Back to Journals » Drug Design, Development and Therapy » Volume 12
Dexmedetomidine protects the developing rat brain against the neurotoxicity wrought by sevoflurane: role of autophagy and Drp1–Bax signaling
Authors Shan Y , Sun S, Yang F, Shang N, Liu H
Received 18 July 2018
Accepted for publication 14 August 2018
Published 30 October 2018 Volume 2018:12 Pages 3617—3624
DOI https://doi.org/10.2147/DDDT.S180343
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Yangyang Shan,1 Shiwei Sun,1 Fan Yang,1 Nan Shang,2 Hongtao Liu1
1Department of Anesthesiology, Shengjing Hospital, China Medical University, Shenyang, 110004, China; 2Department of Respiration, No. 202 Hospital of PLA, Shenyang, 110003, China
Background: The effect of sevoflurane on the nervous system is controversial. As an adjuvant anesthetic, dexmedetomidine has a protective role in various nerve-injury diseases. We investigated the effect of dexmedetomidine on injury to the developing brain induced by sevoflurane anesthesia, and if autophagy and mitochondrial damage are involved in the neuroprotective effects of dexmedetomidine.
Methods: Pregnant rats on gestational day 20 were exposed to 3% sevoflurane for 4 hours. Saline and dexmedetomidine were injected intraperitoneally 15 minutes before exposure to sevoflurane or control gas. Bilateral hippocampi were harvested on postnatal day 1. Hippocampal morphology was observed by Nissl staining and expression of the microtubule-related protein LC3I/II, p62, Drp1, Bax, and Bcl2 were evaluated by Western blotting and immunohistochemistry.
Results: Nissl staining showed that sevoflurane anesthesia during the third trimester caused neuronal damage to the hippocampi of rat pups. Western blotting and immunohistochemistry showed that pregnant rats exposed to sevoflurane during the third trimester led to pups having increased expression of LC3 and p62, suggesting that sevoflurane blocked autophagic flow in the hippocampus. Expression of Drp1 and Bax was increased after sevoflurane exposure, whereas Bcl2 expression was downregulated. All these effects were alleviated by pretreatment with dexmedetomidine.
Conclusion: Sevoflurane exposure during the third trimester caused neurological injury to rat pups. Autophagy and abnormalities in mitochondrial dynamics were involved in this neurotoxic process and were antagonized by dexmedetomidine.
Keywords: dexmedetomidine, sevoflurane, autophagy, Drp1, developing brain
Introduction
In recent years, use of corrective surgery on the fetus during pregnancy has increased gradually, and general anesthesia is necessary for the pregnant women involved. The inhalation anesthetic sevoflurane is used widely for the induction of general anesthesia. Recent studies have shown that sevoflurane can increase the apoptosis of neurons to varying degrees, and even reduce the long-term ability of rats to learn and remember.1 However, few studies have investigated if sevoflurane anesthesia during late pregnancy can elicit neurotoxicity in offspring.
Clinical application of dexmedetomidine has been increasing. Studies have shown that dexmedetomidine can alleviate the damage induced by anesthetics on 7-day-old rats,2,3 but the specific mechanism is not known. Autophagy is type II programmed death, which differs from apoptosis in eukaryotic cells. Deficiency and excess autophagy can induce varying degrees of cell damage. Levels of microtubule-associated LC3 are increased after autophagy activation, and LC3II/I is often used to assess the level of autophagy.4 Levels of p62, which responds to autophagy degradation, are reduced accordingly if the degradation caused by autophagy increases.5
Mitochondria have vital roles in the metabolic functions and survival of cells, because they regulate energy metabolism and calcium storage. Furthermore, these highly dynamic organelles are involved in apoptosis and signal transduction through continuous fission and fusion.6 Mitochondrial fission is mediated by Drp1, which is critical in acute and chronic neurological diseases associated with glutamate toxicity and oxidative stress.7 It has been reported that the membrane remodeling induced by Drp1 stimulates Bax oligomerization.8 However, whether autophagy is involved in the nerve damage induced by anesthetics is largely unknown. We wished to explore the neuroprotective effect of dexmedetomidine against sevoflurane on the developing brain, and whether the neuroprotective mechanism is related to mitochondrial fission and autophagy.
Methods
Instruments and chemicals
An anesthesia machine (Aestiva 5) and gas monitor (F-MCI) were purchased from GE Healthcare UK (Little Chalfont, UK). A digital eclipse modular confocal microscope (C1) was obtained from Nikon (Tokyo, Japan). A BCA protein-assay kit was purchased from Bio-Rad Laboratories (Hercules, CA, USA).
GAPDH monoclonal antibody and Bax monoclonal antibody were obtained from Cell Signaling Technology (Danvers, MA, USA). LC3 monoclonal antibody and p62 monoclonal antibody were from Abcam (Cambridge, UK). Drp1 monoclonal antibody was purchased from Wanleibio (Beijing, China). Bcl2 monoclonal antibody was obtained from R&D Systems (Minneapolis, MN, USA). Nissl staining kits (cresyl violet method) were from Solarbio (Beijing, China).
Animal preparation and grouping
Formal approval to conduct animal experiments was granted by The Laboratory Animal Care Committee of China Medical University (Shenyang, China; no. 2017PS019K). Feeding conditions of animals conformed with the relevant regulations of the National Animal Experiment Center. Sprague Dawley rats (220–260 g) were purchased from the Animal Experimental Center of China Medical University. Female rats were allowed to mate with male rats at a proportion of 3:1. The next morning, female rats found to have sperm cells in vaginal secretions under microscopic observation were removed for separate feeding and recorded as “E0”.
Twenty-day-pregnant rats (E20) were divided randomly into three groups: control (C), sevoflurane anesthesia (S), and sevoflurane anesthesia and dexmedetomidine (D). Pregnant rats from group S were anesthetized with 3% sevoflurane in 33%–35% oxygen for 4 hours, whereas rats in group C received 33%–35% oxygen without sevoflurane. In group D, dexmedetomidine (20 μg/kg body weight) was administered intraperitoneally to pregnant rats 15 minutes before exposure to sevoflurane and was reinjected every 2 hours. All anesthetic procedures were undertaken in a customized “anesthetic box” and monitored through the gas outlet using a gas monitor.
After anesthesia, pregnant rats continued to be fed until spontaneous labor. Postnatal day 1 (P1) rat pups were anesthetized with 10% pentobarbital intraperitoneally and killed. Bilateral hippocampi were removed, placed on ice, and transferred rapidly to a −80°C refrigerator. In addition, 4% paraformaldehyde (PFA) was injected into the left ventricle of rat pups, and then the whole -brain tissue was harvested and soaked in 4% PFA, fixed for 24–48 hour at 4°C, and used to make paraffin sections.
Anesthesia
Pregnant rats were placed in the self-made transparent glass anesthesia box, a rectangular box made of clear glass of length 50 cm, width 30 cm, and height 30 cm. The anesthesia box was divided into two parts with a sieve-like partition, in which the left area contained soda lime (to absorb carbon dioxide) and the right area was used to place pregnant rats. Four holes were drilled in the side walls of the anesthesia box to act as ports for the mixed-gas inlet, the gas outlet, an interface to measure concentrations of sevoflurane and gas, and monitoring of oxygen saturation.
Sevoflurane was volatilized into the anesthesia box. Once the sevoflurane concentration had reached 3%, rats were placed in the anesthesia box. Oxygen concentration was maintained at 33%±2% and sevoflurane concentration maintained at 3% for 4 hours. After anesthesia, the gas supply was stopped. Rats were removed and placed in an air environment for natural revival, and continued feeding until spontaneous delivery. Offspring were weaned on P1.
Western blotting
Isolated hippocampi were added to lysates (radioimmunoprecipitation-assay buffer:phenylmethanesulfonyl fluoride 100:1) to extract total proteins. Hippocampal tissue was cut, lysed on ice for 30 minutes, and centrifuged at 14,000 g for 30 minutes at 4°C. The supernatant was extracted to evaluate protein concentrations using the BCA kit. Proteins (40 μg) from each sample were denatured for detection of Western blots. According to the relative molecular weight of the target proteins, 12% sodium dodecyl sulfate–polyacrylamide gel was prepared and electrophoresis carried out. After electrophoresis, proteins were transferred to polyvinylidene difluoride (PVDF) membranes (0.45 μm) and blocked with 5% BSA (TBST 1× dilution) for 2 hours at room temperature. PVDF membranes were washed thrice with (TBST 1× dilution) for 10 minutes each time. PVDF membranes were incubated with GAPDH monoclonal antibody (1:5,000, G9545; Sigma-Aldrich, St Louis, MO, USA), LC3B antibody (1:1,000, 2775; Cell Signaling Technology), P62/SQSTM1 antibody (1:1,000, P0067; Sigma-Aldrich), Drp1 (1:500, WL03028; Wanleibio), Bcl2 antibody (1:500, MAB8272; R&D Systems) and Bax antibody (1:1,000, 2774s; Cell Signaling Technology) overnight at 4°C. The next day, PVDF membranes were incubated with horseradish peroxidase-labeled goat antirabbit secondary antibody (1:3,000 dilution) for 1.5 hours at room temperature. Protein bands were visualized using an enhanced chemiluminescence system (Thermo Fisher Scientific, Waltham, MA, USA). Semiquantification of target bands was done using ImageJ Pro (National Institutes of Health, Bethesda, MD, USA). Relative expression of LC3, p62, Drp1, Bax, and Bcl2 was normalized to that of GAPDH.
Immunohistochemical staining
Hippocampi fixed in 4% PFA were dehydrated with different concentrations of ethanol, infiltrated with xylene, embedded in paraffin, cooled, and cut into paraffin sections of thickness 2.5 μm in the coronal plane. Paraffin sections were deparaffinized, heated for 7 minutes in a microwave oven, and then allowed to cool to room temperature. Then, sections was incubated with hydrogen peroxide and serum for 30 minutes and washed for 15 minutes with PBS at room temperature. Sections were incubated overnight with anti-LC3 (1:50 dilution) and p62 (1:50) antibodies at 4°C. After rewarming at room temperature for 40 minutes, sections were washed thrice with PBS (5 minutes each time) and incubated with biotin-labeled secondary antibody and streptavidin-peroxidase for 25 minutes at room temperature. Slides were washed with PBS for 10 minutes and stained with hematoxylin after chromogenic staining with 3,3′-diaminobenzidine. Sections were dehydrated through a series of alcohol solutions and coverslipped with neutral resin. Sections were examined and photographed using digital microscopy (BA200; Motic Electric, Xiamen, China). The OD value of sections was measured using Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA).
Nissl staining
The prepared paraffin sections were deparaffinized and blotted with cresyl violet stain for 45 minutes at 37°C. After the reaction, sections were washed and differentiated under microscopy with Nissl differentiation solution. When the tissue was clear, sections were placed immediately in distilled water to stop the reaction. Then, sections were dehydrated through a series of alcohol solutions and coverslipped with neutral resin. Morphologic changes in neurons in hippocampal CA1 regions were observed under optical microscopy.
Statistical analyses
Data are mean ± SEM. Comparisons between groups were evaluated by one-way ANOVA followed by Tukey’s test using SPSS 17.0 (IBM, Armonk, NY, USA) and Prism 7.0 (GraphPad, San Diego, CA, USA). P<0.05 was considered significant.
Results
Dexmedetomidine alleviated sevoflurane-induced nerve injury in rat offspring
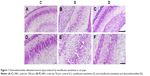
Nissl staining showed that compared with group C, neurons in group S and group D were damaged (Figure 1). In group S, the number of Nissl bodies decreased or Nissl bodies dissolved or disappeared. Decreased neuron numbers, neuronal edema, increase in cell gaps, and nuclear pyknosis were also detected. Neuron morphology in group D was obviously better than that in group S.
Dexmedetomidine inhibited autophagy activation in rat-pup brains induced by sevoflurane anesthesia
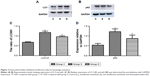
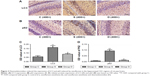
Western blotting showed that expression of LC3 II/I and p62 increased significantly in group S (P<0.05; Figure 2). Compared with group S, expression of LC3 II/I and p62 decreased significantly in group D (P<0.05). Immunohistochemical staining (Figure 3) showed that sevoflurane anesthesia activated autophagy in hippocampal CA1 regions of rat-pup brains. However, combination with dexmedetomidine reduced this effect. LC3 and p62 were located mainly in the cytoplasm of neurons in hippocampal CA1 regions. Compared with group C, expression of LC3 and p62 increased significantly in group S (P<0.01). Compared with group S, expression of LC3 and p62 decreased significantly in group D (P<0.05). These data were consistent with Western blotting results.
Dexmedetomidine attenuated disorders in mitochondrial dynamics in the hippocampi of rat pups induced by sevoflurane
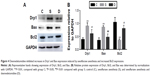
We measured Drp1 (which is associated with mitochondrial fission) expression. Sevoflurane anesthesia induced a significant increase in Drp1 expression, whereas dexmedetomidine inhibited this increase (P<0.01, P<0.05; Figure 4). We also measured expression of Bcl2 and Bax (which are necessary for Drp1-mediated mitochondrial fusion). Compared with group C, Bax expression increased significantly (P<0.01) in group S and Bcl2 expression was downregulated (P<0.01). Furthermore, combination with dexmedetomidine reversed changes in Bax and Bcl2 expression induced by sevoflurane significantly (P<0.01, P<0.05).
Discussion
Our study showed that pregnant rats who received sevoflurane anesthesia during the third trimester had activation of mitochondrial dynamics related to Drp1 and autophagy. Furthermore, dexmedetomidine improved the abnormal morphology of hippocampal CA1 regions of rat-pup brains and inhibited sevoflurane-induced activation of autophagy. Therefore, we propose that mitochondrial dynamics and autophagy may exert major influences on brain development in rat pups, which may be one of the mechanisms of neuroprotection proffered by dexmedetomidine against sevoflurane.
Autophagy is type II programmed death in eukaryotic cells that differs from apoptosis and enables cells to replenish energy and produce substrates for anabolic reactions during starvation. For autophagy to occur, large structures, such as protein aggregates, damaged organelles, and pathogens, must be degraded.9,10 Autophagy can be induced under hunger, ischemic, and hypoxic conditions, and then a bilayer membranous autophagosome is formed in the cytoplasm. The autophagic vesicles that emerge when proteins and organelles are wrapped in autophagosomes can migrate and bind to lysosomes and form “autophagy lysosomes” to digest the encapsulated material.11 Autophagy has a biphasic role in body development and homeostasis. As classic indicators of autophagy, LC3 levels are increased after autophagy activation, and LC3II/I is often used to assess the level of autophagy. p62 levels are reduced accordingly after the degradation wrought by autophagy.
Sevoflurane is the most commonly used general anesthetic for children, but its effects on development of the nervous system are controversial. Several scholars have reported that high-dose sevoflurane can increase neuronal apoptosis and even damage the ability to learn and remember in adult brains.12,13 Sevoflurane neurotoxicity has been studied mainly in postnatal day 7 rats. However, peak formation of synaptic network connections in rats starts from the third trimester of pregnancy. Neurons in the fetal brain continue to differentiate and migrate in the third trimester of pregnancy. During this period, the nervous system is very sensitive to various stimuli. Such factors as ischemia, hypoxia, and narcotic drugs can increase neuronal degeneration and apoptosis.14
We found that rats during the third trimester receiving 3% sevoflurane anesthesia for 4 hours gave birth to pups that had fewer neurons in the hippocampal CA1 region, and that these neurons were swollen and arranged in a nonuniform manner. Simultaneously, expression of LC3 II/I and p62 increased, which suggested that autophagy flow was blocked. This result is in accordance with data from other studies. Li et al demonstrated that sevoflurane anesthesia in the second trimester caused increased autophagy in offspring.15 As such, autophagy could have a vital role in the neurotoxicity of anesthetics in the developing brain. Autophagy can damage mitochondria, inhibit the mitochondrial apoptosis pathway, and reduce cell damage.16 Mitochondria are regarded as dynamic subcellular structures that undergo fission, fusion, biogenesis, and selective degradation constantly.17
As members of the GTPase family, Drp1, Fis1, and the fusion proteins Mfn2 and Opa1 are critical for maintaining the balance between fission and fusion to ensure normal functioning of mitochondria.6 Drp1 is related to the shape, distribution, and remodeling of mitochondria, as well as activation of neuronal damage and synaptic degeneration.18,19 Recently, Zhang et al demonstrated that Drp1 was associated with isoflurane-induced cognitive impairment in developing rats and mitochondrial division inhibitor (Mdivi1; a highly selective and classical antagonist of Drp1) ameliorated the neurotoxicity of isoflurane.20 Consistently with other studies, we demonstrated that Drp1 expression increased in the hippocampi of rat pups after sevoflurane anesthesia during the third trimester. Therefore, we propose that the dynamics of organelles associated with Drp1 are involved in sevoflurane-induced neurotoxicity in the early stages of neurodevelopment.
In general, it is thought that Bcl2 acts as an autophagy inhibitor by binding to beclin 1 and restricting its function, whereas Bax is necessary for autophagy activation.21,22 The reduction in expression of Bcl2 in beclin 1 complexes suggests that beclin 1 release from its inhibitor Bcl2 activates autophagy.23 Recently, Kinarivala et al reported that a combination of Bcl2 induction and autophagy activation is required for aromatic carbamates to engender neuroprotective effects.24 In addition, increasing evidence suggests that Bcl2-family proteins are involved in the stability of mitochondrial morphology.25,26 Bax can stimulate Drp1-dependent mitochondrial fragmentation by combining with Drp1 and Mfn2 at sites on the outer membrane of the mitochondria, which then develop into fission sites. Communication with these proteins stabilizes the association of Drp1 with the mitochondrial membrane and is a prerequisite for inducing organelle fragmentation. Damage due to mitochondrial dynamics related to Drp1 and autophagy had important roles in sevoflurane-induced neurotoxicity in our study, but we did not verify the exact relationship between mitochondrial dynamics and autophagy. We speculate that Bcl2 acts as a key molecule linking mitochondrial injury and autophagy.
We also discovered that dexmedetomidine application reduced the hippocampal damage induced by sevoflurane anesthesia in rat pups. Dexmedetomidine is used commonly as a sedative in patients receiving intensive care and in pediatric surgery. The neuroprotection proffered by dexmedetomidine has received widespread attention. Studies have demonstrated that dexmedetomidine has neuroprotective effects against impairment of learning memory caused by postoperative cognitive dysfunction, traumatic brain injury, or anesthesia.27,28 These neuroprotective mechanisms of dexmedetomidine involve inhibition of inflammatory responses and apoptosis.29,30 In recent years, it has been reported that dexmedetomidine elicits neuroprotective effects by regulating autophagy.
Dexmedetomidine protects mouse brains from ischemia–reperfusion injury by inhibiting neuronal autophagy through upregulation of expression of HIF1α.31 Dexmedetomidine can also exert neuroprotective effects in rats with traumatic brain injury by reducing autophagy.32 Li et al demonstrated that dexmedetomidine can ameliorate the neurotoxicity of anesthetic agents through restraining inflammation and apoptosis.33 We found that dexmedetomidine inhibited activation of LC3 and p62, suggesting that autophagy is involved in the neuroprotection proffered by it. Simultaneously, Drp1-related abnormalities in mitochondrial dynamics induced by sevoflurane anesthesia were inhibited significantly by dexmedetomidine, as manifested by inhibition of expression of Drp1 and Bax and activation of Bcl2 in our study. Demonstrating the exact relationship between autophagy and abnormalities in mitochondrial dynamics and related signaling pathways in the neuroprotection of dexmedetomidine will be the subject of our next study.
Conclusion
We demonstrated that sevoflurane exposure during the third trimester caused neurological injury to rat pups. Autophagy and abnormalities in mitochondrial dynamics were involved in this neurotoxic process, which was antagonized by dexmedetomidine.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81271370).
Disclosure
The authors report no conflicts of interest in this work.
References
Perez-Zoghbi JF, Zhu W, Grafe MR, Brambrink AM. Dexmedetomidine-mediated neuroprotection against sevoflurane-induced neurotoxicity extends to several brain regions in neonatal rats. Br J Anaesth. 2017;119(3):506–516. | ||
Haas DA. Alternative mandibular nerve block techniques: a review of the Gow-Gates and Akinosi-Vazirani closed-mouth mandibular nerve block techniques. J Am Dent Assoc. 2011;142 Suppl 3:8S–12S. | ||
Li Y, Zeng M, Chen W, et al. Dexmedetomidine reduces isoflurane-induced neuroapoptosis partly by preserving PI3K/Akt pathway in the hippocampus of neonatal rats. PLoS One. 2014;9(4):e93639. | ||
Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36(12):2491–2502. | ||
Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4(2):151–175. | ||
Benard G, Bellance N, James D, et al. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120(Pt 5):838–848. | ||
Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev. 2011;67(1–2):103–118. | ||
Montessuit S, Somasekharan SP, Terrones O, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142(6):889–901. | ||
He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. | ||
Ravikumar B, Sarkar S, Davies JE, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90(4):1383–1435. | ||
Abdollahzadeh I, Schwarten M, Gensch T, Willbold D, Weiergräber OH. The Atg8 Family of Proteins-Modulating Shape and Functionality of Autophagic Membranes. Front Genet. 2017;8:109. | ||
Chung W, Park S, Hong J, et al. Sevoflurane exposure during the neonatal period induces long-term memory impairment but not autism-like behaviors. Paediatr Anaesth. 2015;25(10):1033–1045. | ||
Xiao H, Liu B, Chen Y, Zhang J. Learning, memory and synaptic plasticity in hippocampus in rats exposed to sevoflurane. Int J Dev Neurosci. 2016;48:38–49. | ||
Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–338. | ||
Li X, Wu Z, Zhang Y, Xu Y, Han G, Zhao P. Activation of Autophagy Contributes to Sevoflurane-Induced Neurotoxicity in Fetal Rats. Front Mol Neurosci. 2017;10:432. | ||
Shirakabe A, Zhai P, Ikeda Y, et al. Response by Shirakabe et al to Letter Regarding Article, “Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure”. Circulation. 2016;134(6):e75–e76. | ||
Anne Stetler R, Leak RK, Gao Y, Chen J. The dynamics of the mitochondrial organelle as a potential therapeutic target. J Cereb Blood Flow Metab. 2013;33(1):22–32. | ||
Kim H, Perentis RJ, Caldwell GA, Caldwell KA. Gene-by-environment interactions that disrupt mitochondrial homeostasis cause neurodegeneration in C elegans Parkinson’s models. Cell Death Dis. 2018;9(5):555. | ||
Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 2011;20(13):2495–2509. | ||
Zhang C, Yuan XR, Li HY, et al. Downregualtion of dynamin-related protein 1 attenuates glutamate-induced excitotoxicity via regulating mitochondrial function in a calcium dependent manner in HT22 cells. Biochem Biophys Res Commun. 2014;443(1):138–143. | ||
Levine B, Sinha SC, Kroemer G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy. 2008;4(5):600–606. | ||
Lindqvist LM, Heinlein M, Huang DC, Vaux DL. Prosurvival Bcl-2 family members affect autophagy only indirectly, by inhibiting Bax and Bak. Proc Natl Acad Sci U S A. 2014;111(23):8512–8517. | ||
Chauhan S, Ahmed Z, Bradfute SB, et al. Pharmaceutical screen identifies novel target processes for activation of autophagy with a broad translational potential. Nat Commun. 2015;6:8620. | ||
Kinarivala N, Patel R, Boustany RM, Al-Ahmad A, Trippier PC. Discovery of Aromatic Carbamates that Confer Neuroprotective Activity by Enhancing Autophagy and Inducing the Anti-Apoptotic Protein B-Cell Lymphoma 2 (Bcl-2). J Med Chem. 2017;60(23):9739–9756. | ||
Clerc P, Ge SX, Hwang H, et al. Drp1 is dispensable for apoptotic cytochrome c release in primed MCF10A and fibroblast cells but affects Bcl-2 antagonist-induced respiratory changes. Br J Pharmacol. 2014;171(8):1988–1999. | ||
Kim S, Kim C, Park S. Mdivi-1 Protects Adult Rat Hippocampal Neural Stem Cells against Palmitate-Induced Oxidative Stress and Apoptosis. Int J Mol Sci. 2017;18(9):E1947. | ||
Liao Z, Cao D, Han X, et al. Both JNK and P38 MAPK pathways participate in the protection by dexmedetomidine against isoflurane-induced neuroapoptosis in the hippocampus of neonatal rats. Brain Res Bull. 2014;107:69–78. | ||
Wang Y, Wu C, Han B, et al. Dexmedetomidine attenuates repeated propofol exposure-induced hippocampal apoptosis, PI3K/Akt/Gsk-3β signaling disruption, and juvenile cognitive deficits in neonatal rats. Mol Med Rep. 2016;14(1):769–775. | ||
Pan W, Lin L, Zhang N, et al. Neuroprotective Effects of Dexmedetomidine Against Hypoxia-Induced Nervous System Injury are Related to Inhibition of NF-κB/COX-2 Pathways. Cell Mol Neurobiol. 2016;36(7):1179–1188. | ||
Wu GJ, Chen JT, Tsai HC, Chen TL, Liu SH, Chen RM. Protection of Dexmedetomidine Against Ischemia/Reperfusion-Induced Apoptotic Insults to Neuronal Cells Occurs Via an Intrinsic Mitochondria-Dependent Pathway. J Cell Biochem. 2017;118(9):2635–2644. | ||
Luo C, Ouyang MW, Fang YY, et al. Dexmedetomidine Protects Mouse Brain from Ischemia-Reperfusion Injury via Inhibiting Neuronal Autophagy through Up-Regulating HIF-1α. Front Cell Neurosci. 2017;11:197. | ||
Shen M, Wang S, Wen X, et al. Dexmedetomidine exerts neuroprotective effect via the activation of the PI3K/Akt/mTOR signaling pathway in rats with traumatic brain injury. Biomed Pharmacother. 2017;95:885–893. | ||
Li J, Xiong M, Nadavaluru PR, et al. Dexmedetomidine Attenuates Neurotoxicity Induced by Prenatal Propofol Exposure. J Neurosurg Anesthesiol. 2016;28(1):51–64. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.