Back to Journals » Infection and Drug Resistance » Volume 16
Diagnostic Value and Clinical Application of Metagenomic Next-Generation Sequencing for Infections in Critically Ill Patients
Authors He Y, Geng S, Mei Q , Zhang L, Yang T, Zhu C, Fan X, Wang Y, Tong F, Gao Y, Fang X, Bao R, Sheng X, Pan A
Received 7 June 2023
Accepted for publication 14 September 2023
Published 25 September 2023 Volume 2023:16 Pages 6309—6322
DOI https://doi.org/10.2147/IDR.S424802
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Sandip Patil
Yuxi He,1,* Shike Geng,1,* Qing Mei,1,* Lei Zhang,1,* Tianjun Yang,1 Chunyan Zhu,1 Xiaoqin Fan,1 Yinzhong Wang,1 Fei Tong,1 Yu Gao,1 Xiaowei Fang,1 Renren Bao,2 Ximei Sheng,3 Aijun Pan1– 3
1Department of Intensive Care Unit, the First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, People’s Republic of China; 2Department of Intensive Care Unit, the Affiliated Provincial Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China; 3Department of Intensive Care Unit, the Training Center of Anhui Provincial Hospital, Wannan Medical College, Wuhu, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Aijun Pan; Qing Mei, Department of Intensive Care Unit, the First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, No. 17, Lu Jiang Road, Hefei, Anhui Province, People’s Republic of China, Tel +86-0551-62286175, Email [email protected]; [email protected]
Objective: To evaluate the diagnostic value and clinical application of metagenomic next-generation sequencing (mNGS) for infections in critically ill patients.
Methods: Comparison of diagnostic performance of mNGS and conventional microbiological testing for pathogens was analyzed in 234 patients. The differences between immunocompetent and immunocompromised individuals in mNGS-guided anti-infective treatment adjustment were also analyzed.
Results: The sensitivity and specificity of mNGS for bacterial and fungal detection were 96.6% (95% confidence interval [CI], 93.5%– 99.6%) and 83.1% (95% CI, 75.2%– 91.1%), and 85.7% (95% CI, 71.9%– 99.5%) and 93.2% (95% CI, 89.7%– 96.7%), respectively. Overall, 152 viruses were detected by mNGS, but in which 28 viruses were considered causative agents. The proportion of mNGS-guided beneficial anti-infective therapy adjustments in the immunocompromised group was greater than in the immunocompetent group (48.5% vs 30.1%; P=0.008). In addition, mNGS-guided anti-infective regimens with peripheral blood and BALF specimens had the highest proportion (39.0%; 40.0%), but the proportion of patients not helpful due to peripheral blood mNGS was also as high as 22.0%.
Conclusion: mNGS might be a promising technology to provide precision medicine for critically ill patients with infection.
Keywords: next-generation sequencing, critically ill patients, community-acquired infection, hospital-acquired infection, immunosuppression
Introduction
Infection is one of the greatest challenges for critically ill patients and intensivists. Previous investigations reported that 30% to 60% of antimicrobials used in ICUs are unnecessary, inappropriate, or suboptimal,1,2 which might be partly attributed to the many defects of conventional microbiological tests (CMT). Culture is the most commonly used method to identify bacteria and fungi, but some species are fastidious or even unculturable, which leads to a low detection rate.3 Even worse, the use of antimicrobial treatment before microbiological tests will reduce the detection rate further. Poor timeliness of culture is also a cause for the late induction of timely targeted treatment. Although culture-independent approaches such as antigen detection, serological testing, and molecular detection tests are time-saving, a priori hypothesis is necessary, and targeting a limited number of pathogens limits their diagnostic value.4 Therefore, CMT cannot fully satisfy the needs for the diagnosis of critically ill patients.
Metagenomic next-generation sequencing (mNGS) is a hypothesis-free, or unbiased revolutionary technology, allowing the fast and extensive identification of common, unexpected, or novel organisms.4 It has been tentatively used for the diagnosis and treatment adjustment of various infectious diseases including those of the central nervous system,5,6 bloodstream,7 respiratory,8,9 gastrointestinal10,11 and ocular systems,12 and periprosthetic joints.13 Guidelines recommend that mNGS is suitable for critically ill patients with rapid disease progression and frequent antimicrobial use because it is rapid and accurate.14 However, mNGS still has some hurdles remain to be addressed. For instance, the interpretation of mNGS results were often inappropriate or misleading due to a lack of unified interpretation standards. More importantly, patients in ICU have complex conditions and various infectious sites. It is still unknown what type of clinical specimens can maximize the benefit to the patient, and what kind of patients benefit most from mNGS. Therefore, mNGS is an expensive method that does not fulfill the characteristics of a first-line test currently.
To date, comprehensive reports on the application of mNGS in critically ill patients are scarce. This study aimed to evaluate the diagnostic efficacy of mNGS compared with CMT in ICU patients with infections using various specimens. We also aimed to better understand the microbial etiology and the impact of mNGS on anti-infective treatment in immunocompetent or immunocompromised patients.
Materials and Methods
Study Design and Patient Selection
We retrospectively reviewed 358 critically ill patients suspected of infection at the First Affiliated Hospital of the University of Science and Technology of China (USTC), a tertiary care hospital with 130 beds in four ICU centers, between April 2020 to September 2021 (Supplementary Figure 1). The following inclusion criteria were used: (1) CMT and mNGS tests were conducted at an interval of less than 24 hours; (2) the specimens used for mNGS and CMT were of the same type. The exclusion criteria for patients were as follows: (1) cases retrospectively diagnosed as non-infectious diseases; (2) the specimen collection of patients with community-acquired infection was completed more than 48 hours after ICU admission; (3) for patients with a hospital-acquired infection, specimens were collected more than 48 hours after the diagnosis of infection was determined; (4) incomplete medical record; (5) specimen failing to pass quality control of mNGS; and (6) specimen leakage or pollution. Data collection was performed retrospectively from the electronic medical records of patients using a standardized data collection sheet. For all enrolled patients, we collected demographic data, immune status, complications, treatment interventions, laboratory tests, imaging information, and patient outcomes. The severity of illness was assessed using the Acute Physiology and Chronic Health Evaluation (APACHE) II score and Sequential Organ Failure Assessment (SOFA) score. This study was approved by the institutional review board (approval number: 2022-RE-001). Because of the retrospective nature of the study, informed written consent was waived.
Specimen Collection
Different types of specimen collection for testing were based on the site of the suspected infection. Bronchoalveolar lavage fluid (BALF) was collected by electronic bronchoscope, and endotracheal aspirate (ETA) was collected by a sterile aspirator from patients with a respiratory infection. For patients with central nervous system infection, cerebrospinal fluid (CSF) was obtained by lumbar puncture or from a drainage tube. Other puncture or drainage specimens included bile, pleural fluid, ascitic fluid, and abscess. When the lesional specimen was difficult to obtain or the site of infection was difficult to diagnose, a peripheral blood specimen was collected. All specimens were transported in dry ice after collection and delivered to the laboratory within 1 hour.
Conventional Microbiological Tests
The CMT used in this study included bacterial and fungal cultures, smear microscopy, antigen detection, serological tests, and polymerase chain reaction (PCR) (Supplementary Box 1). Culture and smear microscopy (except for special staining) were performed for each sample. Other CMT were conducted according to the specimen type and necessity of clinical assessment.
Metagenomic Next-Generation Sequencing and Analysis
The corresponding specimens were delivered on dry ice to the Matridx Biotechnology Company (Hangzhou, China) for mNGS detection. Deoxyribonucleic acid (DNA) sequencing was conducted for each patient, but ribonucleic acid (RNA) sequencing was conducted only for patients with suspected RNA viral infection. After receiving the specimen, a 1.2 mL homogenized sample was centrifuged at 12,000 rpm for 3 minutes at 4°C and 400 μL of the supernatant was added to the prepared matching cassette. Next, the cassette was placed into an NGSmaster™ automation workstation for the following procedure. The internal ambient temperature of the machine is maintained at 4°C. DNA libraries were constructed by automated nucleic acid extraction, reverse transcription (only for RNA), nucleic acid fragmentation, terminal repair, adding A-tailing, primer ligation, and purification. Finished libraries were quantified by real-time PCR and sequenced by a shotgun approach using the Illumina Nextseq™ high-throughput sequencing platform.
After filtering out low-quality and low complexity data using fastp (https://github.com/OpenGene/fastp), approximately 20 million 75 bp single-end reads were generated for each library. Bioinformatics analysis was conducted on the library sequence data, and then the human genome sequence data (GRCh38.p13) was filtered using Bowtie 2 software (http://bowtie-bio.sourceforge.net/bowtie2/index.shtml). The remaining reads were aligned to bacterial, viral, fungal, and parasitic reference databases (NCBI GenBank and an in-house curated genomic database) using Kraken (https://github.com/DerrickWood/kraken) to determine microbial species and read counts. The alignment results were further verified using the NCBI BLAST software (https://blast.ncbi.nlm.nih.gov/Blast.cgi). For every sequencing run, two negative controls (approximately 104 and 106 human immortalized cells) and one positive control (a mixture of inactivated bacteria, fungi, and pseudovirus particles) were included.
Quality control of mNGS: (1) library concentration > 50 pmol/L; (2) Q20 (proportion of bases with sequencing quality value above 20) > 85%; (3) Q30 (proportion of bases with sequencing quality values above 30) > 80%; (4) guanine-cytosine content < 45%; (5) total reads > 10 million.
Criteria for a Positive mNGS Result
- A database was established of background microorganisms, which contains microorganisms appearing in more than 50% of the specimens in the laboratory in the past three months, then compare the detected microorganisms with the in-house database to delete the suspected background microorganisms.
- To exclude false positive results originating from microbial contamination introduced during experimental operations, the stringently mapped read number (SMRN) of the detected microorganism in a sample must be greater than 2-fold above that in the negative control.
- Bacteria (mycobacteria excluded), fungi (molds excluded), viruses, and parasites: the relative abundance at the species level was more than 30%.
Mycobacterium: Given the difficulty of DNA extraction and low possibility of contamination, the SMRN at the species level was more than 3.
Molds: Given the balance of environmental contamination and the difficulty of DNA extraction from fungal thick polysaccharide cell walls, the SMRN at the species level was more than 10 and the result complied with the clinician’s judgment based on clinical symptoms.
Gold Standard for Causative Pathogens
The final determination of the causative pathogen (the gold standard) was based on comprehensive clinical diagnostic criteria, which have been determined by comprehensive analysis of microbiological results and other relevant information (such as clinical features, laboratory tests, imaging studies, and the observation of treatment effects). Microorganisms detected by CMT or mNGS but not considered causative pathogens according to the gold standard were defined as false positives. Two infection management experts independently reviewed each patient’s electronic medical records and resolved any disagreements through in-depth discussions.
Clinical Evaluation
The presence or absence of infection and the site of infection was evaluated by the two experts. Any controversy between them was resolved by further discussion, and if a consensus could not be reached, a senior intensivist was consulted. The diagnosis of infection was based on the CDC/NHSN surveillance definitions for specific types of infections.15
Immunocompromised status was defined as any of the following: (1) recent chemotherapy or neutropenia (<1000/μL) during the last month; (2) long-term therapy with steroids (>0.3 mg/kg/d of prednisone equivalent dose for more than 3 weeks); (3) hematological malignancy; or (4) immunosuppressive therapy after solid organ transplantation.
The impact of mNGS detection on clinical antimicrobial treatment adjustment was divided into four aspects. The initiation of targeted treatment or the de-escalation of treatment were considered the clinical beneficial impact. mNGS also helped to confirm existing anti-infective treatment in concordance with the etiologic diagnosis. mNGS could also show no clinical treatment benefit when the pathogen could not be identified.
Statistical Analysis
Continuous variables were expressed as the median and interquartile range if they did not satisfy the normal distribution. Then, two independent samples were compared by the Mann–Whitney U-test. Categorical variables were expressed as the constituent ratio or rate, and a comparison of two independent samples was conducted by the chi-square test or Fisher’s exact test. Results from the gold standard assessment were used as the reference to assess the diagnostic efficacy of mNGS and CMT. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated by the McNemar test. 95% confidence intervals for these proportions were determined using Wilson’s method. IBM SPSS Version 26.0 software (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. All statistical tests were two-tailed, and a p-value < 0.05 was considered significant.
Results
Specimens and Patient Characteristics
Ultimately, 234 cases were included for final analysis. The clinical characteristics of the patients are provided in Table 1. Overall, 193 patients (82.5%) received mechanical ventilation and 142 patients (60.7%) were treated with vasoactive medications related to shock. The median of inflammatory markers (including white blood cell count, C-reactive protein, and procalcitonin) exceeded the upper limit of normal. Most patients (229/234, 97.9%) received antimicrobial treatment before the collection of specimens. Nearly half of the patients (47.0%) died in the ICU.
 |
Table 1 Demographic Characteristics of the Patients in the Present Study (n=234) |
Overall, 141 patients (60.2%) underwent mNGS related to lower respiratory tract infection (including pneumonia and empyema). Central nervous system infection (33/234, 14.1%) and gastrointestinal system infection (26/234, 11.1%) were also common infection sites (Supplementary Figure 2A). The types of specimens submitted for mNGS are shown in Supplementary Figure 2B. Because some patients with lower respiratory tract infection were tested for mNGS using peripheral blood, blood specimens accounted for the largest proportion of samples (82/234, 35.0%), followed by BALF (70/234, 29.9%), CSF (33/234, 14.1%), and ETA (26/234, 11.1%).
Diagnostic Performance Comparison of mNGS and CMT
The positive rate of mNGS for pathogenic bacterial detection (155/234, 66.2%) was significantly higher than that of CMT (87/234, 37.2%) (P < 0.001). When the gold standard assessment was used as the reference for correctness, the diagnostic sensitivity of mNGS for bacterial detection was 96.6%, whereas the sensitivity of CMT was only 55.2%. For fungal detection, 15/234 patients (6.4%) were positive for CMT and the results of mNGS were positive in 38 patients (16.2%), which was statistically significant (P = 0.001). The diagnostic sensitivity of mNGS for fungal detection was 85.7%, whereas the sensitivity of CMT was only 42.9% (Supplementary Table 1).
The positive rates of pathogens vary among different specimen types. The detection rates of bacteria and fungi in the BALF and ETA were generally higher than those in sterile body fluids (blood and CSF). The positive rate of bacterial detection in various specimens by mNGS was higher than that by CMT. There was a significant difference between these two methods for the detection of bacteria in blood (56.1% vs 18.3%, P < 0.001), BALF (80.0% vs 58.6%, P = 0.006), CSF (36.4% vs 15.2%, P = 0.049), and ascites (92.9% vs 28.6%, P < 0.001), but there was no statistical difference in the positive rate of ETA (73.1% vs 61.5%, P = 0.375). Similarly, the positive rate of fungi in different specimens by mNGS was also higher than that by CMT, although significant differences were only determined for blood (15.9% vs 1.2%, P =0.001). The diagnostic performance of mNGS for all specimens was also different. The sensitivity of mNGS was generally high for bacterial detection; however, mNGS did not show a significant advantage in specificity. Generally, the sensitivity of mNGS for fungi detection in different types of specimens varied greatly, whereas the specificity of fungal detection was high except for BALF (Supplementary Table 1).
Concordance Between mNGS and CMT for Pathogen Detection
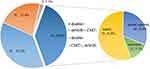
For bacterial and fungal detection, mNGS and CMT were both positive in 91/234 (38.9%) cases and were both negative in 51/234 (21.8%) cases. Of 91 patients who were positive by both methods, the results of 51 patients were completely matched and the results of 12 patients were completely mismatched (Figure 1). The remaining 28 patients were “partially matched”, indicating that at least one pathogen detected by the two methods overlapped. The consistency of pathogen detection by mNGS and CMT (including double negative, double positive with complete matching, and double positive with partial matching) was relatively high in the BALF (67.1%), ETA (76.9%), and CSF (69.7%), and relatively low in the blood and ascites (39.0% and 21.4%, respectively). In addition, the proportion of double negative results was highest in the blood (21/82, 25.6%) and CSF (19/33, 57.6%), but the proportion of complete matching double positive results was also highest in the blood (10/13, 76.9%) and CSF (4/4, 100%) (Supplementary Figure 3).
Identification of Pathogens Using the mNGS and CMT Methods
Among the 280 pathogenic microorganisms (excluding viruses) detected, Acinetobacter baumannii (45/280) was the most commonly isolated pathogen, followed by Klebsiella pneumoniae (37/280) and Pneumocystis jirovecii (21/280) (Figure 2). The percentage of mNGS-positive specimens was significantly higher than the CMT-positive specimens when detecting bacteria (P < 0.001), fungi (P < 0.001), obligate anaerobes (P < 0.001), Acinetobacter baumannii (P = 0.031), Enterococcus faecium (P < 0.001), Corynebacterium striatum (P = 0.039), and P. jirovecii (P < 0.001). Although the difference was not significant (100% vs 16.7%; P = 0.063), mNGS detected all Streptococcus pneumoniae, whereas CMT detected only 1/6 cases. mNGS only detected three cases of Mycobacterium spp. (two cases of Mycobacterium tuberculosis complex and one case of nontuberculosis mycobacteria), two cases of Legionella pneumophila, one case of Chlamydia psittaci, and one case of Rickettsia japonica. When different types of specimens were analyzed separately, there was little difference between mNGS and CMT for the detection of specific species (Supplementary Figure 4). In terms of bacteria, there were significant differences between the two methods only for the detection of K. pneumoniae in blood (P = 0.021) and obligate anaerobes in ascites (P = 0.003). In terms of fungi, the detection rates of P. jirovecii (P = 0.012) in blood by mNGS were significantly higher than those detected by CMT. In addition, one case of Aureobasidium umbelliforme and one case of Rhizomucor pusillus in the blood were detected only by mNGS. mNGS detected all P. jirovecii infections in the BALF (n=8) and ETA (n=4), whereas only three cases were detected in the BALF and no cases was identified in the ETA by CMT, although there were no statistically significant differences (P = 0.063 and P = 0.219, respectively). In the CSF, one case of Candida spp. and one case of Aspergillus spp. were detected only by mNGS. Only Candida spp. was detected in the ascites, and there was no significant difference in detection between the two methods (P = 0.625). Bile drainage fluid was examined in three patients with biliary tract infection. The etiological results suggested Gram-negative bacteria, two of which were K. pneumoniae. Among five patients with skin and soft tissue infection who submitted abscess puncture fluid for microbiological detection, oral obligate anaerobes were detected in three patients. Oral anaerobes were also detected in the pleural puncture fluid of a patient with empyema. Four patients with the detection of oral anaerobes were diagnosed as mixed infection.
Among 234 patients, 152 viruses were detected in 105 patients by mNGS. However, when combining clinical manifestations, imaging results, sequencing data, and literature searches, only 11 different types of viruses in 28 patients were considered as causative agents of infection. The most common pathogenic viruses were herpes simplex virus type 1 (HSV-1), cytomegalovirus (CMV), and Epstein-Barr virus (EBV) (Figure 3). HSV-1 and CMV were considered pathogens of lung infection and EBV was considered a pathogen of viral encephalitis. Herpes virus and torque teno virus (TTV) were the most common viruses detected in all specimens (Supplementary Figure 5). Viruses detected in the CSF of 11 patients with central nervous infection were all considered causative agents except for TTV. mNGS identified one CMV and one EBV in one ascites specimen, but they were not considered causative pathogens of the infection. The virus detection rates in the blood and ETA were the highest (57.3% (47/82) and 65.4% (17/26) respectively), but pathogenic viruses accounted for only 12.2% (10/82) and 3.8% (1/26) of corresponding specimens. The virus detection rate in the BALF (40.0%, 28/70) was significantly lower than that in the ETA (65.4%,17/26) (P = 0.027), and pathogenic viruses accounted for 10% of all BALF specimens (7/70). RNA sequencing was conducted only for patients (n = 25) with suspected RNA viral infection. Twenty-two of them presented with systemic symptoms (fever, headache, sore throat, myalgia, diarrhea, etc.) and imaging findings suggestive of viral infection, but only 1 human parainfluenza virus (hPIV) and 1 respiratory syncytial virus (RSV) were detected in BALF specimens. Two patients had a history of field work or insect bites and laboratory tests suggested thrombocytopenia, and severe fever with thrombocytopenia syndrome virus (SFTSV) was detected in 1 CSF specimen. One patient presented with neurological disorders (impaired consciousness, convulsion, etc.) and had a history of suspected mosquito bites, but the results was negative. All three RNA viruses detected were identified as pathogenic viruses.
Infection with more than one pathogen is termed mixed infection or co-infection. mNGS identified 134 (57.3%) patients with one pathogen infection, 44 (18.8%) patients with two pathogen infections, 12 (5.1%) patients with three pathogen infections, and 4 (1.7%) patients with at least four pathogen infections. Only 24 patients (10.3%) were diagnosed as mixed infection by CMT whereas 60 patients (25.6%) were identified as mixed infection by mNGS. The detection rate of mixed infection by mNGS alone was significantly higher than that by CMT alone (P < 0.001). The proportion of patients with mixed infection was different for different specimen types. The percentage of mixed infection in BALF, ETA, and ascites specimens was high by mNGS (37.1%, 34.6%, and 57.1% respectively). By contrast, no mixed infection was identified in the CSF in our study. The diagnostic ratio of mixed infection by mNGS was significantly higher than that by CMT in the blood (66.7% vs 16.7%, P = 0.006), BALF (72.2% vs 38.9%, P = 0.009), and ascitic fluid (80.0% vs 10.0%, P = 0.005) (Supplementary Figure 6).
Pathogen Spectrum Differ Between Immunocompetent and Immunocompromised Patients
Patients were categorized into two groups, immunocompetent and immunocompromised groups, according to the immune status of patients. The differences of clinical characteristics between the two groups were listed in Supplementary Table 2. There was a difference in the pathogen spectrum between the immunocompetent and immunocompromised groups when combining the CMT and mNGS results. The proportion of immunocompetent patients with bacterial infection (75.3% vs 57.4%; P = 0.006) was significantly higher than that in the immunocompromised group, but the proportion of fungal (7.2% vs 40.0%; P < 0.001) and viral (8.0% vs 18.0%; P = 0.02) infections was higher in immunocompromised individuals (Figure 4A). In the immunocompetent group, the most common fungus was Candida spp. and the most common virus was HSV-1. In contrast, the most common fungus and virus in the immunocompromised group were P. jirovecii and CMV, respectively. The proportion of P. jirovecii (OR, 76.5; 95% CI, 10.1–582.5; P < 0.001) and CMV (OR, 15.9; 95% CI, 18.4–135.3; P = 0.003) infection in the immunocompromised group was significantly higher than that in the immunocompetent group and the proportion of obligate anaerobes (OR, 0.1; 95% CI, 0.02–1.00; P = 0.022) infection in the immunocompromised group was significantly lower than that in the immunocompetent group (Figure 4B). Although there was no statistically significant difference in the proportion of mixed infection between the two groups (36.8% vs 28.9%, P=0.239), bacterial-bacterial infections were the most common types of mixed infection both in the immunocompetent and immunocompromised groups (36/48, 75.0% vs 8/25, 32.0%). Fungal-fungal mixed infection (3/25) was only present in the immunocompromised group. The proportion of co-infection with fungi and other pathogens (64.5% vs 30.6%; P = 0.002) or viruses and other pathogens (32.3% vs 8.1%; P = 0.003) in the immunocompromised group was significantly higher than that in the immunocompetent group. Additionally, a mixed infection pattern of CMV and P. jirovecii was unique in immunocompromised patients (OR, 10.9; 95% CI, 1.2–97.2; P = 0.019) (Figure 4C).
Pathogen Spectrum Differ Across Disease Type
Pathogen Spectrum in the different disease groups are shown in Supplementary Figure 7. In general, each group shows its characteristic, different from the others. Although both were lower respiratory tract infections, infection by conditional pathogenic bacteria represented by A. baumannii and P. aeruginosa, were more common in the hospital acquired pneumonia group, whereas infection by P. jirovecii was predominant in patients with community acquired pneumonia; K. pneumoniae holds a dominant position in both groups. A. baumannii and S. pneumoniae are the most common pathogens of central nervous system infection. Obligate anaerobes are the main culprits of gastrointestinal system infection and skin and soft tissue infection. For bloodstream infections, no fungi or viruses were identified as responsible pathogens. The proportion of mixed infections vary among different diseases groups. 24 (36.4%) mixed infections occurred in CAP patients, 34 (45.3%) in the HAP, 13 (50.0%) in the gastrointestinal infection, 5 (35.7%) in the skin and soft tissue infection, and 2 (15.4%) in the bloodstream infection. However, none occurred in the nervous system infection group.
Impact on Anti-Infective Treatment
mNGS led to a clinically beneficial impact on treatment adjustment in 83 (35.5%) patients. The clinically beneficial therapeutic impact included the initiation of targeted treatment (77/234, 32.9%) and the de-escalation of treatment (6/234, 2.6%) (Figure 5A). mNGS provided a greater proportion of clinically beneficial anti-infective adjustment for the immunocompromised group compared with the immunocompetent group (48.5% vs 30.1%; P = 0.008) (Figure 5B). Regarding different specimen types, the clinically beneficial impact based on mNGS results was greater when using the blood (39.0%) and BALF (40.0%), and the lowest benefit was for ascites (21.4%). The percentage of no clinical benefit was highest for sterile specimens (blood, 22.0%; CSF, 30.3%), and the lowest was for ascites (0.0%). In respiratory specimens, the proportion of no clinical benefit in the BALF by mNGS was lower than in ETA, although this did not reach statistical significance (5.7% vs 15.4%, P = 0.268) (Supplementary Figure 8).
Discussion
In our study, mNGS was superior for bacterial identification compared with CMT. The positive rate was significantly higher than that for CMT (66.2% vs 37.2%) with a sensitivity of 96.6%. This advantage was observed for common ICU bacteria (eg, Acinetobacter baumannii, Enterococcus faecium, etc.), but also for bacteria that are difficult to diagnose by CMT or are under-recognized by laboratory physicians and clinicians such as Mycobacterium spp., anaerobes, Corynebacterium striatum, and others. These results are concordant with a study by Miao et al,16 except for the adjudication of C. striatum infection. Although most C. striatum were considered colonizing bacteria, some cases were identified as pathogenic bacteria in this study. Indeed, C. striatum can cause pneumonia, especially in patients with invasive mechanical ventilation;17 however, few patients enrolled in the study by Miao et al were from the ICU (including respiratory ICU). Because target detection is not performed related to a lack of a priori consideration and high false-negative rates of conventional detection techniques occur, obligate intracellular atypical pathogens such as Legionella pneumophila, Chlamydia psittaci, and Rickettsia are easily missed and misdiagnosed.18,19 Conversely, mNGS allows for universal pathogen detection without the need for a priori knowledge of a specific pathogen, which can overcome these limitations.
The positive rate of fungal detection by mNGS was higher than that of CMT (16.2% vs 6.4%), especially for Aspergillus spp. and P. jirovecii, but its sensitivity was only 85.7%. It is difficult to extract DNA from thick polysaccharide cell walls, making the identification of Aspergillus spp. challenging.20 However, technology to break cell walls by mechanical homogenization and enzymolysis, enrichment technology, and continuously optimized sequencing depth will greatly improve the low detection limit of Aspergillus spp. by mNGS.21 Currently, the identification of fungal infection requires the combined use of mNGS and CMT. Notably, most cases of Candida spp. from respiratory specimens were judged as colonizations instead of infection, although it was reported that the presence of Candida spp. may be associated with poor prognosis in patients with ventilator-associated pneumonia.22,23
Previous studies have shown that mNGS is more sensitive than CMT for virus detection.9,24 However, we could not objectively compare the diagnostic efficacy of the two methods for virus detection in this study, because most specimens were not sent for virus PCR or serological detection synchronously with mNGS. Although mNGS detected many viruses in various specimens, only a few patients with suspected virus infection received RNA based mNGS detection, which might have led to the missed diagnosis of some RNA viruses. Common viruses reported in this study include herpes virus, torque teno virus, and other DNA viruses. These viruses always remain latent in target cells, which can colonize the respiratory tract or integrate their genetic material into host cell chromosomes.25 In this study, few viruses were judged to be pathogenic causing symptoms; however, the remaining asymptomatic reactivated viruses might have clinical implications. It was reported that the seroprevalence rate of HSV-1 in the human population is 54%, and that HSV-1 is latent in sensory neurons and is reactivated when the host is in an immunosuppressed state.26 The reactivation and replication of HSV-1 in the respiratory tract are common in ICU patients with invasive mechanical ventilation, even in those who are not immunodeficient, with a reported incidence range of 5% to 64%.27 HSV detection in the lower respiratory tract does not indicate true HSV broncho pneumonitis. It may reflect downward contamination by saliva or tracheobronchial reactivation without parenchymal involvement,28 requiring clinicians to make the correct diagnosis in combination with clinical manifestations. A prospective study detected herpes viremia in 68% of immunocompetent patients presenting with septic shock, including EBV viremia in 48% of cases and CMV viremia in 18% of cases, indicating the reactivation of EBV may be associated with poor prognosis.29 The pathogenicity of EBV in the lower respiratory tract is unclear and the detection of EBV is more a marker of disease severity rather than evidence of pathogenicity.30
In ICU wards, infections can occur at various sites in the body. The type of specimens submitted for examination directly affects the diagnostic efficiency of pathogen identification and treatment options. In principle, clinicians collect specimens from the primary lesion as much as possible. However, it is difficult to obtain specimens from primary sites or sometimes the infection site is unknown, so peripheral blood specimens are the suboptimal choice. In this study, although the most common site of infection was the lower respiratory tract, the most frequently submitted specimen was blood. The positive rate of bacterial and fungal detection using blood for mNGS tends to be lower than that of most primary sites, leading to a higher percentage of no clinical treatment benefit. Chen et al31 compared the microbiological detection performance between BALF and blood by mNGS in 20 patients with severe pneumonia: 17 BALF specimens were considered positive whereas only 10 blood specimens were mNGS-positive. Although the pathogens detected in the two specimen types were highly consistent, we still do not recommend using blood as an alternative specimen for mNGS detection, because of the low positive rate and because patients may not benefit sufficiently from this expensive technology. In addition, lower respiratory tract specimens are relatively easy to obtain from intubated ICU patients. However, plasma mNGS has some clinical value. The clinical beneficial therapeutic impact of mNGS-positive blood specimens is higher than for other types of positive specimens. Similar results were found when using the same sterile CSF specimens. For double positive reported cases, the proportion of pathogens identified by mNGS and CMT with complete consistency was highest in the blood and CSF. Accordingly, it is reasonable to speculate that the positive results of mNGS using specimens from sterile environments are more reliable than specimens from non-sterile environments, although the positive proportion is lower.
Unbiased mNGS assays can detect multiple microorganisms simultaneously, which is conducive to a diagnosis of mixed infection. However, intensivists are generally unexperienced with interpretating mNGS results because it is not easy to distinguish between background microorganisms, colonized microorganisms, and pathogenic microorganisms among the detected species, especially for specimens from non-sterile environments such as the respiratory tract. Moreover, the contamination of respiratory specimens by oral or pharyngeal microorganisms during sampling is possible. Thus, pathogenic microorganisms should be judged with caution. The combination of clinical manifestations, pulmonary imaging features, and treatment response is necessary. The interpretation of mNGS reports can be affected by subjective factors, including an understanding of the clinical microbiology and local epidemiology of infection among clinicians and laboratory physicians.32 The detection of bacteria from the BALF and ETA, which are respiratory tract samples, had similar sensitivity and specificity. However, BALF was slightly better than ETA when considering the impact of mNGS on treatment because the proportion of no clinical benefit using BALF mNGS was lower than for ETA mNGS (5.7% vs 15.4%). This difference did not reach statistical significance (P = 0.268) but considering the small sample size and low incident rate, some non-pathogenic microbes in might have been present in the ETA specimens because the anatomical position is close to the oral cavity, which might interfere with the subjective judgment of physicians.
In our study, most of the included ascites specimens originated from patients after abdominal surgery, leading to enteropathogenic infections being the prominent manifestation. We noted that large amounts of obligate anaerobes were detected in ascites by mNGS, which were rarely detected by CMT, implying that anaerobic cultures are neglected in our center. Fortunately, these patients received anti-anaerobic treatments at the beginning of disease because multiple guidelines and consensuses recommend that the empiric anti-infective treatment of intraabdominal infection should include agents with activity against enteric Gram-negative aerobic and facultative bacteria, enteric Gram-positive streptococci, and obligate enteric anaerobic bacteria.32–35 This led to anti-infective treatment impact based on mNGS results is mostly confirming existing treatment. Therefore, we do not recommend mNGS as a first-line microbiological detection method for intraabdominal enteropathogenic infection, although it has advantages for the diagnosis of anaerobic bacteria. We also found many anaerobic infections in pus specimens from several patients with skin and soft tissue infections and one patient with empyema, most of which were from the oral cavity. Skin and soft tissue infections caused by anaerobes are not uncommon, and it was reported that the predominant anaerobes causing infection include oral anaerobes such as Peptostreptococcus spp., Prevotella spp., and Porphyromonas spp.36 Additionally, periodontal disease was present in 61% of patients with a community-acquired lung abscess where large amounts of anaerobes had colonized the oral cavity and then spread into the lungs to develop the infection.37 Interestingly, most odontogenic infections are caused by more than one bacterium, possibly because individual members of the oral flora produce metabolites essential for the growth of other microorganisms in the oral flora, promoting the synergistic metabolism and interdependence of diverse microorganisms.38
Immunocompromised patients are often admitted to the ICU and the diagnosis of infection in such patients is challenging. Immunocompromised patients are more susceptible to being infected by rare pathogens. Relatively low antibody titers and a high proportion of preventive anti-infective therapies make microbiological identification difficult by CMT, including culture and serological detection. Our study showed that immunocompromised patients are vulnerable to fungal and/or viral infections. P. jirovecii and CMV are often coinfected with other pathogens. Furthermore, co-infection of P. jirovecii and CMV is a unique mode of mixed infection in immunocompromised patients. Another phenomenon of concern is that the positive rate of mNGS detection in peripheral blood was only 30.0% in immunocompetent patients, whereas this ratio was 60.0% in immunocompromised patients (P = 0.004), indicating that plasma mNGS may be more valuable for immunocompromised patients. From a curative perspective, the proportion of beneficial anti-infective adjustments in the immunocompromised group was significantly higher than in the immunocompetent group (48.5% vs 30.1%, P = 0.008). Together, these results indicated that immunocompromised patients might benefit more from mNGS detection. However, we should recognize that this study may have underestimated the diagnostic efficiency of CMT. Peng et al (Peng et al, 2021) argued the diagnostic performance of BALF mNGS in immunocompromised patients with pneumonia was similar to that of CMT, which is inconsistent with our results and other most studies. One possible explanation is that the BALF specimens included in the study of Peng et al were sent for all available CMT methods including the PCR detection of P. jirovecii and other viruses. Conversely, CMT methods used in our and other studies were selectively performed according to the pathogens suspected by the treating clinicians or because some centers lack the relevant technology.
Although this was a study on mNGS conducted in an ICU with a relatively large sample size, there were several limitations. First, there are no widely accepted quantitative cutoffs for mNGS results. Thus, pathogen diagnosis is still based on the judgment of clinicians, which has the potential to introduce bias. Second, we noticed that the time from collection to reporting was only 17–28 hours in our study. mNGS, as a rapid pathogen detection method, allow an earlier and more optimal anti-infective treatment for critically ill patients. However, the impact of shorter reporting time on clinical prognosis cannot be confirmed in this retrospective study, and a well-designed prospective study is necessary.
In summary, irrespective of the type of specimen, mNGS has significant advantages for the detection of both common and uncommon pathogen compared with CMT. mNGS is expected to be a promising technology that provides precision medicine for critically ill patients with infection, especially in those who are immunocompromised. However, reasonable usage scenarios, appropriate specimen types, and cautious data interpretation are necessary.
Ethics Approval and Informed Consent
Ethical approval was obtained from the Ethics Committee of The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, China (approval number: 2022-RE-001). Due to the retrospective nature of the study, the Ethics Committee waived the requirement for patient consents. The patients were anonymized, and their information was nonidentifiable. In general, all data in this study were obtained in accordance with the Helsinki declaration.
Acknowledgment
We thank the patients for cooperating with our investigation and acknowledge the professionalism and compassion demonstrated by all the healthcare workers involved in patients’ care. We also thank Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the language of a draft of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Key Research and Development Program of Anhui Province (grant number 202104j07020043). The funder had no role in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Luyt CE, Bréchot N, Chastre J. What role do viruses play in nosocomial pneumonia? Curr Opin Infect Dis. 2014;27(2):194–199. doi:10.1097/QCO.0000000000000049
2. Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–1083. doi:10.1093/cid/ciu027
3. Phua J, Ngerng W, See K, et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit Care. 2013;17(5):R202. doi:10.1186/cc12896
4. Gu W, Miller S, Chiu CY. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu Rev Pathol. 2019;14(1):319–338. doi:10.1146/annurev-pathmechdis-012418-012751
5. Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–2417. doi:10.1056/NEJMoa1401268
6. Zhang Y, Cui P, Zhang HC, et al. Clinical application and evaluation of metagenomic next-generation sequencing in suspected adult central nervous system infection. J Transl Med. 2020;18(1):199. doi:10.1186/s12967-020-02360-6
7. Grumaz S, Stevens P, Grumaz C, et al. Next-generation sequencing diagnostics of bacteremia in septic patients. Genome Med. 2016;8(1):73. doi:10.1186/s13073-016-0326-8
8. Langelier C, Kalantar KL, Moazed F, et al. Integrating host response and unbiased microbe detection for lower respiratory tract infection diagnosis in critically ill adults. Proc Natl Acad Sci U S A. 2018;115(52):E12353–E12362. doi:10.1073/pnas.1809700115
9. Chen H, Yin Y, Gao H, et al. Clinical Utility of In-house Metagenomic Next-generation Sequencing for the Diagnosis of Lower Respiratory Tract Infections and Analysis of the Host Immune Response. Clin Infect Dis. 2020;71(Suppl 4):S416–S426. doi:10.1093/cid/ciaa1516
10. Zhou Y, Wylie KM, El Feghaly RE, et al. Metagenomic Approach for Identification of the Pathogens Associated with Diarrhea in Stool Specimens. J Clin Microbiol. 2016;54(2):368–375. doi:10.1128/JCM.01965-15
11. Kujiraoka M, Kuroda M, Asai K, et al. Comprehensive Diagnosis of Bacterial Infection Associated with Acute Cholecystitis Using Metagenomic Approach. Front Microbiol. 2017;8:685. doi:10.3389/fmicb.2017.00685
12. Doan T, Wilson MR, Crawford ED, et al. Illuminating uveitis: metagenomic deep sequencing identifies common and rare pathogens. Genome Med. 2016;8(1):90. doi:10.1186/s13073-016-0344-6
13. Goswami K, Parvizi J. Culture-negative periprosthetic joint infection: is there a diagnostic role for next-generation sequencing? Expert Rev Mol Diagn. 2020;20(3):269–272. doi:10.1080/14737159.2020.1707080
14. Clinical Microbiology Group of Chinese Society of Laboratory Medicine, Clinical Microbiology Group of Chinese Society of Microbiology and Immunology, Society of Clinical Microbiology and Infection of China International Exchange and Promotion Association for Medical and Healthcare. Chinese expert consensus on metagenomics next‐generation sequencing application on pathogen detection of infectious diseases. Chin J Lab Med. 2021;44(2):107–120.
15. Centers for Disease Control and Prevention. CDC/NHSN Surveillance Definitions for Specific Types of Infections. Surveillance Definitions. 2022;17:1–30.
16. Miao Q, Ma Y, Wang Q, et al. Microbiological Diagnostic Performance of Metagenomic Next-generation Sequencing When Applied to Clinical Practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. doi:10.1093/cid/ciy693
17. Verroken A, Bauraing C, Deplano A, et al. Epidemiological investigation of a nosocomial outbreak of multidrug-resistant Corynebacterium striatum at one Belgian university hospital. Clin Microbiol Infect. 2014;20(1):44–50. doi:10.1111/1469-0691.12197
18. Chahin A, Opal SM. Severe Pneumonia Caused by Legionella pneumophila: differential Diagnosis and Therapeutic Considerations. Infect Dis Clin North Am. 2017;31(1):111–121. doi:10.1016/j.idc.2016.10.009
19. Teng XQ, Gong WC, Qi TT, et al. Clinical Analysis of Metagenomic Next-Generation Sequencing Confirmed Chlamydia psittaci Pneumonia: a Case Series and Literature Review. Infect Drug Resist. 2021;14:1481–1492. doi:10.2147/IDR.S305790
20. Peng JM, Du B, Qin HY, et al. Metagenomic next-generation sequencing for the diagnosis of suspected pneumonia in immunocompromised patients. J Infect. 2021;82(4):22–27. doi:10.1016/j.jinf.2021.01.029
21. Zinter MS, Dvorak CC, Mayday MY, et al. Pulmonary Metagenomic Sequencing Suggests Missed Infections in Immunocompromised Children. Clin Infect Dis. 2019;68(11):1847–1855. doi:10.1093/cid/ciy802
22. Azoulay E, Timsit JF, Tafflet M, et al. Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest. 2006;129(1):110–117. doi:10.1378/chest.129.1.110
23. Delisle MS, Williamson DR, Albert M, et al. Impact of Candida species on clinical outcomes in patients with suspected ventilator-associated pneumonia. Can Respir J. 2011;18(3):131–136. doi:10.1155/2011/827692
24. van Boheemen S, van Rijn AL, Pappas N, et al. Retrospective Validation of a Metagenomic Sequencing Protocol for Combined Detection of RNA and DNA Viruses Using Respiratory Samples from Pediatric Patients. J Mol Diagn. 2020;22(2):196–207. doi:10.1016/j.jmoldx.2019.10.007
25. Osterrieder N, Wallaschek N, Kaufer BB. Herpesvirus Genome Integration into Telomeric Repeats of Host Cell Chromosomes. Annu Rev Virol. 2014;1(1):215–235. doi:10.1146/annurev-virology-031413-085422
26. Schuierer L, Gebhard M, Ruf HG, et al. Impact of Acyclovir use on survival of patients with ventilator-associated pneumonia and high load herpes simplex virus replication. Crit Care. 2020;24(1):12. doi:10.1186/s13054-019-2701-5
27. Luyt CE, Bréchot N, Trouillet JL, Chastre J. Antibiotic stewardship in the intensive care unit. Crit Care. 2014;18(5):480. doi:10.1186/s13054-014-0480-6
28. Luyt CE, Combes A, Deback C, et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175(9):935–942. doi:10.1164/rccm.200609-1322OC
29. Ong DSY, Bonten MJM, Spitoni C, et al. Epidemiology of Multiple Herpes Viremia in Previously Immunocompetent Patients With Septic Shock. Clin Infect Dis. 2017;64(9):1204–1210. doi:10.1093/cid/cix120
30. Libert N, Bigaillon C, Chargari C, et al. Epstein-Barr virus reactivation in critically ill immunocompetent patients. Biomed J. 2015;38(1):70–76. doi:10.4103/2319-4170.132905
31. Chen J, Zhao Y, Shang Y, et al. The clinical significance of simultaneous detection of pathogens from bronchoalveolar lavage fluid and blood samples by metagenomic next-generation sequencing in patients with severe pneumonia. J Med Microbiol. 2021;70(1). doi:10.1099/jmm.0.001259
32. Casto AM, Fredricks DN, Hill JA. Diagnosis of infectious diseases in immunocompromised hosts using metagenomic next generation sequencing-based diagnostics. Blood Rev. 2022;53:100906. doi:10.1016/j.blre.2021.100906
33. Sartelli M, Coccolini F, Kluger Y, et al. WSES/GAIS/SIS-E/WSIS/AAST global clinical pathways for patients with intra-abdominal infections. World J Emerg Surg. 2021;16(1):49. doi:10.1186/s13017-021-00387-8
34. Sartelli M, Weber DG, Ruppé E, et al. Antimicrobials: a global alliance for optimizing their rational use in intra-abdominal infections (AGORA). World J Emerg Surg. 2016;11:33. doi:10.1186/s13017-016-0089-y
35. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133–164. doi:10.1086/649554
36. Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg. 2008;6(4):328–338. doi:10.1016/j.ijsu.2007.07.001
37. Guo W, Gao B, Li L, et al. A community-acquired lung abscess attributable to odontogenic flora. Infect Drug Resist. 2019;12:2467–2470. doi:10.2147/IDR.S218921
38. Singh M, Kambalimath DH, Gupta KC. Management of odontogenic space infection with microbiology study. J Maxillofac Oral Surg. 2014;13(2):133–139. doi:10.1007/s12663-012-0463-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.