TIA. J Am Coll Cardiol. 2022;79(22):2189–2199. doi:10.1016/j.jacc.2022.03.376
16. Shinohara Y, Sakamoto M, Kuya K, et al. Assessment of carotid plaque composition using fast-kV switching dual-energy CT with gemstone detector: comparison with extracorporeal and virtual histology-intravascular ultrasound. Neuroradiology. 2015;57(9):889–895. doi:10.1007/s00234-015-1541-5
17. Demirler Simsir B, Danse E, Coche E. Benefit of dual-layer spectral CT in emergency imaging of different organ systems. Clin Radiol. 2020;75(12):886–902. doi:10.1016/j.crad.2020.06.012
18. Rassouli N, Etesami M, Dhanantwari A, Rajiah P. Detector-based spectral CT with a novel dual-layer technology: principles and applications. Insights Imaging. 2017;8(6):589–598. doi:10.1007/s13244-017-0571-4
19. Huang J, Chen J, Wang X, et al. The diagnostic value of quantitative parameters on dual-layer detector-based spectral CT in identifying ischaemic stroke. Front Neurol. 2023;14:1056941. doi:10.3389/fneur.2023.1056941
20. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis [published correction appears in N Engl J Med. 2011 Nov 24;365(21):2040]. N Engl J Med. 2011;364(3):226–235. doi:10.1056/NEJMoa1002358
21. Funayama H, Ishikawa SE, Kubo N, et al. Increases in interleukin-6 and matrix metalloproteinase-9 in the infarct-related coronary artery of acute myocardial infarction. Circ J. 2004;68(5):451–454. doi:10.1253/circj.68.451
22. Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107(12):1579–1585. doi:10.1161/01.CIR.0000058700.41738.12
23. Fabiani I, Palombo C, Caramella D, Nilsson J, De Caterina R. Imaging of the vulnerable carotid plaque: role of imaging techniques and a research agenda [published correction appears in Neurology. 2021 Nov 16;97(20):968]. Neurology. 2020;94(21):922–932. doi:10.1212/WNL.0000000000009480
24. Mech P, Makowski M, Kawiak A, Chylewska A. When biomolecules meet 2-hydrazinopyrazine: from theory through experiment to molecular levels using a wide spectrum of techniques. RSC Adv. 2020;10(67):40673–40688. doi:10.1039/d0ra06239a
25. Chung JH. Update on thyroid hormone levels and thyroid dysfunction in the Korean population based on data from the Korea National Health and Nutrition Examination Survey VI (2013 to 2015). Endocrinol Metab. 2020;35(1):7–13. doi:10.3803/EnM.2020.35.1.7
26. Bai X, Gao P, Zhang D, et al. Plaque burden assessment and attenuation measurement of carotid atherosclerotic plaque using virtual monoenergetic images in comparison to conventional polyenergetic images from dual-layer spectral detector CT. Eur J Radiol. 2020;132:109302. doi:10.1016/j.ejrad.2020.109302
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
