Back to Journals » Infection and Drug Resistance » Volume 16
Diagnosis of Two Meningitis Cases Caused by Rickettsia Felis in China, with Metagenomic Next-Generation Sequencing: A Case Report
Authors Wang J, Zhou H, Dong Z, Wang J, Wang R, Guan Y
Received 19 April 2023
Accepted for publication 4 November 2023
Published 14 November 2023 Volume 2023:16 Pages 7239—7245
DOI https://doi.org/10.2147/IDR.S417787
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Jie Wang,1,* Hong Zhou,2,* Zan Dong,3 Jing Wang,1 Rui Wang,1 Yuanlin Guan4
1Department of Neurology, First Hospital of Shanxi Medical University, Taiyuan, 030000, People’s Republic of China; 2Graduate School, the First Clinical School of Medicine, Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 3Department of Neurology, Yuncheng Central Hospital, Yuncheng, 044000, People’s Republic of China; 4Department of Scientific Affairs, Hugobiotech Co, Ltd, Beijing, 100176, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jie Wang, Department of Neurology, First Hospital of Shanxi Medical University, No. 85 of Jiefang South Road, Taiyuan, Shanxi, 030000, People’s Republic of China, Tel +8613753198116, Email [email protected]
Background: Rickettsia felis is a kind of zoonotic pathogen. Rickettsia felis infections of the central nervous system are rare with only a few cases reported worldwide. The early diagnosis of R. felis is difficult due to its nonspecific clinical features and laboratory tests. Here, we report two meningitis cases caused by R. felis using metagenomic next-generation sequencing (mNGS).
Methods: The clinical data of patients with meningitis who were diagnosed to have R. felis through cerebrospinal fluid culture, nuclear magnetic imaging, mNGS detection from January 2019 to December 2019 in The First Clinical Hospital of Shanxi Medical University, were retrospectively analyzed, and their clinical characteristics and disease regression findings were summarized.
Case Presentation: The first case was a female patient aged 23 years who was admitted to our hospital presenting with symptoms of headache, fever, and weakness in both lower limbs. Upon examination of spinal imaging, myelitis was diagnosed. However, routine examination and culture of cerebrospinal fluid did not identify the pathogen responsible. Subsequently, metagenomic second-generation sequencing (mNGS) revealed that the infection was caused by R. felis. The patient responded well to standard treatment and showed signs of recovery. The second case was a male patient aged 29 years who was admitted to our hospital with a headache and fever that had persisted for 4 days within a month. Routine examination and culture of the cerebrospinal fluid did not reveal any identifiable pathogens. However, metagenomic second-generation sequencing (mNGS) determined that the patient had a Rickettsial infection likely transmitted by a cat. The patient showed significant improvement after 14 days of doxycycline treatment. Tests for herpes simplex virus, cytomegalovirus, Epstein-Barr virus and tubercle bacillus nucleic acid in the CSF and blood were negative.Therefore mNGS of the cerebrospinal fluid was used, which identified the pathogen as R. felis. One case was diagnosed as subacute meningitis with immune-associated myelitis and the other as subacute meningitis.
Conclusion: mNGS of cerebrospinal fluid can be used as a fast and effective method to identify intracranial R. felis infections.
Keywords: Rickettsia felis, meningitis, myelitis, metagenomic next-generation sequencing, case report
Introduction
Rickettsia felis belongs to the transitional group in the genus Rickettsia and is mainly transmitted by the cat flea (Ctenocephalides felis) but has been detected in a variety of arthropods including mosquitoes, ticks, and mites.1 Flea-borne spotted fever is caused by R. felis, with symptoms including fever, headache, and myalgia.2,3 R. felis infections involving the central nervous system were extremely rare and may present as photophobia, hearing loss, glove-and-stocking numbness, or subacute meningitis.4,5 Moreover, R. felis infection is difficult to diagnose due to its atypical clinical manifestations.
Metagenomic next-generation sequencing (mNGS) is a culture-independent approach, which can unbiasedly detect almost all species, including bacteria, viruses, fungi, and parasites present in clinical samples and distinguish the possible pathogen with an unbiased sequencing approach.6 Here, we report two human cases of R. felis infections identified by mNGS using cerebrospinal fluid.
The positive rate of R. felis disease in cats was low by PCR and serum antibody detection. mNGS was superior to traditional clinical methods in early diagnosis of rickettsial disease.
Case Presentation
Case No. 1
The patient was a 23-year-old woman with a history of thrombocytopenia. She owned a dog. On October 15, 2019, she suffered from headache (whole head), nausea, and vomiting, without any disturbance of consciousness. Before admission, she considered it to be “gastritis” and took Rabeprazole (10mg twice a day) orally, but her symptoms did not improve. And after 13 days, she developed fever, with a maximum temperature of 39°C. A lumbar puncture showed an intracranial pressure of 41.5 cm H2O, Cerebrospinal fluid (CSF) pleocytosis (260 leukocytes/mm3), glucose 1.5 mmol/L, chlorine 116.5 mmol/L, and protein 1513 mg/L. Gram staining, Ziehl-Neelsen staining, and CSF culture were negative. A GeneXpert tuberculosis test was negative (Cepheid AB, Solna Sweden). The local hospital administered mannitol (250mL every 8h), meropenem (2g every 8h), and levofloxacin (200mg twice a day) treatment. At 15 days after onset, the patient showed weakness in both lower limbs and could not stand. Magnetic resonance imaging (MRI) of the cervical and thoracic medullas showed abnormal signals in the spinal cord from the third segment of the cervical spinal cord to the 11th segment of the thoracic spinal cord, indicating myelitis (Figure 1). Therefore, empirical anti-tuberculosis treatment combined steroid (30 mg once a day) therapy was administered at the tuberculosis hospital for 10 days, and the patient’s fever and headache gradually resolved.
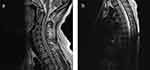 |
Figure 1 The key slices of the spinal cord MRI images of the case 1. (a) Cervical magnetic resonance imaging; (b)Thoracic magnetic resonance imaging. |
On November 13, she was transferred to our hospital due to weakness in both lower limbs. After receiving steroid therapy with a gradually decreased doses, her lower limb weakness and paresthesia improved. Her erythrocyte sedimentation rate (ESR) was 110 mm/h, and the anti-ribosomal P protein, anti-nuclear ribonuclear protein (anti-nRNP), anti-Sm antibodies and phosphorylated anti-neutrophil cytoplasmic antibody (ANCA) results were positive. The result of the anti-nuclear antibody spot test was 1:80. On November 18, the patient’s blood and CSF were negative for anti-aquaporin 4 (anti-AQP4), anti-myelin oligodendrocyte glycoprotein (anti-MOG), anti-myelin basic protein (anti-MBP), and anti-oligoclonal bands (anti-OB) antibodies. We analyzed the CSF with mNGS, and twenty unique sequence reads of R. felis were identified, (Supplement File). Other detected sequences were within the ranges of laboratory references. The patient, who was able to walk with the help of others, was diagnosed with R. felis meningitis with immune-related myelitis. She was not given doxycycline and other antibiotics, and was discharged on November 22, 2019.
The patient could walk by herself at 2 and 4 months follow-ups after discharge, and immunity-related tests and MRI of the spinal cord showed no abnormalities at follow-ups 2 months (Table 1).
 |
Table 1 Clinical Laboratory Investigations of Two Patients with R. Felis Infection |
Case No. 2
A 29-year-old man was in good health and did not have any contact with dogs or cats. On August 25, 2019, he was admitted to a local hospital due to severe headache and a sense of periorbital edema who did not have the funduscopy examination. After 26 days, he was hospitalized again with aggravated headache and fever. A brain MRI revealed a Chiari malformation with syringomyelia. A neurological examination showed no abnormality. Lumbar puncture procedures showed increased intracranial pressure and pleocytosis: 29 cm H2O, 46 leukocytes/mm3 on September 29, as well as 35 cm H2O and 76 leukocytes/mm3 on September 30. After empirical treatment with mannitol (250mL every 8h) for 10 days, acyclovir (0.5g every 8h) and ceftriaxone sodium (2g every 12h) for 5 days, his fever improved but his headache continued.
The patient was diagnosed with an “intracranial infection” and was transferred to the Department of Neurology on October 9, 2019 (at 46 days after onset). His funduscopy showed papilledema. He was positive for anti-cardiolipin IgM antibodies, with elevated anti-cyclic peptide containing citrulline (anti-CCP) antibodies, and his ESR was 25 mm/h. Lumbar puncture procedures revealed 33 cm H2O and 46 leukocytes/mm3 on October 10. The CSF was negative for antibodies against herpes simplex virus, human cytomegalovirus, Rubella virus, Toxoplasma gondii, Epstein-Barr virus, and parainfluenza virus. Patients were continued to be treated with mannitol (125mL every 8h), acyclovir (0.5g every 8h) and ceftriaxone sodium (2g every 12h). On October 17, another lumbar puncture was performed, and the intracranial pressure was 25 cm H2O. mNGS of from the CSF detected 38 unique sequences of R. felis (Supplement File). The other detected sequences were within the laboratory reference ranges. The patient was diagnosed with R. felis meningitis and given doxycycline (100 mg every 12 h) for 14 days. The patient was discharged on October 22 with improved headache and reduced cranial pressure.
At 2 and 4 months follow-ups after discharge, the patient had no discomfort and was working normally (Table 1).
mNGS Detection
About 2 mL CSF of each patient was collected and stored at −20 °C. Then, the samples were shipped on dry ice for PACEseq mNGS detection (Hugobiotech, Beijing, China). The DNA of CSF was extracted using TIANamp Micro DNA Kit (TIANGEN). The libraries were built using QIAseq™ Ultralow Input Library Kit (Illumina). Qubit (Thermo Fisher) and agarose gel electrophoresis were used to detect the DNA concentration and quality. The qualified libraries were finally sequenced on a Nextseq 550 platform (Illumina). Adapters, short, low-quality, and low-complexity reads were first removed from raw data. Human DNA reads were also filtered out after aligning to human reference database (hg38). The remaining reads were finally mapped to Microbial Genome Databases (ftp://ftp.ncbi.nlm.nih.gov/genomes/) using Burrows-Wheeler Alignment software (Figure 2).The probable pathogens of samples were distinguished.
 |
Figure 2 Results of mNGS in cerebrospinal fluid. (a) Sequencing of R. felis yielded a total coverage of 0.1751%. (b) Sequencing of R. felis yielded a total coverage of 0.0698%. |
Discussion
Biological information of R. felis has been found in 16 provinces of China,7 mainly in cat fleas and mosquitoes.8,9 Although cases of R. felis infection have been reported worldwide, in China it has only been reported in Taiwan and Shandong,10,11 and asymptomatic infections have been found in Jiangsu province.12 The CNS infection of R. felis was found in Shandong, Taiwan, Mexico, Indonesia, and Sweden.5,10,11,13,14 Here, we report two human cases of R. felis infection of the CNS in Shanxi, China, which represent an increase in the endemic region of the disease.
The clinical manifestations of R. felis infection of the CNS include fever, headache, and myalgia, and even photophobia, hearing loss, glove-and-stocking numbness, or subacute meningitis.5,10,11,13,14 Most patients were reported to have contact with dogs or cats.15 Both our patients developed fever and headache; the first patient who had a dog also presented with unreported secondary spinal cord injury, with weakness and paresthesia in both lower limbs. Immune-related tests of the two patients produced abnormal results in the acute stage of the disease, and results turned normal in the recovery stage. One patient developed post-infection myelitis with a normal spinal MRI during convalescence, suggesting that the immune response was activated and led to myelitis after infection. Moreover, multiple lumbar punctures revealed pleocytosis and persistently high intracranial pressure, which may be a characteristic of R. felis infection of the CNS. These findings were consistent with previous reports.16
R. felis can persist in the immunocompromised central nervous system and reappear after a long time to cause fatal neuroinflammation, which suggests a potential neuropathic effect and also explains the abnormal detection of immune indicators in both patients in this study.
The diagnosis of R. felis infection can be confirmed with an immunofluorescence assay (IFA) or PCR analysis of the blood, CSF, or a skin biopsy sample.1 Antigen selection with IFA is limited because the antigens of different rickettsias are susceptible to cross reactions.17 mNGS detection of CSF can identify the genetic information of viruses, bacteria, fungi, and parasites in the CSF, which can be compared with the microbial database to identify specific pathogenic microorganisms. The mNGS assay of the CSF can distinguish other pathogens and the different genera of Rickettsia. Studies have confirmed that rickettsia can penetrate the blood-brain barrier.17 The number of specific sequences detected by mNGS in the two patients was relatively small, but the identity of all the detected sequences was higher than 97% (Supplement File). The small number of R. felis reads may be related to the late acquisition of CSF samples (both were longer than one month from the onset time). At the time of CSF sample collection, the patients had received a variety of antibacterial drugs and immunoregulatory treatment, especially the use of steroid and ceftriaxone, which were reported to be effective for R. felis infection treatment. So, the amount of R. felis in CSF might have already decrease before mNGS. Their symptoms had improved, which also confirmed our diagnosis. Therefore, R. felis infection should be considered when an intracranial infection is suspected, or the nature of the infection is unclear. The early mNGS detection of CSF can quickly identify the pathogen.
The symptoms of feline Rickettsial infection and the routine detection of cerebrospinal fluid are very similar to viral infection, which is difficult to distinguish clinically, so it is often misdiagnosed. Rickettsial infection in cats is also self-limiting, which is another reason why its incidence is underestimated. mNGS is highly sensitive to pathogen diagnosis and has certain advantages for detection of rare pathogens. Rickettsial infection can be diagnosed by serological testing and microimmunofluorescence (MIF) methods. However, the disadvantage of MIF testing is that it can cross-react with different Rickettsias and is not sufficient to determine the cause. The culture sensitivity of cerebrospinal fluid was poor. Second-generation sequencing is highly sensitive for the diagnosis of Rickettsial in blood samples and skin biopsy specimens.
The most effective treatment for R. felis infection is doxycycline (100 mg every 12 h), with symptoms usually improving after two days of treatment.1 Rifampicin, chloramphenicol, and macrolide can also be used to treat R. felis infection.1,10 Other patients have improved after treatment with ceftriaxone or steroids.15,16 The improvement in our two patients might have resulted from their treatment with steroid, rifampicin, or ceftriaxone. Both patients had a long disease course and slow recovery. The first patient developed myelitis two weeks after onset, which might be related to the late diagnosis of the pathogen and the lack of early doxycycline treatment. Thus, it is important to identify the pathogen as early as possible and to give precise treatment.
In conclusion, R. felis infection of the CNS can caused meningitis and myelitis, characterized by pleocytosis and a persistently high intracranial pressure. The clinical manifestations and routine tests are similar to viral meningitis, which is easy to miss diagnosis. The diagnosis was based on the detection of R. felis DNA in the CSF. Most patients have a good prognosis. mNGS detection of the CSF can quickly identify R. felis and facilitate the initiation of early precision therapy.
Abbreviations
R. felis, Rickettsia felis; SFG, spotted fever group; CNS, central nervous system; NGS, next-generation sequencing; CSF, cerebrospinal fluid; DNA, deoxyribonucleic acid; MRI, magnetic resonance imaging; PCR, Polymerase chain reaction; ESR, erythrocyte sedimentation rate; IFA, immunofluorescence assay; AQP4, aquaporin 4; MOG, myelin oligodendrocyte glycoprotein; MBP, myelin basic protein; OB, oligoclonal bands; ANCA, anti-neutrophil cytoplasmic antibody; CCP, cyclic peptide containing citrulline antibody; nRNP, nuclear ribonuclear protein; Sm: Smith.
Data Sharing Statement
All data generated or analysed during this study are included in this published article. The CSF was detected by mNGS (Hugobiotech Co., Ltd.).
Ethics Approval and Consent to Participate
This study was approved by the ethical review committee of the First Hospital of Shanxi Medical University. Written informed consent was obtained from the patient.
Consent for Publication
Written informed consent was obtained from the patients for publication of this case report. Copies of the written consent for publication from the patients are available for review by the Editor-in-Chief of this journal.
Acknowledgments
We thank Huirong Liu for her invaluable suggestions during manuscript preparation.
Funding
This paper was not funded.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Angelakis E, Mediannikov O, Parola P, et al. Rickettsia felis: the Complex Journey of an Emergent Human Pathogen. Trends Parasitol. 2016;32(7):554–564. doi:10.1016/j.pt.2016.04.009
2. Adams JR, Schmidtmann ET, Azad AF. Infection of colonized cat fleas, Ctenocephalides felis (Bouche), with a rickettsia-like microorganism. Am J Trop Med Hyg. 1990;43(4):400–409. doi:10.4269/ajtmh.1990.43.400
3. Schriefer ME, Sacci JB Jr, Dumler JS, et al. Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J Clin Microbiol. 1994;32(4):949–954. doi:10.1128/jcm.32.4.949-954.1994
4. Zavala-Castro J, Zavala-Velazquez J, Walker D, et al. Severe human infection with Rickettsia felis associated with hepatitis in Yucatan, Mexico. Int J Med Microbiol. 2009;299(7):529–533. doi:10.1016/j.ijmm.2009.03.002
5. Mawuntu AHP, Johar E, Anggraeni R, et al. Rickettsia felis identified in two fatal cases of acute meningoencephalitis. PLoS Negl Trop Dis. 2020;14:e0007893.
6. Wilson MR, Sample HA, Zorn KC, et al. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. N Engl J Med. 2019;380(24):2327–2340. doi:10.1056/NEJMoa1803396
7. Zhang J, Lu G, Li J, et al. Molecular Detection of Rickettsia felis and Rickettsia bellii in Mosquitoes. Vector Borne Zoonotic Dis. 2019;19(11):802–809. doi:10.1089/vbz.2019.2456
8. Zhang J, Lu G, Kelly PJ, et al. Seasonal and Gender Differences in Presence of Rickettsia felis and Blood meals Provide Additional Evidence of a Vector Role for Mosquitoes. Can J Infect Dis Med Microbiol. 2019;2019:8543460. doi:10.1155/2019/8543460
9. Slapeta J, Lawrence A, Reichel MP. Cat fleas (Ctenocephalides felis) carrying Rickettsia felis and Bartonella species in Hong Kong. Parasitol Int. 2018;67(2):209–212. doi:10.1016/j.parint.2017.12.001
10. Tsai KH, Lu HY, Tsai JJ, et al. Human Case of Rickettsia felis Infection, Taiwan. Emerg Infect Dis. 2008;14(12):1970–1972. doi:10.3201/eid1412.080515
11. Zeng Z, Wang C, Liu C, et al. Follow-up of a Rickettsia felis encephalitis: some new insights into clinical and imaging features. Int J Infect Dis. 2021;104:300–302. doi:10.1016/j.ijid.2020.12.090
12. Zhang J, Lu G, Kelly P, et al. First report of Rickettsia felis in China. BMC Infect Dis. 2014;14(1):682. doi:10.1186/s12879-014-0682-1
13. Zavala-Velazquez JE, Ruiz-Sosa JA, Sanchez-Elias RA, et al. Rickettsia felis rickettsiosis in Yucatan. Lancet. 2000;356(9235):1079–1080. doi:10.1016/S0140-6736(00)02735-5
14. Lindblom A, Severinson K, Nilsson K. Rickettsia felis infection in Sweden: report of two cases with subacute meningitis and review of the literature. Scand J Infect Dis. 2010;42(11–12):906–909. doi:10.3109/00365548.2010.508466
15. Oteo JA, Portillo A, Santibanez S, et al. Cluster of Cases of Human Rickettsia felis Infection from Southern Europe (Spain) Diagnosed by PCR. J Clin Microbiol. 2006;44(7):2669–2671. doi:10.1128/JCM.00366-06
16. Dittrich S, Phommasone K, Anantatat T, et al. Rickettsia felis Infections and Comorbid Conditions, Laos, 2003–2011. Emerg Infect Dis. 2014;20(8):1402–1404. doi:10.3201/eid2008.131308
17. Rydkina E, Sahni SK, Santucci LA, et al. Selective modulation of antioxidant enzyme activities in host tissues during Rickettsia conorii infection. Microb Pathog. 2004;36(6):293–301. doi:10.1016/j.micpath.2004.01.002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
