Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 10
Diagnostic discrepancy between bronchoalveolar lavage and transbronchial biopsy from bronchoscopies of HIV patients with pneumonia: toward an integral diagnosis
Authors Sánchez-Cabral O, Martínez-Mendoza D, Flores-Bello ÁP, Martínez-Orozco JA, Rivera-Rosales RM, Luna-Rivero C, Santillán-Doherty P , Reyes-Terán G
Received 8 January 2018
Accepted for publication 21 March 2018
Published 6 July 2018 Volume 2018:10 Pages 115—123
DOI https://doi.org/10.2147/HIV.S161899
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Olivia Sánchez-Cabral,1 Dina Martínez-Mendoza,1,2 Ángel Paul Flores-Bello,3 José Arturo Martínez-Orozco,4 Rosa María Rivera-Rosales,5 César Luna-Rivero,5 Patricio Santillán-Doherty,6 Gustavo Reyes-Terán7
1Interventional Pulmonology Unit, 2Hospital Epidemiology Surveillance Unit, 3Clinic of Tuberculosis and Pleural Diseases, 4Department of Infectious Diseases and Clinical Microbiology, 5Anatomic Pathology Service, 6Medical Direction, 7Department of Research in Infectious Diseases, National Institute of Respiratory Diseases Ismael Cosío Villegas, Mexico City, Mexico
Background: The key diagnostic method for the evaluation of lung diseases associated with HIV infection is bronchoscopy, with bronchoalveolar lavage (BAL) being the most commonly used sampling technique. Transbronchial biopsy (TBB) is often complementary.
Setting: This is a retrospective cross-sectional study to determine the diagnostic usefulness of bronchoscopy with simultaneous samples obtained through BAL and TBB in HIV-infected patients with pneumonia at the National Institute of Respiratory Diseases Ismael Cosío Villegas.
Methods: In this cross-sectional study (January 2014–December 2015), the diagnostic yield of bronchoscopic samples from all HIV-positive patients with pneumonia aged >18 years, from procedures performed in the Interventional Pulmonology Unit, was analyzed and recorded in its database. The diagnostic yield concordance between BAL and TBB samples was evaluated by kappa index calculation.
Results: A total of 198 procedures on 189 HIV-infected patients with pneumonia were performed. A total of 167/189 (88.4%) patients were male, and the mean age was 34.7 years (SD ±9.0). Overall, the diagnostic yield for either technique was 87.9% (174/198), but it was higher for TBB, its yield being 78.8% (156/198). In contrast, that of BAL was 62.1% (123/198) (P=0.001). The overall diagnostic yield concordance between TBB and BAL was insignificant (κ=0.213, P<0.001). It improved for fungal infections, pneumocystosis, and tuberculosis (κ=0.417, 0.583, and 0.462, respectively, all P<0.001).
Conclusion: Our results show that the simultaneous obtainment of BAL and TBB samples is useful and complementary in the diagnosis of infections and malignancies in HIV-infected patients. Additionally, they are safe procedures in this group of patients.
Keywords: bronchoalveolar lavage, transbronchial biopsy, HIV, BAL, TBB, interventional bronchoscopy, bronchoscopy
Background
Respiratory diseases are frequent in HIV-infected patients and are a leading cause of morbidity and mortality despite combined antiretroviral therapy (ART). The spectrum of pulmonary manifestations is wide and includes infectious and noninfectious diseases, which frequently have overlapping clinical and radiological manifestations. Additionally, coinfections are frequent and their isolation with conventional methods is difficult. The most frequent infections are bacterial pneumonia, tuberculosis, Pneumocystis jirovecii pneumonia (PJP), and fungal infections (coccidioidomycosis, histoplasmosis, and cryptococcosis).1–6 The key diagnostic method for the evaluation of lung diseases associated with HIV is bronchoscopy, with bronchoalveolar lavage (BAL) being the most commonly used sampling technique, while transbronchial biopsy (TBB) is often complementary. Both are useful and safe procedures.2,7 However, the utility of each of these techniques in infectious diseases is different, especially for PJP and cytomegalovirus (CMV) infection, and TBB is more useful for bacterial infections and noninfectious disease such as malignancies.2,8 Our objective was to determine the diagnostic usefulness of bronchoscopy with simultaneous sampling obtained with BAL and TBB in HIV-infected patients with pneumonia.
Methods
This is a retrospective cross-sectional study, which was approved by the Research and Ethics Committees of the National Institute of Respiratory Diseases Ismael Cosío Villegas of Mexico, with code number C72-16. Informed consent waiver from the Institutional Research and Ethics Committees was requested, since this was a retrospective study, and the confidentiality of the patients included in the study was strictly protected. A total of 189 patients older than 18 years diagnosed with HIV and registered in the database of the Interventional Pulmonology Unit of our institution were subjected to a diagnostic bronchoscopy, which included BAL and TBB. The patients were admitted to the External Consultation Service, the Emergency Department and Clinical Services of the INER, or were referred by medical interconsultation from other institutions because they presented with pneumonia diagnosed either clinically or by chest tomography, with indication to perform diagnostic bronchoscopy by means of BAL and TBB study during the period from January 2014 to December 2015. The population size was 198 procedures, considering 1) the finite population correction factor (N), an estimated 400 outpatients or hospitalized patients with HIV and pneumonia treated within 2 years at the institute; 2) an overall diagnostic yield of 50% (±5), according to the published variability of the diagnostic yields of the different pulmonary samples; 3) 95% CIs; and 4) the formula: N = (EDFF × Np [1- p])/(d2/Z21- α/2× [N -1] + p × [1- p]), which was obtained through the OpenEpi software, Version 3.
BAL and TBB samples were cultured as follows. In fungal infection, the samples were cultured on sabouraud and sabouraud with antibiotics culture media and the species were identified under the cotton blue microscopy. For the diagnosis of P. jirovecii, direct immunofluorescence microscopy, as the gold standard, was performed. Special staining with grocott, calcofluor white, and Wright stains for fungal identification was also performed. In mycobacteria, the samples were cultured on liquid (BACTEC MGIT 960) and solid (Löwenstein–Jensen) media and the species were identified using the line probe assay (HAIN) tests. For drug susceptibility testing in Mycobacterium tuberculosis, BACTEC MGIT 960 SIRE kit was used. GeneXpert® MTB/RIF for M. tuberculosis diagnosis was also performed in all samples. In bacteria, the samples were cultured on liquid and solid agar media and automated VITEK 2 was used for identification and drug susceptibility testing. In viral infection, multiplex real-time polymerase chain reaction (PCR) LUMINEX assay for respiratory viral diagnosis and Anyplex™ II multiplex real-time PCR RPB5 for respiratory bacteria platforms were performed. In CMV, tissue real-time PCR and histopathological analysis were performed.
Samples for histopathological analysis obtained transbronchially (TBB) were kept in formaldehyde buffer, pH 7.1 (EMD Millipore, Billerica, MA, USA), and BAL samples were kept in 10 cc carbowax (polyethyleneglycol 4000; Merck Millipore, Billerica, MA, USA).
All procedures were performed and supervised by an expert interventional pulmonologist.
BAL is performed using the pulmonary bronchoscopic wedging technique in the most affected lung subsegment with 180 mL saline instillation, recovering at least 45% of instillate. The material collected in vial-like containers is sent to the Microbiology and Anatomic Pathology Department.
In TBB, lung biopsy is performed using the bronchoscopic wedging technique at the most affected site under fluoroscopic guidance and is sent to the Microbiology and Anatomic Pathology Department.
Helical computed tomography of the chest with contrast was performed, with reconstruction in window for the lung and mediastinum, with a section thickness of 3 mm with SIEMENS SOMATOM tomograph definition 128. Prior to tomography, the site with the greatest damage is identified, and TBB and BAL are performed.
Variables were evaluated according to their distribution. The diagnostic yield of the samples obtained by bronchoscopy (BAL and TBB) was analyzed with chi-square and Fisher’s exact tests for categorical variables, and t-test was used for continuous independent variables. The kappa index was used to evaluate the diagnostic yield concordance between the samples obtained with BAL and TBB. Results were significant at a P-value of <0.05. The statistical analysis was performed using the IBM SPSS Statistics 21 software.
Results
A total of 198 procedures on 189 HIV-infected patients with pneumonia were performed. A total of 167/189 (88.4%) patients were male, with a mean age of 34.7 years (SD ±9.0) (Table 1). Information about previous ART or antibiotic therapy prior to bronchoscopy was available from 167 patients (84.3%). A total of 92.8% (155/167) of patients had taken antibiotics for a mean of 5.4 days (SD ±5.8), and 36.6% (60/164) of patients were on ART prior to evaluation, with a mean ART duration of 7.3 months (SD ±22.6) prior to bronchoscopy.
  | Table 1 Summary of procedures performed on 189 HIV-infected patients with pneumonia subjected to 198 bronchoscopies Note: aTBB. Abbreviations: BAL, bronchoalveolar lavage; TBB, transbronchial biopsy. |
Overall diagnostic yield
Overall diagnostic yield for either technique was 87.9% (174/198), but it was higher for TBB, the yield of this method being 78.8% (156/198) in contrast to 62.1% (123/198) for BAL (P=0.001). The most frequent diagnosis was infections, with etiologic agent identification in 79.3% (157/198) (Tables 1 and 2).
  | Table 2 Diagnostic yield according to the type of sample in HIV-infected patients with pneumonia Note: *P-value for χ2. Abbreviations: BAL, bronchoalveolar lavage; TBB, transbronchial biopsy. |
BAL and TBB yields according to specific diagnosis
Of all samples, 247 samples were useful for diagnosis, 173 (70%) samples were obtained with TBB, and 161 (65.2%) samples were obtained with BAL; 92.3% (228) led to the identification of an infectious cause, while 13 (5.3%) corresponded to benign neoplasia and 6 (2.4%) corresponded to malignant neoplasia. The most frequent infection was fungal infection in 57.5% (131/228), followed by bacterial infection in 18.9% (43/228), mycobacterial infection in 16.2% (37/228), and viral infection in 7.5% (17/228). P. jirovecii was the most frequently identified pathogen in 91.6% (107), followed by M. tuberculosis complex in 93.8% (32) (Table 2).
BAL was a better tool for the identification of mycobacteria (94.6% vs 32.4%, P<0.001) and viruses (70.6% vs 35.3%, P=0.001) except CMV. Regarding the latter, TBB identified all cases of CMV infection, with only 1/6 being isolated through BAL. As for fungal infection, TBB performed better than BAL (87.0% vs 67.2%, P<0.001). With respect to bacteria, there was no difference between both techniques, except Escherichia coli (Gram-negative Enterobacteriaceae) for which TBB was superior (82.4% vs 52.9%, P<0.001) (Table 3). TBB was the only method that led to the diagnosis of neoplasia either benign or malignant (Table 4).
  | Table 4 Noninfectious diagnoses according to sample type in HIV-infected patients with pneumonia Abbreviations: BAL, bronchoalveolar lavage; TBB, transbronchial biopsy. |
The overall concordance between TBB and BAL was nonsignifcant (k=0.213, P<0.001); however, it improved for the following diagnostic categories: fungal infections (k=0.417, P<0.001), PJP (k=0.583, P<0.001), and tuberculosis (k=0.462, P<0.001) (Table 5).
Coinfections
Positive isolates were obtained from 79.3% (157) procedures, and 35.0% (55) of them occurred as coinfections. The agents more frequently isolated from coinfections were P. jirovecii in 44/55 (80.0%) and M. tuberculosis complex in 19/54 (34.5%). In contrast, of all patients who had P. jirovecii, 41.1% (44/107) had a coinfection, and of all patients who had M. tuberculosis complex, 59.4% (19/32) had also coinfection. The following data were found to be nonsignificant regarding the development of infections with one or more organisms (coinfections) or the type of infectious agent: previous antibiotic treatment, length of treatment, and previous ART.
PJP
Regarding PJP, BAL had a sensitivity of 71.9%, with a negative predictive value (NPV) of 75.2% and a diagnostic precision of 84.9%, while TBB for PJP diagnosis had a sensitivity of 89.7%, an NPV of 89.2%, and a diagnostic precision of 94.4%.
Tuberculosis
The prevalence of tuberculosis in our population was 16.2% (32/198). Diagnosis was performed through samples obtained by culture in BAL in 93.8% (30/32), by GeneXpert in BAL in 53.1% (17/32), and by culture in TBB in 37.5% (12/32). TBBs added the 6.3% (2) to the overall prevalence that would have not been obtained by BAL. The sensitivity of GeneXpert in BAL compared with culture in BAL, as the gold standard, was 56.7% (39.2–72.6), with a specificity of 100% (96.5–100), a positive predictive value of 100% (81.6–100), an NPV of 89.2% (82.3–93.6), a diagnostic precision of 90.5% (84.4–94.4), and Cohen’s kappa concordance 0.67 (0.5132–0.8295, P<0.001). When the sum of tuberculosis diagnoses by culture and GeneXpert® by BAL and TBB culture was used as gold standard, sensitivity was 53.1% (35–70.5), with a specificity of 100% (97–99.9), a positive predictive value of 100% (77.1–99.5), an NPV of 91.2% (85.7–94.8), and a diagnostic precision of 92.0% (85.7–94.8) (Table 5).
Yield of BAL and TBB according to tomographic imaging
We could retrieve tomographic studies from 155 (78.3%) patients. A better correlation was observed for TBB than for BAL in ground glass opacities (84.3% vs 66.1%, P=0.008), consolidation (88.3% vs 63.6%, P=0.04) or cysts (90.9% vs 68.2%, P=0.03). These differences were significant.
Complications
Safety was a crucial concern for the implementation of the BAL and TBB procedures. Complications occurred in 8.1% (16/198) of patients; in total, they accounted for 22 events. All cases were fully resolved, and no perioperative deaths occurred. The complications and their management for each one were as follows: pneumothorax with chest tube placement (4.5%); desaturation with supplemental oxygen (3%); bronchospasm with bronchodilator nebulization (0.5%); moderate bleeding (2.5%), which was controlled with cold saline solution instillation, argon plasma cauterization balloon occlusion, and/or saline solution plus adrenaline instillation. Finally, patients who required intubation were 0.5% (Table 6).
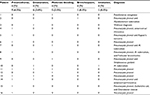  | Table 6 Complications after transbronchial biopsy and bronchoalveolar lavage in HIV-infected patients with pneumonia |
Discussion
Globally, microorganism isolation with all methods used was 87.9%, with TBB being superior to BAL (78.8% vs 62.1%, P=0.001); these results are comparable to those from Cazzadori et al.9 In their study, 79 HIV-infected patients with lung infiltrates were subjected to 84 bronchoscopies with a positive yield of 79.7%, being higher for TBB than for BAL (77.3% vs 47.6%, P<0.001), and TBB increased the diagnostic yield of BAL in 32.1%. Salzman et al10 also reported an additional 26% yield when TBB was obtained in 205 bronchoscopies performed in 182 HIV-infected patients. In general, both methods are useful in these patients. Regarding PJP, BAL can detect this infection in up to 90% of cases.11 This figure can reach 100% when it is combined with TBB.12
In our population, 57% had CD4 counts <50 cells/mL and 85.5% had CD4 counts <200 cells/mL; at CD4 levels >400 cells/mL, patients are at risk of infection by relatively virulent organisms, such as bacteria and tuberculosis (TB). Lung cancer can also occur at this stage. This explains coinfections in HIV-infected patients, where etiologic agents were isolated in 79.3% of procedures and 34.4% had an additional coinfection, with P. jirovecii being the most frequent followed by M. tuberculosis complex. The most frequent coinfection was P. jirovecii with M. tuberculosis complex followed by P. jirovecii with Histoplasma capsulatum. With CD4 cell counts between 200 and 400 cells/mL, patients may experience recurrent infection, as well as lymphoma. Opportunistic infections and Kaposi’s sarcoma are rare at CD4 levels >200 cells/mL, and in fact, most PJP cases occur at CD4 cell counts <100 cells/mL, together with Mycobacterium avium complex (MAC), fungal infections, and CMV.13 The population in the study by Cattamanchi et al14 had a median CD4+ T-lymphocyte count of 60 cells/mL (IQR 22–200 cells/mL), and 16% were receiving ART. TB prevalence by culture was 38%, but it was positive in only 5.3% in BAL culture. The incidence of bacterial pneumonia increases starting from CD4 cell counts <200 cells/mL in HIV-infected patients.1,15
In our population with known history of antibiotic use prior to bronchoscopy or antiretroviral treatment, 92.8% took antibiotics and 36.6% took ART; no statistically significant differences were found between patients with previous antibiotic use, the days with previous antibiotic, or with previous ART with the identification of etiologic agent, the presence of coinfections, or the types of etiologic agents identified. Bronchoscopic procedures are useful even after the initiation of empirical treatment if treatment duration has not exceeded 1 or 2 weeks.11
Regarding BAL yield for the diagnosis of PJP, Golden et al16 reported a 97% sensitivity, although considering their sample size, a 75% NPV is obtained (95% CI 30.1–95.4), and therefore, consideration must be given to their conclusions, in which they suggest the replacement of TBB by BAL. In fact, they did not directly study the role of TBB. Our results, with the sensitivities of 71.9% (95% CI 62.8–79.6) for BAL and 89.7% (95% CI 82.5–84.2) for TBB and k=0.583 for both methods, support the opposite. The latter is similar to the results obtained by Broaddus et al,17 who reported an 86% yield for BAL and 87% for TBB. When both procedures were performed in the same study, the combined yield for all lung infections was 96%. In contrast, Coleman et al18 reported the global sensitivities of 79 and 55% for the diagnosis of PJP by TBB and BAL, respectively.
In other series, the additive yield of TBB seems to be more important when other infections, besides PJP, are present. In this setting, Batungwanayo et al19 reported the diagnostic yields of 82% and 26%, respectively, for TBB and BAL, but this reflects the wide spectrum of lung complications in African populations, where PJP is rare, but nonspecific interstitial pneumonia, TB, and Cryptococcal pneumonia were the most common diagnoses. In a retrospective analysis of 205 bronchoscopies in 182 patients, Salzman et al20 demonstrated that 26% of diagnoses were exclusively obtained with TBB, which was also the only source for the diagnosis of noninfectious conditions in over half of those cases (54%). In other series of HIV-infected patients, the global yield of TBB is superior to that of BAL (80% vs 57.1%), but sample sizes are small.21
Diagnoses of granulomatous inflammation were classified as benign, as no microbiological confirmation of infection was obtained. Nonetheless, in some studies, this finding is regarded as either tuberculosis or histoplasmosis, which can be overestimated.20 Miro et al22 concluded that in tuberculosis-infected patients, neither TBB nor bronchial brushings increased the diagnostic yield. In contrast to their study, our study included not only TB but also other diagnoses, which were demonstrated from TBB and BAL sampling.
The prevalence of tuberculosis in our study was higher than that previously reported in other series, even when a low sensitivity of BAL GeneXpert was observed and even though sputum production in these patients was negligible.23 This was overcome by the combination of BAL culture and BAL GeneXpert, which we did to obtain optimal samples. In contrast, TBB added some TB cases, which were not isolated by BAL. Based on this assertion, it is necessary to consider TBB samples to be routinely sent to mycobacterial culture.24,25
We found lower frequencies of CMV in BAL and of coinfections in the setting of PJP, as observed in other series. However, some of these did not look for this agent by TBB, in contrast to our study, in which diagnosis was supported with TBB findings in all six cases, with only one case showing CMV in BAL. All CMV cases were, in fact, coinfected with other pathogens (M. tuberculosis, Haemophilus influenzae, Enterococcus faecalis, Candida albicans, enterovirus, and parainfluenza type 3 virus), and five of the six cases also had PJP.11,26–30
Regarding specific diagnosis in relation to the sampling method, Shafiek et al31 found a 93% concordance of BAL with laser confocal endomicroscopy in 32 patients, but they did not compare this with TBB. Stover et al11 took as gold standard the samples obtained via bronchoscopy from BAL, TBB, cytology, BAL and/or postmortem. They found that 65% of patients had specific germ isolation. Specific figures for each diagnosis were as follows: 94% for PJP, 67% for CMV, 62% for MAC, and 0% for SK. For PJP, the highest yield was obtained when combining TBB and BAL. As for PJP, TBB had an 88% yield with an 85% yield for BAL, but the combination of both methods led to the best yield. Our figures also support that performance of both techniques in combination yields better results. Specifically, 54% of the patients we studied had PJP. The differences found by using each method, BAL 51 vs TBB 91.6%, k=0.583 (P<0.001), show that both sampling techniques are complementary.
Cancer prevalence in our population was 2.4%, and the diagnoses were demonstrated solely by TBB. It is important to remember that HIV causes a 3.5-fold increase in the risk of lung cancer and that it also occurs earlier, and its mean survival is only 3–4 weeks.6,13,32
We found alveolar hemorrhage (AH) in 4.5% of patients, diagnosed solely by TBB. This finding occurred in association with various infections (PJP, M. tuberculosis, H. influenzae, Coccidioides immitis, MAC, and enterovirus). This was lower than that reported in the study by Vincent et al,33 who found a 32% prevalence of AH. In their article, associations of AH with other conditions (CMV and Kaposi’s sarcoma) were also observed. Regarding granulomatous inflammation and pneumonia without isolation of specific pathogens, these were suggested by the histopathological findings of TBB.
Among benign diagnoses, AH had a 4.5% (nine patients) prevalence in our study and the diagnosis was obtained solely by TBBs; however, since six of these patients had some other diagnosis, at the end, the specific diagnosis was considered and the AH diagnosis was given to only three of the nine patients. Each six patients had an infection, including PJP, M. tuberculosis, H. influenzae, C. immitis, MAC, and enterovirus. These results contrast with those reported by Vincent et al33 in their study of HIV-infected patients with pulmonary symptoms, in which the prevalence of AH was 32% but only in BAL samples, where CMV infection (OR 9.8 [95% CI 1–91], P=0.05) and pulmonary Kaposi’s sarcoma (OR 5.3 [95% CI 1.8–16.7], P=0.003) were among the main associated factors. Regarding pneumonia without isolation of the etiologic agent and granulomatous inflammation, the diagnoses were obtained by TBB through histopathological observation.
Finally, our complication figures were comparable to others, with pneumothorax in 4.5% and mild-to-moderate bleeding in 2.5%.10,11,18
We acknowledge the following limitations, which could have increased the diagnostic yield of each sample (BAL and TBB): lack of a larger panel for viral diagnosis via PCR and lack of specific antigen search of other microorganisms, including TB. This study has internal validity, but the external one will depend on the experience of the center in the care of patients with HIV and pneumonia and in pulmonary interventionism.
Conclusion
Our results show that the diagnosis through BAL and TBB samples is discrepant; so the simultaneous study of both samples would mean a greater diagnostic opportunity, their obtainment is safe, and they are very useful techniques to specifically diagnose infections and malignancies in HIV-infected patients with pneumonia.
Disclosure
The authors report no conflicts of interest in this work.
References
Hirschtick RE, Glassroth J, Jordan MC, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. N Engl J Med. 1995;333(13):845–851. | ||
Narayanswami G, Salzman S. Bronchoscopy in the human immunodeficiency virus-infected patient. Semin Respir Infect. 2003;18(2):80–86. | ||
Kumar T, Epstein M, Markovskaya Y, Narasimhan M, Rosen M, Talwar A. Bronchoscopy and endobronchial disease in patients with human immunodeficiency virus infection. Indian J Chest Dis Allied Sci. 2011;53(2):99–105. | ||
Kanmogne GD, Kennedy RC, Grammas P. Is HIV involved in the pathogenesis of non-infectious pulmonary complications in infected patients? Curr HIV Res. 2003;1(4):385–393. | ||
de Leon FC, Britt EJ. The noninfectious respiratory complications of infection with HIV. Curr Opin Pulm Med. 1995;1(3):223–233. | ||
Tokman S, Huang L. Evaluation of respiratory disease. Clin Chest Med. 2013;34(2):191–204. | ||
Wallace J. A reassessment of indications and diagnostic algorithms bronchoscopy in HIV disease: an update in the era of HAART. J Respir Dis. 2007;28(6):244–252. | ||
Kvale PA. How much bronchoscopic sampling is enough (for HIV-infected patients)? J Broncol. 1996;3(2):83–84. | ||
Cazzadori A, Di Perri G, Todeschini G, et al. Transbronchial biopsy in the diagnosis of pulmonary infiltrates in immunocompromised patients. Chest. 1995;107(1):101–106. | ||
Salzman SH, Bernstein LE, Villamena PC, Schneider RF, Mayo PH, Rosen MJ. Bronchoscopic lung biopsy improves the diagnostic yield of bronchoscopy in patients with known or suspected HIV infection. J Bronchol. 1996;3:88–95. | ||
Stover DE, White DA, Romano PA, Gellene RA. Diagnosis of pulmonary disease in acquired immune deficiency syndrome (AIDS). Am Rev Respir Dis. 1984;130(4):659–662. | ||
Kirk GD, Merlo C, O’ Driscoll P, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45(1):103–110. | ||
Parker MS, Leveno DM, Campbell TJ, Worrell JA, Carozza SE. AIDS-related bronchogenic carcinoma: fact or fiction? Chest. 1998;113(1):154–161. | ||
Cattamanchi A, Ssewenyana I, Nabatanzi R, et al. Bronchoalveolar lavage enzyme-linked immunospot for diagnosis of smear-negative tuberculosis in HIV-infected patients. PLoS One. 2012;7(6): e39838. | ||
Colangelo G, Baughman RP, Dohn MN, Frame PT. Follow-up bronchoalveolar lavage in AIDS patients with Pneumocystis carinii pneumonia: Pneumocystis carinii burden predicts early relapse. Am Rev Respir Dis. 1991;143(5 pt 1):1067–1071. | ||
Golden JA, Hollander H, Stulbarg MS, Gamsu G. Bronchoalveolar lavage as the exclusive diagnostic modality for Pneumocystis carinii pneumonia. A prospective study among patients with acquired immunodeficiency syndrome. Chest. 1986;90(1):18–22. | ||
Broaddus C, Dake MD, Stulbarg MS, et al. Bronchoalveolar lavage and transbronchial biopsy for the diagnosis of pulmonary infections in the acquired immunodeficiency syndrome. Ann Intern Med. 1985;102(6):747–752. | ||
Coleman DL, Dodek PM, Luce JM, Golden JA, Gold WM, Murray JF. Diagnostic utility of fiberoptic bronchoscopy in patients with Pneumocystis carinii pneumonia and the acquired immune deficiency syndrome. Am Rev Respir Dis. 1983;128(5):795–799. | ||
Batungwanayo J, Taelman H, Lucas S, et al. Pulmonary disease associated with the human immunodeficiency virus in Kigali, Rwanda: a fiberoptic bronchoscopy study of 111 cases of undetermined etiology. Am J Respir Crit Care Med. 1994;149(6):1591–1596. | ||
Salzman SH, Schindel ML, Aranda CP, Smith RL, Lewis ML. The role of bronchoscopy in the diagnosis of pulmonary tuberculosis in patients at risk for HIV infection. Chest. 1992;102(1):143–146. | ||
Williams D, Yungbluth M, Adams G, Glassroth J. The role of fiberoptic bronchoscopy in the evaluation of immunocompromised hosts with diffuse pulmonary infiltrates. Am Rev Respir Dis. 1985;131(6):880–885. | ||
Miro AM, Gibilara E, Powell S, Kamholz SL. The role of fiberoptic bronchoscopy for diagnosis of pulmonary tuberculosis in patients at risk for AIDS. Chest. 1992;101(5):1211–1214. | ||
Kirk O, Gatell JM, Mocroft A, et al. Infections with Mycobacterium tuberculosis and Mycobacterium avium among HIV-infected patients after the introduction of highly active antiretroviral therapy. EuroSIDA Study Group JD. Am J Respir Crit Care Med. 2000;162(3 pt 1):865–872. | ||
Agrawal M, Bajaj A, Bhatia V, Dutt S. Comparative study of GeneXpert with ZN stain and culture in samples of suspected pulmonary tuberculosis. J Clin Diagn Res. 2016;10(5):DC09–DC12. | ||
Walters E, Goussard P, Bosch C, Hesseling AC, Gie RP. GeneXpert MTB/RIF on bronchoalveolar lavage samples in children with suspected complicated intrathoracic tuberculosis: a pilot study. Pediatr Pulmonol. 2014;49(11):1133–1137. | ||
Tamm M, Reichenberger F, McGandy CE, et al. Diagnosis of pulmonary Kaposi’s sarcoma by detection of human herpes virus 8 in bronchoalveolar lavage. Am J Respir Crit Care Med. 1998;157(2):458–463. | ||
Millar AB, Patou G, Miller RF, et al. Cytomegalovirus in the lungs of patients with AIDS: respiratory pathogen or passenger? Am Rev Respir Dis. 1990;141(6):1474–1477. | ||
Miles PR, Baughman RP, Linnemann CC. Cytomegalovirus in the bronchoalveolar lavage fluid of patients with AIDS. Chest. 1990;97(5):1072–1076. | ||
Tamm M, Traenkle P, Grilli B, et al. Pulmonary cytomegalovirus infection in immunocompromised patients. Chest. 2001;119(3):838–843. | ||
Mann M, Shelhamer JH, Masur H, et al. Lack of clinical utility of bronchoalveolar lavage cultures for cytomegalovirus in HIV infection. Am J Respir Crit Care Med. 1997;155(5):1723–1728. | ||
Shafiek H, Fiorentino F, Cosio BG, et al. Usefulness of bronchoscopic probe-based confocal laser endomicroscopy in the diagnosis of Pneumocystis jirovecii pneumonia. Respiration. 2016;92(1):40–47. | ||
Hanson DL, Chu SY, Farizo KM, Ward JW. Distribution of CD4+ T lymphocytes at diagnosis of acquired immunodeficiency syndrome-defining and other human immunodeficiency virus-related illnesses. The Adult and Adolescent Spectrum of HIV Disease Project Group. Arch Intern Med. 1995;155(14):1537–1542. | ||
Vincent B, Flahault A, Antoine M, et al. AIDS-related alveolar hemorrhage. A Prospective Study of 273 BAL Procedures. Chest. 2001;120(4):1078–1084. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


