Back to Journals » Infection and Drug Resistance » Volume 16
Diagnosis of Non-Tuberculous Mycobacterial Pulmonary Disease by Metagenomic Next-Generation Sequencing on Bronchoalveolar Lavage Fluid
Authors Zhang X, Chen H, Lin Y, Yang M, Zhao H, Hu J, Han D
Received 4 May 2023
Accepted for publication 15 June 2023
Published 26 June 2023 Volume 2023:16 Pages 4137—4145
DOI https://doi.org/10.2147/IDR.S417088
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xuan Zhang,1 Huixin Chen,1 Yaqing Lin,2 Meifang Yang,1 Hong Zhao,1 Jianhua Hu,1 Dongsheng Han3– 5
1State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, Hangzhou, Zhejiang, People’s Republic of China; 3Department of Laboratory Medicine, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 4Key Laboratory of Clinical in vitro Diagnostic Techniques of Zhejiang Province, Hangzhou, Zhejiang, People’s Republic of China; 5Institute of Laboratory Medicine, Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Dongsheng Han, Centre of Clinical Laboratory, First Affiliated Hospital, College of Medicine, Zhejiang University, 79 Qingchun Road, Hangzhou, 310003, People’s Republic of China, Tel/Fax +86-571-87236394, Email [email protected]
Purpose: Metagenomic next-generation sequencing (mNGS) has been extensively used in the diagnosis of infectious diseases but has rarely been applied in non-tuberculous mycobacterial pulmonary disease (NTMPD). This study analyzed the diagnostic performance of mNGS in bronchoalveolar lavage fluid (BALF) samples to identify non-tuberculous mycobacteria (NTM).
Patients and Methods: A total of 231 patients with suspected NTMPD were recruited from the First Affiliated Hospital, School of Medicine, Zhejiang University, from March 2021 to October 2022. A total of 118 cases were ultimately included. Of these patients, 61 cases were enrolled in the NTMPD group, 23 cases were enrolled in the suspected-NTMPD group, and 34 cases were enrolled in the non-NTMPD group. The diagnostic performance of traditional culture, acid-fast staining (AFS), and mNGS for NTMPD was assessed.
Results: Patients in the NTMPD group had a higher proportion of bronchiectasis (P=0.007). Among mNGS-positive samples in the NTMPD group, a significantly higher reads number of NTM was observed in AFS-positive patients [61.50 (22.00, 395.00) vs 15.50 (6.00, 36.25), P=0.008]. Meanwhile, mNGS demonstrated a sensitivity of 90.2%, which was far superior to AFS (42.0%) and culture (77.0%) (P< 0.001). The specificity of mNGS in detecting NTM was 100%, which was the same as that of traditional culture. The area under the receiver operating characteristic curve of mNGS was 0.951 (95% CI 0.906– 0.996), which was higher than that of culture (0.885 [95% CI 0.818– 0.953]) and AFS (0.686 [95% CI 0.562– 0.810]). In addition to NTM, other pulmonary pathogens were also found by mNGS.
Conclusion: mNGS using BALF samples is a rapid and effective diagnostic tool for NTMPD, and mNGS is recommended for patients with suspected NMTPD or NTM coinfected pneumonia.
Keywords: non-tuberculous mycobacterial, culture, metagenomic next-generation sequencing, bronchoalveolar lavage fluid
Introduction
Non-tuberculous mycobacteria (NTM) is a collective name given to a group of more than 190 species of Mycobacterium other than Mycobacterium tuberculosis and Mycobacterium leprae.1 NTM are labeled as environmental mycobacteria as they are widely distributed in the environment, such as in soil, marshland, streams, rivers, estuaries, dust, domestic and wild animals, and food.2 Most NTM species are non-pathogenic, but some can cause diseases in humans. Pulmonary manifestations account for 80–90% of all NTM-associated diseases.3 Recent data have shown an increasing incidence and mortality of non-tuberculous mycobacterial pulmonary disease (NTMPD) worldwide.4–6 Early diagnosis and timely treatment are critical in NTMPD patients.
However, the traditional diagnostic methods of NTM have some deficiencies. Acid-fast staining (AFS) can rapidly identify mycobacteria, but cannot distinguish between M. tuberculosis and NTM. Mycobacterial culture on growth media is considered a “gold standard” diagnostic method,7,8 but this process takes several weeks and has a low positive rate. Furthermore, traditional culture cannot identify the specific strains of NTM, which is valuable information in clinical practice. Therefore, a rapid and accurate method for NTM detection would benefit clinical practice.
Metagenomic next-generation sequencing (mNGS) is a promising new technology that is highly effective in diagnosing infectious diseases.9–11 Xu et al reported that 23 patients with NTMPD were all confirmed using mNGS.12 However, the main research target of most previous studies was M. tuberculosis,12,13 while only a few studies have focused on NTM. The current retrospective study was performed in China to analyze the diagnostic performance of mNGS in the identification of NTMPD in comparison to traditional culture and AFS.
Materials and Methods
Study Design and Participants
A retrospective observational study was carried out on patients with suspicion of NTMPD admitted to the First Affiliated Hospital, School of Medicine, Zhejiang University, from March 2021 to October 2022. The inclusion criteria were as follows: (1) patients aged over 18 years; (2) patients who visited the First Affiliated Hospital, School of Medicine, Zhejiang University, from March 2021 to October 2022; (3) patients with pulmonary lesions in accordance with the imaging changes of NTMPD, including thin-walled cavities, multifocal bronchiectasis, multiple nodules, and mass shadow; (4) patients with one or more positive AFS results in sputum or BALF, but no response to regular antituberculosis therapy; and (5) patients with persistent symptoms (for example fever, respiratory symptoms) that did not respond to traditional anti-bacterial therapy. Exclusion criteria were as follows: (1) patients with incomplete clinical data; (2) patients without mNGS or culture on bronchoalveolar lavage fluid (BALF). The enrollment and screening process of patients is displayed in Figure 1. The NTMPD group was confirmed according to the guidelines of the American Thoracic Society/European Respiratory Society/European Society of Clinical Microbiology and Infectious Diseases/Infectious Diseases Society of America (ATS/ERS/ESCMID /IDSA) (2020).14 In the suspected-NTMPD group, the patients met the clinical and radiographic criteria of ATS/ERS/ ESCMID/IDSA guidelines, and the mNGS results in BALF was positive, which met the diagnosis criteria of NTMPD in the guidelines formulated by the Chinese Medical Association,15 but not the guidelines of ATS/ERS/ ESCMID/IDSA. The patients in the non-NTMPD group did not meet the ATS/ERS/ ESCMID/IDSA or Chinese guidelines.
 |
Figure 1 Flowchart of patient enrollment and grouping. Abbreviation: NTMPD, non-tuberculous mycobacterial pulmonary disease. |
Data were extracted from the electronic patient record system of the hospital and the results of AFS, conventional culture, and mNGS in BALF of all patients were collected. This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University. Due to the retrospective nature of the study and as no identifiable patient information was included in this manuscript, the need for consent was waived.
mNGS Workflow
DNA-based mNGS testing for BALF samples of each patient was performed in the clinical laboratory. BALF DNA was extracted from 1 mL of the sample using the QIAamp® UCP Pathogen DNA Kit (catalog number: 50214, Qiagen, Duesseldorf, Germany) according to the manufacturer’s instructions. Human DNA was removed using 1U Benzonase (Sigma) and 0.5% Tween 20 (Sigma) and incubated at 37°C for 5 min. The extracted DNA was then quantified using a Qubit dsDNA HS Assay Kit (catalog number: Q32854, Invitrogen, Carlsbad, CA, USA). Subsequently, the quantified unique DNA fragments (named UMSI) were spiked for each sample as an identity and internal control, which were PCR products of Oryza sativa of 400 to 600 bp in length.16,17 Thirty microliters of the eluate were used to generate libraries using the Nextera DNA Flex kit (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. Library pools were then loaded onto the Illumina Nextseq CN500 sequencer for 50 cycles of single-end sequencing (SE-50), generating approximately 20 million reads for each library. For negative controls, peripheral blood mononuclear cell (PBMC) samples were also prepared with 105 cells/mL from healthy donors in parallel with each batch using the same protocol, and sterile deionized water was extracted alongside the specimens to serve as non-template controls.15
Low-quality reads, adapter contamination, duplicate reads, and low-complexity reads were removed by fastp (version 0.20.0) with default parameters.18 Human sequence data were excluded by mapping to a human reference genome (hg38) using Burrows-Wheeler Aligner (version 0.7.17).18 The remaining sequencing data were aligned to an in-house microbial database for microbial identification with SNAP v1.0 beta.18.19 Virus-positive detection results (DNA viruses) were defined as the coverage of three or more non-overlapping regions on the genome. A positive detection was reported for a given species or genus if the reads per million (RPM) ratio, or RPM-r was ≥5, where the RPM-r was defined as the RPMsample/RPMNC (ie, the RPM corresponding to a given species or genus in the clinical sample divided by the RPM in the NC/negative control).20 In addition, the RPM of microorganisms sharing a genus or family designation was penalized (reduced) if the species or genus appeared in non-template controls to minimize cross-species misalignments among closely related microorganisms. A penalty of 5% was used for species.21
Statistical Analysis
Continuous variables following normal distribution were expressed as mean ± standard deviation (SD). Continuous variables with non-normal distribution were expressed as median (P25, P75). ANOVA test was used for the comparison of the means, and the χ2 test was used for the comparison of the categorical data between groups. The reads number of NTM between groups was analyzed by the Mann–Whitney U-test. The diagnostic performance of mNGS, AFS and culture for NTMPD was calculated, including the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Moreover, the receiver operating characteristic (ROC) curves were drawn and the area under the curve (AUC) was calculated. All statistical analyses were performed using SPSS software 25.0 (IBM, Armonk, NY, USA) and GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA), P < 0.05 was considered to be statistically significant.
Results
Patient Characteristics
As displayed in Figure 1, 231 patients with suspicion of NTMPD were selected from March 2021 to October 2022. However, 108 patients were excluded due to the lack of BALF sample analysis, and five patients were excluded due to incomplete clinical data. Therefore, 118 cases were ultimately included in the study. Based on the BALF sample analysis results, the patients were divided into the NTMPD group (61 cases), the suspected-NTMPD group (23 cases), and the non-NTMPD group (34 cases).
The patient characteristics are listed in Table 1. There were 24 males (39.3%) and 37 females (70.7%) in the NTMPD group, with an average age of 59.26 ± 12.21 years. The average age of non-NTMPD patients was 59.65±13.14 years and 52.9% (18/34) were female. Patients in the NTMPD group exhibited a higher rate of bronchiectasis (P=0.007). No significant difference in sex, age, imaging findings (except bronchiectasis), and other baseline characteristics (except bronchiectasis) was found between the three groups.
 |
Table 1 Demographic and Clinical Characteristics Between the NTMPD and Non-NTMPD Groups |
In the NTMPD group, M. intracellulare was the most common species isolated in NTMPD patients (31/61, 50.8%), followed by M. abscessus (12/61, 19.7%), M. kansasii (13.1%), M. avium (7/61, 11.5%), M. colombiense (2/61, 3.3%), M. chimaera (1/61, 1.6%), M. maeseillense (1/61, 1.6%), M. chelonae (1/61, 1.6%) and M. xenopi (1/61, 1.6%).
Results of Culture and mNGS in BALF Samples on NTM Detection
As shown in Figure 2, 70.5% (43/61) of the NTMPD patients had positive results in both mNGS and culture, while 6.6% (4/61) were culture-positive and mNGS-negative and 19.7% (12/61) were mNGS-positive and culture-negative; 3.3% (2/61) received negative results in both mNGS and culture. A total of 14 (23.0%) cases had positive AFS, culture, and mNGS results.
Among the mNGS-positive patients in the NTMPD group, eight patients had a reads number of five or less. As shown in Figure 3a, the reads number of NTM in the AFS-positive patients of the NTMPD group was significantly higher than in the AFS-negative patients [61.50 (22.00, 395.00) vs 15.50 (6.00, 36.25)]. There was a significant difference in different smear grades (P=0.008). However, the reads number of NTM in the culture-positive patients and the culture-negative patients was 30.00 (10.25, 116.25) and 43.50 (22.75, 97.00), respectively, showing no statistically significant difference (P=0.482). The above results are shown in Figure 3b.
In the suspected-NTMPD group, mNGS revealed that every sample was positive. Two samples had only 1 read referring to either the species or genus of NTM and two samples had 3 reads. The reads number of NTM in the suspected-NTMPD group was lower than that in the mNGS-positive samples of the NTMPD group [17.50 (5.50, 55.25) vs 30.00 (11.00, 90.00)], but statistically significant difference was not observed (P=0.433).
Diagnostic Performance of AFS, Culture, and mNGS in NTM
The diagnostic performance of AFS, traditional culture, and mNGS for NTMPD was estimated in BALF specimens (Table 2). mNGS displayed a sensitivity of 90.2%, which was significantly superior to AFS (42.0%) and culture (77.0%) (P<0.001). The specificity of mNGS in detecting NTM was 100%, which was the same as that of traditional culture. However, due to a positive result in one tuberculosis patient, the specificity of AFS was 95.2%, which was slightly lower than that of culture and mNGS. In addition, the PPV and NPV of mNGS in the analysis of NTMPD were calculated as 100% and 85.0%, respectively, which was significantly better than the NPV of AFS and culture (40.8% and 70.8%, respectively, P<0.001). Notably, the combination of culture and mNGS showed a higher specificity and NPV (96.7%, 94.4%), but it was not significantly superior to mNGS alone (P=0.273, 0.334).
 |
Table 2 Diagnostic Performance of AFS, Traditional Culture, and mNGS in NTMPD |
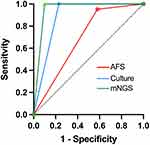
The ROC curves of mNGS and culture are shown in Figure 4. The AUC of mNGS was 0.951 (95% CI 0.906–0.996), which was higher than that of culture (0.885 [95% CI 0.818–0.953]) and AFS (0.686 [95% CI 0.562–0.810]).
Clinical mNGS Testing for Other Pathogens
In the NTMPD group, mNGS detected 5 bacteria, 3 fungi, and 4 viruses in the 61 clinical samples, including Staphylococcus aureus (3, 4.9%), Pseudomonas aeruginosa (4, 6.6%), Stenotrophomonas maltophilia (1, 1.6%), Moraxella catarrhalis (1, 1.6%), Nocardia (1, 1.6%), Pneumocystis carinii (2, 3.3%), Aspergillus fumigatus (1, 1.6%), Candida albicans (1, 1.6%), torque teno virus (5, 8.2%), rhinovirus (2, 3.3%), cytomegalovirus (3, 4.9%), and Epstein-Barr virus (3, 4.9%). In the non-NTMPD group, M. tuberculosis was detected in six patients.
Discussion
This study comprehensively evaluated the performance of mNGS for the etiological diagnosis of suspected NTMPD patients. In addition, the traditional culture method was performed in the whole cohort, among whom 50 patients also underwent AFS testing. In the NTMPD group, women accounted for the majority (60.7%), and the mean age was nearly 60 years old, which was consistent with previous reports.22,23 Meanwhile, M. intracellulare was the most common species isolated in the NTMPD group (68.9%). A study by Fang et al also showed that most of the pulmonary NTM diseases in the Zhejiang province of China were caused by M. intracellulare.24 The risk of NTMPD was significantly higher in patients with bronchiectasis (P = 0.007), which was consistent with the results of a previous study.25
NTMPD patients often lack specific clinical manifestations and pulmonary imaging features can be easily mistaken for a common pulmonary infection. Furthermore, doctors in primary hospitals had a relatively low awareness of NTM, posing challenges to NTMPD diagnosis. A major advantage of mNGS technology is in testing clinical samples without any prior suspicion of specific pathogens.26 In the diagnosis of NTMPD, the diagnostic sensitivity of mNGS (90.2%) was significantly higher than those of the conventional diagnostic methods culture (77.0%) and AFS (42.0%) (P<0.001). Moreover, mNGS showed good specificity, PPV, NPV, and Youden index, which suggested that mNGS was suitable to diagnose NTMPD in BALF. Compared with the two conventional detection methods, mNGS has a shorter processing time and can identify species of NTM. Previous reports on the diagnostic value of mNGS in NTMPD patients are rare,13,27 and all concluded that mNGS of BALF represents a potentially effective tool for the diagnosis of NTMPD. Additionally, mNGS combined with culture (96.7%) showed the highest diagnostic sensitivity, which also demonstrated the best specificity, NPV, and other aspects. However, combined testing showed no significant advantage, but required additional time. Therefore, combined mNGS and culture testing for the diagnosis of NTMPD is not recommended.
As NTM are intracellular parasitic microorganisms, their detection is complicated. Therefore, NTM is considered a low-abundance mNGS detection of high-priority pathogens. In this study, eight samples in the NTMPD group had only a few reads (≤5) referring to either the species or genus of NTM. However, seven samples had positive culture results, and one patient was diagnosed by lung biopsy. Furthermore, the reads number of NTM was significantly higher in AFS-positive patients of the NTMPD group (P=0.008), probably due to the higher bacterial load in smear-positive patients. In the suspected-NTMPD group, NTM were all detected by mNGS, but not tested by culture in BALF as most patients received antibiotic therapy, which limited the culture results.
In addition to NTM, other pathogens were also detected in BALF, including viruses such as cytomegalovirus and Epstein-Barr virus. A previous study also detected the viruses, which may be associated with poor outcomes in ICU patients.28 Mycobacterium tuberculosis was detected in six patients. Due to the similarities in the clinical manifestations of pulmonary tuberculosis and NTMPD, NTM was also often detected in previous studies investigating suspected tuberculosis.13,29,30
Despite the encouraging results, the limitations of the present study should be acknowledged. This was a retrospective, single-center study with a relatively small sample size, which may lead to statistical bias. Therefore, a large-scale multicenter prospective study is required to further investigate the diagnostic value of mNGS in NTMPD.
Conclusion
In this study, the diagnostic performance of mNGS using BALF was not inferior to that of traditional culture in patients with clinical suspicion of NTMPD. mNGS has a shorter processing time, thereby contributing to early diagnosis and precise treatment. Moreover, mNGS of BALF samples can detect pulmonary coinfection. Therefore, mNGS of BALF samples is a rapid and effective diagnostic tool for NTMPD, and is recommended for patients with suspected NTMPD or NTM coinfected pneumonia.
Ethics Approval and Informed Consent
Approval was obtained from the ethics committee of the First Affiliated Hospital of Zhejiang University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki. This was a retrospective observational study and informed consent to participate was waived in accordance with the ethics approval.
Acknowledgments
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Funding
This work was supported by National Key R&D Program of China (2022YFC2304500, 2022 YFC2304505) and Zhejiang Province Natural Science Foundation (LY23H200001).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Porvaznik I, Solovic I, Mokry J. Non-tuberculous mycobacteria: classification, diagnostics, and therapy. Adv Exp Med Biol. 2017;944:19–25. doi:10.1007/5584_2016_45
2. Mathewos B, Kebede N, Kassa T, Mihret A, Getahun M. Characterization of mycobacterium isolates from pulmomary tuberculosis suspected cases visiting Tuberculosis Reference Laboratory at Ethiopian Health and Nutrition Research Institute, Addis Ababa Ethiopia: a cross sectional study. Asian Pac J Trop Med. 2015;8(1):35–40. doi:10.1016/S1995-7645(14)60184-X
3. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi:10.1164/rccm.200604-571ST
4. Brode SK, Marchand-Austin A, Jamieson FB, Marras TK. Pulmonary versus nonpulmonary nontuberculous mycobacteria, Ontario, Canada. Emerg Infect Dis. 2017;23:1898–1901. doi:10.3201/eid2311.170959
5. Prevots DR, Loddenkemper R, Sotgiu G, Migliori GB. Nontuberculous mycobacterial pulmonary disease: an increasing burden with substantial costs. Eur Respir J. 2017;49(4):1700374. doi:10.1183/13993003.00374-2017
6. Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18(1):206. doi:10.1186/s12879-018-3113-x
7. Ryu YJ, Koh WJ, Daley CL. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians’ perspectives. Tuberc Respir Dis. 2016;79(2):74–84. doi:10.4046/trd.2016.79.2.74
8. Gopalaswamy R, Shanmugam S, Mondal R, Subbian S. Of tuberculosis and non-tuberculous mycobacterial infections - A comparative analysis of epidemiology, diagnosis and treatment. J Biomed Sci. 2020;27(1):74. doi:10.1186/s12929-020-00667-6
9. Chiang AD, Dekker JP. From the pipeline to the bedside: advances and challenges in clinical metagenomics. J Infect Dis. 2020;221(Suppl 3):S331–S340. doi:10.1093/infdis/jiz151
10. Deurenberg RH, Bathoorn E, Chlebowicz MA, et al. Application of next generation sequencing in clinical microbiology and infection prevention. J Biotechnol. 2017;243:16–24. doi:10.1016/j.jbiotec.2016.12.022
11. Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. 2018;66(5):778–788. doi:10.1093/cid/cix881
12. Xu P, Yang K, Yang L, et al. Next-generation metagenome sequencing shows superior diagnostic performance in acid-fast staining sputum smear-negative pulmonary tuberculosis and non-tuberculous mycobacterial pulmonary disease. Front Microbiol. 2022;13:898195. doi:10.3389/fmicb.2022.898195
13. Shi CL, Han P, Tang PJ, et al. Clinical metagenomic sequencing for diagnosis of pulmonary tuberculosis. J Infect. 2020;81(4):567–574. doi:10.1016/j.jinf.2020.08.004
14. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56:2000535. doi:10.1183/13993003.00535-2020
15. Tuberculosis Branch of Chinese Medical Association. Guidelines for Diagnosis and treatment of nontuberculous mycobacteriosis (2020 edition). Chin J Tuberculosis Respir Dis. 2020;43:918–946. Chinese. doi:10.3760/cma.j.cn112147-20200508-00570
16. Han D, Yu F, Zhang D, et al. The real-world clinical impact of plasma mNGS testing: an observational study. Microbiol Spectr. 2023;11(2):e0398322. doi:10.1128/spectrum.03983-22
17. Diao Z, Lai H, Han D, Yang B, Zhang R, Li J. Validation of a metagenomic next-generation sequencing assay for lower respiratory pathogen detection. Microbiol Spectr. 2023;11(1):e0381222. doi:10.1128/spectrum.03812-22
18. Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884–i890. doi:10.1093/bioinformatics/bty560
19. Naccache SN, Federman S, Veeraraghavan N, et al. A cloud-compatible bioinformatics pipeline for ultrarapid pathogen identification from next-generation sequencing of clinical samples. Genome Res. 2014;24(7):1180–1192. doi:10.1101/gr.171934.113
20. Zhang X, Chen H, Han D, Wu W. Clinical usefulness of metagenomic next-generation sequencing for Rickettsia and Coxiella burnetii diagnosis. Eur J Clin Microbiol Infect Dis. 2023;42(6):681–689. doi:10.1007/s10096-023-04586-w
21. Gu W, Deng X, Lee M, et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. 2021;27(1):115–124. doi:10.1038/s41591-020-1105-z
22. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary non-tuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. 2010;182(7):977–982. doi:10.1164/rccm.201003-0503OC
23. Lai CC, Tan CK, Chou CH, et al. Increasing incidence of non-tuberculous mycobacteria, Taiwan, 2000–2008. Emerg Infect Dis. 2010;16(2):294–296. doi:10.3201/eid1602.090675
24. Fang H, Shangguan Y, Wang H, et al. Multicenter evaluation of the biochip assay for rapid detection of mycobacterial isolates in smear-positive specimens. Int J Infect Dis. 2019;81:46–51. doi:10.1016/j.ijid.2019.01.036
25. Cowman S, van Ingen J, Griffith DE, Loebinger MR. Non-tuberculous mycobacterial pulmonary disease. Eur Respir J. 2019;54(1):1900250. doi:10.1183/13993003.00250-2019
26. Zhou X, Wu H, Ruan Q, et al. Clinical evaluation of diagnosis efficacy of active mycobacterium tuberculosis complex infection via metagenomic next-generation sequencing of direct clinical samples. Front Cell Infect Microbiol. 2019;9:351. doi:10.3389/fcimb.2019.00351
27. Wei W, Cao J, Wu XC, et al. Diagnostic performance of metagenomic next-generation sequencing in non-tuberculous mycobacterial pulmonary disease when applied to clinical practice. Infection. 2023;51(2):397–405. doi:10.1007/s15010-022-01890-z
28. Huang L, Zhang X, Pang L, et al. Viral reactivation in the lungs of patients with severe pneumonia is associated with increased mortality, a multicenter, retrospective study. J Med Virol. 2023;95(1):e28337. doi:10.1002/jmv.28337
29. Liu X, Chen Y, Ouyang H, et al. Tuberculosis diagnosis by metagenomic next-generation sequencing on bronchoalveolar lavage fluid: a cross-sectional analysis. Int J Infect Dis. 2021;104:50–57. doi:10.1016/j.ijid.2020.12.063
30. Chen P, Sun W, He Y. Comparison of metagenomic next-generation sequencing technology, culture and GeneXpert MTB/RIF assay in the diagnosis of tuberculosis. J Thorac Dis. 2020;12(8):4014–4024. doi:10.21037/jtd-20-1232
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.