Back to Journals » Drug Design, Development and Therapy » Volume 16
Dezocine Has the Potential to Regulate the Clinical and Biological Features of Tumors
Authors Hu X , Luo B, Qiu L , Chen S, Wu Q, Chen Q, Liu X , Ling C, Deng S, Yuan M, Hu P
Received 8 January 2022
Accepted for publication 9 April 2022
Published 20 April 2022 Volume 2022:16 Pages 1121—1129
DOI https://doi.org/10.2147/DDDT.S356863
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Xudong Hu,1,* Bing Luo,2,* Lei Qiu,2 Shaosen Chen,3 Qing Wu,2 Qingbiao Chen,2 Xingqing Liu,1 Chen Ling,1 Shuping Deng,4 Manjuan Yuan,3 Peicun Hu3
1Department of Anesthesiology, The Second People’s Hospital of Foshan, Foshan, 528000, People’s Republic of China; 2Department of Surgery, The Second People’s Hospital of Foshan, Foshan, 528000, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, The Second People’s Hospital of Foshan, Foshan, 528000, People’s Republic of China; 4Department of Internal Medicine, Huanshi Hospital, People’s Hospital of Chancheng District, Foshan, 528000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peicun Hu, Department of Respiratory and Critical Care Medicine, The Second People’s Hospital of Foshan, No. 78 of Weiguo Road, Foshan, Guangdong, 528000, People’s Republic of China, Tel +86 13902845621, Fax +86 757-88032008, Email [email protected]
Abstract: Cancer is the second leading cause of death following ischemic heart disease in the world and the primary clinical, social and economic burden. Surgical resection is the main measure for the treatment of the vast majority of solid tumors. However, the recurrence and metastasis of tumors occur at different periods after surgery in many cases undergoing radical tumor surgery, which is the main cause of death of tumor patients. Moreover, tumor patients are prone to suffer from mental depression, which may increase the morbidity and mortality of tumors. Tumors have a series of clinical biological signs with the following five main features: postoperative pain and cancerous pain; suppression of antitumor immunity; angiogenesis in tumors; proliferation, growth and metastasis of tumors; and mental depression. Surgery is the first treatment in the majority of cancer patients with solid tumors. Opioids are required for anesthesia and postoperative analgesia. For cancerous pain control, patients undergo surgery, and their quality of life of is improved. However, traditional opioids, such as morphine, may inhibit antitumor immunity, induce vascular growth of tumors and promote the proliferation, invasion and migration of cancer cells, and traditional opioids can induce a risk of somatic dependence. However, studies have found that not all opioids share the effects of immunosuppression, tumor proliferation promotion and angiogenesis induction. Dezocine, a novel opioid with specific pharmacological mechanisms, has been demonstrated to regulate the five clinical and biological features of tumors. We reviewed the preclinical and clinical studies of dezocine on postoperative pain and cancer pain in tumor patients as well as the immune system, tumor angiogenesis, tumor proliferation, tumor growth, tumor metastasis and mental depression. We proposed that dezocine may be the best choice of opioids for anesthesia and analgesia in cancer patients.
Keywords: dezocine, cancer, anesthesia, postoperative analgesia, cancerous pain, immune, angiogenesis, proliferation, growth and metastasis, mental depression
Cancer poses the highest clinical, social and economic burden in terms of cause-specific disability-adjusted life years among all human diseases. A total of 18 million new cancer patients were diagnosed worldwide in 2018 with the most common cancers being lung cancer (2.09 million), breast cancer (2.09 million) and prostate cancer (1.28 million); cancer is the second largest cause of death (8.97 million deaths per year) worldwide after ischemic heart disease, but it will be the first cause of death (approximately 18.63 million) in 2060.1 In recent years, cancer has become the leading cause of death in China and has been a severe disease burden.2 Surgical resection is the main measure of the vast majority of solid tumor treatments, and it is combined with chemical therapy, radiotherapy, antiangiogenic treatment, immunotherapy and support treatment, resulting in an improved survival rate of tumor patients, especially for thyroid cancer, breast cancer, lung cancer, colorectal cancer, prostate cancer and cervical cancer.2,3 However, many cancer patients undergoing radical tumor surgery suffer from cancer recurrence and metastasis at different periods after surgery, and cancer recurrence and metastasis are the main causes of death in tumor patients.4 Moreover, tumors are accompanied with depression in cancer patients, which aggravates immune function inhibition, promotes tumor proliferation and reduces the quality of life in cancer patients. Studies have found that major depression (MDD) is common in cancer patients, whose prevalence is four times that of the general population, which can directly lead to suicide and death.5–7
Five Clinical and Biological Features of Tumors
The local growth, infiltration and distant metastasis of cancer can induce different degrees of pain through various mechanisms as follows: direct stimulation and compression of neural fibers around the tumor; stimulation of organ ectoblasts caused by tumor swelling and pain-induced factor release by tumor tissue, especially in solid tumors with peritoneal membranes;8,9 and bone metastasis. The prescription of strong opioids, mainly including morphine and oxycodone, is used to control cancer pain and to improve the quality of life in cancer patients.10 Surgical treatment is performed for almost all cancer patients at an early stage, and surgical injury leads to postoperative pain, which may last for several days after surgery. Furthermore, surgical complications, such as anastomotic leakage, intestinal obstruction and wound infection, can occur, which enhances the postoperative pain.11 Pain in cancer patients, including postoperative pain and cancerous pain, constitutes the first clinical biological feature of the tumor.
Immune inhibition to different degrees is found in almost all cancer patients and is aggravated due to the stress response caused by surgery, postoperative pain and cancer pain, including abnormal expression and function of cytotoxic T cells and NK cells as well as T helper cell differentiation and cytokines. These changes in the immune system may result in tumor proliferation, invasion and migration, and they are important factors leading to postoperative tumor recurrence and metastasis.12–14 Currently, immunotherapy, including immunoadaptive infusion of T lymphocytes15 and PD-1/PDL-1 inhibitors,16 has achieved satisfactory results for cancer treatment. Immunosuppression in cancer patients constitutes the second clinical and biological feature of the tumor.
Tumor angiogenesis is a hallmark of cancer and plays a crucial role in providing oxygen and nutrients to tumor cells during cancer progression and metastasis. Many proangiogenic factors and their receptors are upregulated under pathological conditions, such as tumors, and VEGF is generally recognized as a key regulator of tumor angiogenesis among these factors. Anti-VEGF antibodies, such as bevacizumab, have been developed as a cancer therapy to inhibit tumor angiogenesis in cancers, including colorectal cancer, renal cell cancer and non-small-cell lung cancer, as well as senile macular degeneration17,18 despite the drug tolerance phenomenon.19 Tumor angiogenesis constitutes the third clinical and biological feature of tumors.
Tumor proliferation is also related to the biological properties of the tumor cells themselves. Because tumor cells have unlimited growth and proliferative characteristics, tumor lesion growth and infiltration occur, and remote metastasis occurs when the tumor involves the vascular and lymphatic systems. Moreover, the proliferation of cancer consumes the body’s energy reserves, destroys the body’s defense system, leads to the failure of various organs in the body and leads to cancer patients’ death.20 Chemical therapy and radiotherapy focus directly on tumor proliferation, and they inhibit tumor growth and promote the apoptosis of cancer cells.21,22 Therefore, tumor proliferation and growth constitute the fourth clinical and biological feature of the tumor.
Cancer patients suffer from the pain and stress induced by surgical treatment of cancer itself as well as the concerns and fears about cancer, which induce psychological pressure and neuroinflammation in the brain, resulting in a high risk of mental depression. Mental depression is found in 39 to 47% of cancer patients; it seriously affects the recovery of patients and reduces the quality of life of patients and their families, and it may even cause suicide, directly leading to death.5–7 Mental depression constitutes the fifth clinical biological feature of tumors.
Studies have confirmed that pain leads to immunosuppression, vascular growth, tumor proliferation, tumor metastasis and accompanying depression.14,23,24 Immune function suppression exacerbates tumor proliferation and metastasis, and it induces hyperalgesia and promotes vascular growth.14,25 Depression has also been associated with immune function and the inflammatory response; unfortunately, overactivation of the inflammatory response may cause damage to the nervous system, resulting in aggravated depression.26 Vascular growth, tumor proliferation and tumor metastasis contribute to each other,25,27 and concomitant depression increases sensitivity to pain, inhibits immune function, promotes vascular growth and promotes tumor proliferation.5–7,26 Therefore, postoperative pain or cancer pain, immunosuppression, tumor vascular growth, tumor growth/proliferation and mental depression constitute the five clinical and biological characteristics of the tumor. The five clinical and biological characteristics of the tumor interact with each other in a vicious cycle and directly affect the postoperative rehabilitation, quality of life and survival of cancer patients.
In the current treatment of tumors, the five clinical biological characteristics of tumors are regulated by different therapeutic procedures as follows: opioids for the inhibition of postoperative pain and cancerous pain;10 immunotherapy for improving antitumor immunity;15,16 targeted vascular growth factor (VEGF) inhibitors for the inhibition of angiogenesis;17,18 chemical therapy and radiotherapy for the inhibition of tumor proliferation and the direct induction of tumor cell death;21,22 and antidepressants for controlling tumor-associated depression.5–7 However, due to the interaction of the five elements in an overall unity of the body, the treatment for a single clinical and biological characteristic may not be enough and cause other adverse reactions. Opioids are beneficial to pain, but traditional opioids, such as morphine, fentanyl, sufentanil and oxycodone, inhibit immune function and increase the risk of tumor recurrence and metastasis.24 PD-1/PDL-1 inhibitors, mainly upregulating the function of cytotoxic T cells (CD8+ cells), promote the apoptosis of tumor cells but can also promote angiogenesis, induce capillary syndrome, decrease its antitumor effects and reduce the analgesic effect of morphine.28,29 Antidepressant treatment may promote tumor proliferation30 and increase the expression of vascular growth factor (VEGF), which may facilitate the treatment of depression, but VEGF promotes tumor growth and metastasis.31
Therefore, balanced therapy with multiple targets is a new method in tumor treatment. For example, the combination of PD1/PDL1 inhibitors with anti-vascular growth factor monoclonal antibodies is used to treat tumors.32 The idea of multitarget treatment for tumors was proposed as early as 2005.33 Benedetti et al proposed34 in 2015 that cancer should not be considered as a disease caused by a single factor but as a multifactorial disease in which genetic, epigenetic and metabolic factors are all involved in tumor formation, leading to major changes in the molecular networks that control cell growth, development, death and specialization. Thus, many antitumor therapies no longer target individual targets but target the entire biological system, in which regulation determines the function of the physiopathological state and maintenance. At present, for the treatment of tumors, combined treatments of antitumor drugs are advocated, which not only supplement monotherapy but also overcome the resistance to monotherapy drugs and reduce the side effects.35
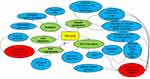
Dezocine Has Potential to Regulate the Clinical and Biological Features of Tumors (Figure 1)
Surgery is the primary and basic treatment for the majority of solid tumors, and anesthesia and postoperative analgesia must be performed to inhibit the surgical stress response and postoperative pain, usually by the use of opioids to protect immune function, improve depressive symptoms, promote patient recovery, improve patient quality and improve survival rate.36 However, traditional opioids, such as morphine, fentanyl and sufentanil, may inhibit immune function, promote tumor vascular growth, promote tumor proliferation, promote tumor migration and simultaneously induce somatic-dependent risk.37,38 Therefore, understanding how to reduce perioperative pain, protect immune function and reduce the risk of tumor recurrence and metastasis has become an important topic in the practice and research of anesthesia and perioperative management in cancer patients.
Recent studies have found that not all opioids share the same effects of immunosuppression, tumor proliferation promotion, vascular growth promotion and habituation induction.39 Novel opioids, represented by dezocine, with specific pharmacological mechanisms have initially demonstrated their potential for better analgesia, immune protection, antitumor proliferation, antitumor migration, vascular growth suppression and antidepressants in cancer.40
Dezocine is an amino-tetrahydronaphthalene derivative similar to opiates, such as morphine, in chemical structure and pharmacological mechanism, and it has the same analgesic effect as morphine.41 Dezocine is widely used in China for anesthesia and postoperative analgesia, including in cancer patients. The analgesic effect of dezocine is comparable to that of morphine but with less respiratory inhibition showing a ceiling effect, less malignant vomiting, less constipation and fewer cardiovascular adverse effects than morphine.40,42–45 There are few additive case reports related to dezocine, and it is not included in the list of controlled drugs in the World Health Organization or in China.40 Therefore, dezocine occupies more than 45% of the market share of opioid analgesics in China, showing its good application prospects.40,42 Recent studies have confirmed dezocine as a virtual opioid with less opioid-induced tolerance and hyperalgesia as well as with effects on immunomodulation, tumor growth, tumor proliferation and mental depression. Previous studies have found that dezocine is a partial μ opioid receptor agonist and later found that it interacts with the κ opioid receptor,44,45 but whether dezocine is an agonist or antagonist is controversial.42,44–48 In 2018, Chinese scholars reported that dezocine has similar efficacy and selectivity for the μ and κ opioid receptors and confirmed that dezocine is a partial μ and partial κ opioid receptor agonist.48 Later, Chinese scholars also found that dezocine binds to norepinephrine (NET) and serotonin (5-HT) transporters to inhibit the reuptake of NET and 5-HT.47,48
Role of Dezocine in Postoperative and Cancerous Pain in Cancer Patients
The role of dezocine in cancer pain was initially demonstrated in two pilot studies. Staquet et al performed two pilot studies on the analgesic effect in cancer patients, in which a double-blind cross-comparison of intramuscular injections of 10 mg dezocine with 10 mg morphine and placebo in ten cancer pain patients showed that a single dose of dezocine is superior to placebo in relieving moderate to severe pain with no side effects.49 A double-blind, placebo-controlled cross-trial was later performed in 20 inpatients with persistent cancer pain to assess the analgesic efficacy and tolerance of a single intramuscular injection of 10 mg dezocine, and the results indicated that dezocine produces a statistically significant greater and sustained pain relief than placebo, reporting only minor side effects.50
Later, in the 1990s, dezocine was introduced in China for many clinical applications. A meta-analysis by Wang Lei et al showed that there is no significant difference between the analgesic efficacy of dezocine and morphine in persistent pain in Chinese cancer patients; however, dezocine injections cause a 56% lower incidence of adverse drug reactions than morphine injections.42
Li et al compared the postoperative analgesic effect of dezocine and oxycodone in patients with radical cervical cancer surgery and found that the analgesic and sedative effects of dezocine are less than those of oxycodone, but the adverse effects of the two opioids are the same.51 However, Wang et al found that the analgesic effect of 2.5 mg/kg dezocine is slightly less than that of 2.5 μg/kg sufentanil for 2 days of postoperative analgesia in breast cancer patients undergoing surgery.52
Stambaugh et al compared the effect of single-dose and multidose intramuscular injection of dezocine (10 mg) with butorphanol (2 mg) for chronic cancerous pain, and they found a similar peak in analgesic efficacy but a longer duration of dezocine than butorphanol after the initial dose, indicating that dezocine is superior to butorphanol in terms of action duration after multiple administrations as well as that dezocine has less toxicity than butorphanol both after single and repeated administration. These results further suggested that dezocine may be beneficial in the treatment of chronic cancer pain.53
Interestingly, Wu et al found that the combination of low-dose dezocine with morphine prevents or delays the development of morphine tolerance in a rat model of bone cancer pain without reducing the analgesic effect of morphine.54 These results suggest the possibility of the combined use of dezocine with morphine in patients with severe cancer pain.
Effects of Dezocine on Angiogenesis in Cancer
Gupta et al found that morphine promotes angiogenesis, enhances VEGF expression and promotes breast tumor growth in vitro in 2002,55 and they found that fentanyl (MOP agonist) has VEGF-like effects in angiogenesis in 2015.56 However, later studies found that opioids may inhibit angiogenesis in opposite ways, which may be explained by their direct toxic effects at high doses, and more scholars have suggested that opioids promote angiogenesis at low doses and clinical doses.57
Feng et al found that morphine, oxycodone and fentanyl all increase endothelial tube formation and proliferation in vitro, suggesting that opioids act on endothelial cells via a non-μ opioid receptor-dependent pathway.58 However, Singleton et al found that methylnaltrexone, a peripheral μ receptor blocker, inhibits angiogenesis induced by opioid drugs, such as morphine, morphine-6-glucuronic acid and DAMGO, as well as encephalopathy and VEGF.59 In addition, Yamamizu et al found that a κ opioid receptor agonist suppresses tumor angiogenesis through inhibition of VEGF signaling in a series of studies.60 Thus, these studies suggest that κ opioid receptor agonists inhibit angiogenesis in cancer, but the effects of the μ opioid receptor on angiogenesis are controversial.
The research on the effect of dezocine on tumor angiogenesis has not been reported to date. However, we hypothesized that dezocine may have antiangiogenic effects based on its characteristics of partial κ opioid receptor agonism.
Effects of Dezocine on Tumor Immune Function
Feng et al found that dezocine regulate immune function, in which dezocine upregulates IL-12 levels and downregulates IL-10 levels by promoting lymphocyte activity during maturation of DCs in human cord blood.61 Feng et al also found that dezocine significantly promotes the morphological maturation of dendritic cells (DCs) and increases the expression of DC-related surface markers, such as CXCL10, CD3G and GRB2, resulting in increased IL-12 and IL-6 cytokines as well as the proliferation and cytotoxicity of CD8+ T cells in postoperative peripheral blood of patients with lung cancer.62 Another study has reported that dezocine promotes BMDC maturation and inhibits tumor metastasis by increasing CD8+ T cell proliferation and cytotoxicity.63 Clinical studies have confirmed that the analgesic efficacy of dezocine was slightly less effective than that of sufentanil, but the activities of NK cells and CD4+ cells are significantly higher than those of sufentanil within 48 h postoperatively in breast cancer patients undergoing surgery, which is more conducive to the recovery of early postoperative immune function in dezocine-treated patients.52 Studies have also found that dezocine maintains the balance of serum Th1/Th2 cytokines (INF-γ/IL-4) better than sufentanil in patients with gastric cancer surgery64 and downregulates the expression of the TNF-α and IL-6 inflammatory factors in patients with liver cancer surgery.65 Our studies also found that dezocine inhibits CRP levels66 and maintains the CD3+/CD8+ balance, the IL-2/IL-10 cytokine balance67,68 and the Th1/Th2 cell balance69 in breast cancer patients undergoing surgery as compared to fentanyl or sufentanil. Interestingly, Dong et al found that the production of IFN-γ and PD-L1 decreases after treatment with 2 µg/mL dezocine but increases after treatment with 8 µg/mL dezocine in lung cancer cells,70 suggesting that clinical doses of dezocine have immune upregulation effects but that large doses lead to immunosuppression, which is consistent with our previous findings.67
The above findings suggest that dezocine protects the immune function of cancer patients with potential antitumor effects.
Effects of Dezocine on Tumor Proliferation and Growth
Dezocine inhibits tumor growth and metastasis by regulating immune function.61–63,70 However, in vitro studies have found that dezocine directly inhibits the viability and migration of HepG2 and Hep 3B HCC cells in a concentration-dependent manner by inhibiting cellular oxyglycolysis and its malignant potential through the serine/threonine kinase 1 (Akt1)-glycogen synthase kinase 3 (GSK-3) pathway,71 and they have found that dezocine exerts an antitumor effect by targeting nicotinamide phosphoribosyltransferase in triple-negative breast cancer.72 Thus, these findings suggest that dezocine has direct antitumor effects in vitro. However, the mechanism and significance are still unknown.
Effects of Dezocine on Tumor Patients with Depression
Because dezocine has inhibitory effects on 5-HT and NOR reuptake, dezocine may have an antidepressant effect.40,46 We first reported that a single epidural small dose of morphine combined with intravenous patient controlled dezocine analgesia is effective in reducing the postoperative Edinburgh depression scores and the incidence of postpartum depression in cesarean delivery pregnant women, and the antidepressant effect may be associated with its ability to increase blood 5-HT levels.73 We also found that dezocine for anesthesia induction and postoperative intravenous analgesia, reduces the postoperative depression self-score in breast cancer patients compared to fentanyl, and the depression self-score is negatively correlated with the blood 5-HT levels.74 Zhao et al also confirmed this finding in patients after colon cancer surgery.75 These results suggest that dezocine has an antidepressant effect both in cancer patients undergoing surgery and in pregnant women undergoing cesarean section. An experimental study has found that dezocine exerts antidepressant-like effects in mice through the involvement of 5-HT1A and κ opioid receptors.76
In summary, dezocine has been used for anesthesia and analgesia in China for more than ten years with billions of people using dezocine, and it has good application prospects. According to previous studies and due to its unique pharmacological role, dezocine has the potential to regulate the clinical biological characteristics of tumors, including postoperative pain, cancer pain, immune function, vascular growth, tumor proliferation, tumor metastasis and mental depression.
However, little is known about the effects of dezocine on cancer. Research on the immune regulation of dezocine is not comprehensive, and there has been no reported study on the angiogenesis effects of dezocine. Studies of the effects of dezocine on tumor proliferation and metastasis as well as mental depression in tumor patients have just begun. The mechanism by which dezocine regulates the clinical biological characteristics of tumors is not completely clear. More research, especially clinical research on tumor prognosis, is needed to confirm the value of dezocine in cancer patients.
Funding
This work was supported by the Fund of Science and Technology Project of Guangdong Province of China (NO.2012A030400042).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019;9(4):217–222. PMID: 31854162; PMCID: PMC7310786. doi:10.2991/jegh.k.191008.001
2. Wu C, Li M, Meng H, et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62(5):640–647. PMID: 30900169. doi:10.1007/s11427-018-9461-5
3. Branter J, Basu S, Smith S. Tumour treating fields in a combinational therapeutic approach. Oncotarget. 2018;9(93):36631–36644. PMID: 30564303; PMCID: PMC6290966. doi:10.18632/oncotarget.26344
4. Dubowitz JA, Sloan EK, Riedel BJ. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin Exp Metastasis. 2018;35(4):347–358. PMID: 28894976. doi:10.1007/s10585-017-9862-x
5. Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. PMID: 29695476. doi:10.1136/bmj.k1415
6. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin North Am. 2017;101(6):1099–1113. PMID: 28992857; PMCID: PMC5915316. doi:10.1016/j.mcna.2017.06.005
7. Bortolato B, Hyphantis TN, Valpione S, et al. Depression in cancer: the many biobehavioral pathways driving tumor progression. Cancer Treat Rev. 2017;52:58–70. PMID: 27894012. doi:10.1016/j.ctrv.2016.11.004
8. Portenoy RK, Ahmed E. Cancer pain syndromes. Hematol Oncol Clin North Am. 2018;32(3):371–386. PMID: 29729775. doi:10.1016/j.hoc.2018.01.002
9. Zajączkowska R, Kocot-Kępska M, Leppert W, Wordliczek J. Bone pain in cancer patients: mechanisms and current treatment. Int J Mol Sci. 2019;20(23):6047. PMID: 31801267; PMCID: PMC6928918. doi:10.3390/ijms20236047
10. Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37(37):705–713. doi:10.1200/EDBK_180469 PMID: 28561731.
11. Cata JP, Corrales G, Speer B, Owusu-Agyemang P. Postoperative acute pain challenges in patients with cancer. Best Pract Res Clin Anaesthesiol. 2019;33(3):361–371. PMID: 31785721. doi:10.1016/j.bpa.2019.07.018
12. Market M, Tennakoon G, Auer RC. Postoperative natural killer cell dysfunction: the prime suspect in the case of metastasis following curative cancer surgery. Int J Mol Sci. 2021;22(21):11378. PMID: 34768810; PMCID: PMC8583911. doi:10.3390/ijms222111378
13. Peng YP, Qiu YH. [Surgical stress and immunosuppression]. Sheng Li Ke Xue Jin Zhan. 2006;37(1):31–36. Chinese. PMID: 16683542.
14. Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol. 2019;19(7):433–447. PMID: 30874629; PMCID: PMC6700742. doi:10.1038/s41577-019-0147-2
15. Ruella M, Kalos M. Adoptive immunotherapy for cancer. Immunol Rev. 2014;257(1):14–38. doi:10.1111/imr.12136
16. Kruger S, Ilmer M, Kobold S, et al. Advances in cancer immunotherapy 2019 - latest trends. J Exp Clin Cancer Res. 2019;38(1):268. PMID: 31217020; PMCID: PMC6585101. doi:10.1186/s13046-019-1266-0
17. Pandya NM, Dhalla NS, Santani DD. Angiogenesis–a new target for future therapy. Vascul Pharmacol. 2006;44(5):265–274. PMID: 16545987. doi:10.1016/j.vph.2006.01.005
18. Kong DH, Kim MR, Jang JH, Na HJ, Lee S. A review of anti-angiogenic targets for monoclonal antibody cancer therapy. Int J Mol Sci. 2017;18(8):1786. PMID: 28817103; PMCID: PMC5578174. doi:10.3390/ijms18081786
19. Ribatti D. Tumor refractoriness to anti-VEGF therapy. Oncotarget. 2016;7(29):46668–46677. PMID: 27081695; PMCID: PMC5216828. doi:10.18632/oncotarget.8694
20. Cardano M, Tribioli C, Prosperi E. Targeting Proliferating Cell Nuclear Antigen (PCNA) as an effective strategy to inhibit tumor cell proliferation. Curr Cancer Drug Targets. 2020;20(4):240–252. PMID: 31951183. doi:10.2174/1568009620666200115162814
21. Qin SY, Zhang AQ, Zhang XZ. Recent advances in targeted tumor chemotherapy based on smart nanomedicines. Small. 2018;14(45):e1802417. PMID: 30247806. doi:10.1002/smll.201802417
22. Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193–199. PMID: 22408567; PMCID: PMC3298009. doi:10.7150/ijms.3635
23. Seifert O, Baerwald C. Interaction of pain and chronic inflammation. Z Rheumatol. 2021;80(3):205–213. English. PMID: 33373022. doi:10.1007/s00393-020-00951-8
24. Michaelides A, Zis P. Depression, anxiety and acute pain: links and management challenges. Postgrad Med. 2019;131(7):438–444. PMID: 31482756. doi:10.1080/00325481.2019.1663705
25. Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. 2019;25(18):5449–5457. PMID: 30944124. doi:10.1158/1078-0432.CCR-18-1543
26. Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107(2):234–256. PMID: 32553197; PMCID: PMC7381373. doi:10.1016/j.neuron.2020.06.002
27. Kosciuczuk U, Knapp P, Lotowska-Cwiklewska AM. Opioid-induced immunosuppression and carcinogenesis promotion theories create the newest trend in acute and chronic pain pharmacotherapy. Clinics. 2020;75:e1554. PMID: 32215455; PMCID: PMC7074583. doi:10.6061/clinics/2020/e1554
28. Wang DY, Johnson DB, Davis EJ. Toxicities associated with PD-1/PD-L1 blockade. Cancer J. 2018;24(1):36–40. PMID: 29360726; PMCID: PMC5784852. doi:10.1097/PPO.0000000000000296
29. Zhang Y, La B, Liang B, Gu Y. Treatment-related adverse events with PD-1 or PD-L1 inhibitors: a systematic review and meta-analysis. Life. 2021;11(11):1277. PMID: 34833153; PMCID: PMC8618590. doi:10.3390/life11111277
30. Lavergne F, Jay TM. Antidepressants Promote and Prevent Cancers. Cancer Invest. 2020;38(10):572–598. PMID: 32866046. doi:10.1080/07357907.2020.1817481
31. Clark-Raymond A, Halaris A. VEGF and depression: a comprehensive assessment of clinical data. J Psychiatr Res. 2013;47(8):1080–1087. PMID: 23684549. doi:10.1016/j.jpsychires.2013.04.008
32. Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis. 2017;20(2):185–204. PMID: 28361267; PMCID: PMC5439974. doi:10.1007/s10456-017-9552-y
33. German AI. Multi- target-tumortherapie [Multi-target tumor therapy]. Krankenpfl J. 2005;43(1–3):38–39. German. PMID: 15912831.
34. Benedetti R, Conte M, Iside C, Altucci L. Epigenetic-based therapy: from single- to multi-target approaches. Int J Biochem Cell Biol. 2015;69:121–131. PMID: 26494003. doi:10.1016/j.biocel.2015.10.016
35. Bhatia K, Bhumika B, Das A. Combinatorial drug therapy in cancer - New insights. Life Sci. 2020;258:118134. PMID: 32717272. doi:10.1016/j.lfs.2020.118134
36. Amaram-Davila J, Davis M, Reddy A. Opioids and cancer mortality. Curr Treat Options Oncol. 2020;21(3):22. PMID: 32095929. doi:10.1007/s11864-020-0713-7
37. Tregubenko P, Zvonarev V. Impact of opioid use in hematological malignancies: clinical, immunological and concomitant aspects. J Hematol. 2020;9(3):41–54. PMID: 32855752; PMCID: PMC7430860. doi:10.14740/jh689
38. Moyano J, Aguirre L. Opioids in the immune system: from experimental studies to clinical practice. Rev Assoc Med Bras. 2019;65(2):262–269. PMID: 30892453. doi:10.1590/1806-9282.65.2.262
39. Franchi S, Moschetti G, Amodeo G, Sacerdote P. Do all opioid drugs share the same immunomodulatory properties? A review from animal and human studies. Front Immunol. 2019;10:2914. PMID: 31921173; PMCID: PMC6920107. doi:10.3389/fimmu.2019.02914
40. Childers WE, Abou-Gharbia MA. “I’ll Be Back”: the resurrection of dezocine. ACS Med Chem Lett. 2021;12(6):961–968. PMID: 34141081; PMCID: PMC8201756. doi:10.1021/acsmedchemlett.1c00233
41. Rowlingson JC, Moscicki JC, DiFazio CA. Anesthetic potency of dezocine and its interaction with morphine in rats. Anesth Analg. 1983;62(10):899–902. doi:10.1213/00000539-198310000-00008
42. Wang L, Liu X, Wang J, Sun Y, Zhang G, Liang L. Comparison of the efficacy and safety between dezocine injection and morphine injection for persistence of pain in Chinese cancer patients: a meta-analysis. Biosci Rep. 2017;37(3):BSR20170243. doi:10.1042/BSR20170243
43. Romagnoli A, Keats AS. Ceiling respiratory depression by dezocine. Clin Pharmacol Ther. 1984;35(3):367–373. PMID: 6421529. doi:10.1038/clpt.1984.45
44. Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB. The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sci. 1993;52(4):389–396. doi:10.1016/0024-3205(93)90152-s
45. O’Brien JJ, Benfield P. Dezocine. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1989;38(2):226–248. doi:10.2165/00003495-198938020-00005
46. Liu R, Huang XP, Yeliseev A, Xi J, Roth BL. Novel molecular targets of dezocine and their clinical implications. Anesthesiology. 2014;120(3):714–723. doi:10.1097/ALN.0000000000000076
47. Wang YX, Mao XF, Li TF, Gong N, Zhang MZ. Dezocine exhibits antihypersensitivity activities in neuropathy through spinal μ-opioid receptor activation and norepinephrine reuptake inhibition. Sci Rep. 2017;7(1):43137. doi:10.1038/srep43137
48. Wang YH, Chai JR, Xu XJ, et al. Pharmacological characterization of dezocine, a potent analgesic acting as a κ partial agonist and μ partial agonist. Sci Rep. 2018;8(1):14087. doi:10.1038/s41598-018-32568-y
49. Staquet M. A double-blind study of dezocine in cancer pain. J Clin Pharmacol. 1979;19(7):392–394. PMID: 479384. doi:10.1002/j.1552-4604.1979.tb02497.x
50. Staquet M. Effect of parenteral dezocine and placebo in cancer pain. Curr Med Res Opin. 1980;6(9):634–637. PMID: 6996934. doi:10.1185/03007998009109501
51. Li Z, Wu H, He R, Hu X, Liu S. Oxycodone versus dezocine for postoperative analgesia in patients with cervical cancer treated with radical surgery. J Cancer Res Ther. 2016;12(5):27–29. doi:10.4103/0973-1482.191624 PMID: 27721247.
52. Wang F, Zhang X, Wang H, Liu Y. Effects of dezocine and sufentanyl for postoperative analgesia on activity of NK, CD4+ and CD8+ cells in patients with breast cancer. Oncol Lett. 2019;17(3):3392–3398. PMID: 30867775; PMCID: PMC6396157. doi:10.3892/ol.2019.9964
53. Stambaugh JE Jr, McAdams J. Comparison of intramuscular dezocine with butorphanol and placebo in chronic cancer pain: a method to evaluate analgesia after both single and repeated doses. Clin Pharmacol Ther. 1987;42(2):210–219. PMID: 3301154. doi:10.1038/clpt.1987.134
54. Wu LX, Dong YP, Zhu QM, et al. Effects of dezocine on morphine tolerance and opioid receptor expression in a rat model of bone cancer pain. BMC Cancer. 2021;21(1):1128. PMID: 34670518; PMCID: PMC8529774. doi:10.1186/s12885-021-08850-0
55. Gupta K, Kshirsagar S, Chang L, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62(15):4491–4498.
56. Gupta M, Poonawala T, Farooqui M, Ericson ME, Gupta K. Topical fentanyl stimulates healing of ischemic wounds in diabetic rats. J Diabetes. 2015;7:21.
57. Mahbuba W, Lambert DG. Opioids and neovascularization; pro or anti? Br J Anaesth. 2015;115(6):821–824. PMID: 26537630. doi:10.1093/bja/aev357
58. Feng T, Zeng S, Ding J, et al. Comparative analysis of the effects of opioids in angiogenesis. BMC Anesthesiol. 2021;21(1):257. PMID: 34702181; PMCID: PMC8549314. doi:10.1186/s12871-021-01475-7
59. Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvasc Res. 2006;72(1–2):3–11. PMID: 16820176. doi:10.1016/j.mvr.2006.04.004
60. Yamamizu K, Furuta S, Hamada Y, et al. к Opioids inhibit tumor angiogenesis by suppressing VEGF signaling. Sci Rep. 2013;3(1):3213. doi:10.1038/srep03213 PMID: 24225480; PMCID: PMC3827603.
61. Feng C, Feng M, Jiao R, et al. Effect of dezocine on IL-12 and IL-10 secretion and lymphocyte activation by culturing dendritic cells from human umbilical cord blood. Eur J Pharmacol. 2017;796:110–114. PMID: 28017828. doi:10.1016/j.ejphar.2016.12.035
62. Feng M, Jiang Y, Liu D, Cheng G, Zhang W, Feng C. RNA-seq analysis of peripheral blood dendritic cells maturated by dezocine in patients with lung cancer. Mol Immunol. 2022;143:85–93. PMID: 35091230. doi:10.1016/j.molimm.2022.01.003
63. Song Q, Liu G, Liu D, Feng C. Dezocine promotes T lymphocyte activation and inhibits tumor metastasis after surgery in a mouse model. Invest New Drugs. 2020;38(5):1342–1349. PMID: 32170576. doi:10.1007/s10637-020-00921-6
64. Feng M, Feng Q, Chen Y, et al. Effect of dezocine on the ratio of Th1/Th2 cytokines in patients receiving postoperative analgesia following laparoscopic radical gastrectomy: a prospective randomised study. Drug Des Devel Ther. 2021;15:2289–2297. PMID: 34079227; PMCID: PMC8166330. doi:10.2147/DDDT.S306120
65. Jia Q, Tian F, Duan WN, Jia YF, Wang HX, Xia ZY. Effects of dezocine-remifentanil intravenous anaesthesia on perioperative signs, serum TNF-αand IL-6 in liver cancer patients undergoing radiofrequency ablation. J Coll Physicians Surg Pak. 2019;29(1):4–7. PMID: 30630559. doi:10.29271/jcpsp.2019.01.4
66. Hu XD, Zhang LG, Ling C, et al. Effect of dezocine or fentanyl applied in full perioperative period on postoperative pain and C response proteins in breast cancer patients undergoing radical section. Chin Physician J. 2013;17(z1):1–4.
67. Ling C, Zou L, Deng AH, et al. Effects of different doses of dezocine on postoperative cellular immune function and cytokines in breast cancer patients undergoing radical section. J Pract Med. 2015;23:3944–3946.
68. Ling C, Hu XD, Zhang LG, et al. Effects of different analgesic methods on cellular immune function and inflammatory mediators in breast cancer patients undergoing radical section. Guangdong Med. 2015;04:551–553.
69. Hu X, Luo B, Wu Q, et al. Effects of dezocine and sufentanil on Th1/Th2 Balance in breast cancer patients undergoing surgery. Drug Des Devel Ther. 2021;15:4925–4938. PMID: 34880602; PMCID: PMC8648097. doi:10.2147/DDDT.S326891
70. Dong W, Zhang D, Zhu A, Hu Y, Li W. High concentration of dezocine induces immune escape of lung cancer and promotes glucose metabolism through up-regulating PD-L1 and activating NF-κB pathway. Curr Mol Med. 2021;22. PMID: 34951362. doi:10.2174/1566524022666211222155118
71. Zhong ZW, Zhou WC, Sun XF, Wu QC, Chen WK, Miao CH. Dezocine regulates the malignant potential and aerobic glycolysis of liver cancer targeting Akt1/GSK-3β pathway. Ann Transl Med. 2020;8(7):480. PMID: 32395524; PMCID: PMC7210161. doi:10.21037/atm.2020.03.28
72. Xue C, Chen W, Yuan A, et al. Dezocine, an opioid analgesic, exerts antitumor effects in triple-negative breast cancer by targeting nicotinamide phosphoribosyltransferase. Front Pharmacol. 2021;12:600296. PMID: 33912035; PMCID: PMC8072669. doi:10.3389/fphar.2021.600296
73. Liu XQ, Ling C, Mai QL, et al. Effects of a single injection of morphine in the epidural cavity combined with dezocine intravenous analgesia on postpartum depression and serum serotonin levels in puerperant undergoing cesarean section. Hum Med. 2018;29(9):1224–1227.
74. Ling C, Hu XD, Zhang W, Liu XQ, Deng AH, Zhou SX. Preliminary study of the effects of preoperative depression status on the intravenous analgesic efficacy of dezocine and serum serotonin concentrations in breast cancer patients. Chin J Physicians. 2017;07:
75. Zhao P, Wu Z, Li C, et al. Postoperative analgesia using dezocine alleviates depressive symptoms after colorectal cancer surgery: a randomized, controlled, double-blind trial. PLoS One. 2020;15(5):e0233412. doi:10.1371/journal.pone.0233412
76. Shang L, Duan C, Chang S, Chang N, Jia S. Antidepressant-like effects of dezocine in mice: involvement of 5-HT1A and κ opioid receptors. Behav Pharmacol. 2021;32(6):472–478. doi:10.1097/FBP.0000000000000641
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.