Back to Journals » Drug Design, Development and Therapy » Volume 16
Dexmedetomidine Combined with Femoral Nerve Block Provides Effective Analgesia Similar to Femoral Nerve Combined with Sciatic Nerve Block in Patients Undergoing Total Knee Arthroplasty: A Randomized Controlled Study
Authors Xiao R , Liu LF, Luo YR , Liu C, Jin XB, Zhou W , Xu GH
Received 15 August 2021
Accepted for publication 13 December 2021
Published 13 January 2022 Volume 2022:16 Pages 155—164
DOI https://doi.org/10.2147/DDDT.S334415
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Georgios Panos
Rui Xiao,1,* Li-Fang Liu,1,* Ya-Ru Luo,1,* Chang Liu,1 Xiao-Bin Jin,1 Wei Zhou,1 Guang-Hong Xu1,2
1Department of Anesthesiology, First Affiliated Hospital, Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China; 2Department of Neurology, First Affiliated Hospital, Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guang-Hong Xu
Department of Anesthesiology, First Affiliated Hospital, Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China
Tel +86-551-62922344
Fax +86 551 62923704
Email [email protected]
Background: Total knee arthroplasty (TKA) is a severe traumatic procedure, and femoral nerve block (FNB) combined with a sciatic nerve block (SNB) is widely used in TKA. However, injury of the sciatic nerve is clinically reported. Dexmedetomidine (DEX) could reduce stress and inflammation, as well as improve pain in TKA. This study aims to observe the analgesic impact of DEX combined with FNB in TKA.
Methods: Eighty-eight patients undergoing TKA were included and randomly divided into two groups: DF group (FNB combined with DEX 0.6μg/kg before surgery, followed by DEX 0.2– 0.4μg/kg/h until articular closure) and SF group (FNB combined with SNB). Each nerve was blocked with 0.375% ropivacaine 20mL, and all patients received general anesthesia routinely. The primary endpoint was the pain visual analog scale (VAS) score during activities at postoperative 24 hours.
Results: There was no statistical difference in the pain VAS scores at any time point. The mean duration of analgesia for patients with rescue analgesic requests was comparable between the two groups: 25.4 ± 6.3 hours in the DF group vs 24.8 ± 6.4 hours in the SF group (two-sample t-test, p=0.738). The total dose of sufentanil was similar between groups (P=0.355). The maintenance dose of propofol and dose of rescue analgesics were comparable (all P> 0.05). There were no statistical differences in the incidence of adverse events. However, the time to extubate in the DF group was significantly longer than those in the SF group (P< 0.001).
Conclusion: DEX combined with FNB could provide effective analgesia similar to SNB combined with FNB in TKA.
Clinical Trial Registration: The trial was registered at the Chinese Clinical Trial Registry on November 17, 2019 (identifier: ChiCTR1900027552).
Keywords: analgesia, dexmedetomidine, nerve block, postoperative pain, total knee arthroplasty
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
With the emergence of aging and the demand for quality of life, the number of total knee arthroplasty (TKA) increased dramatically over the last decade.1 However, patients undergoing TKA often suffer moderate-to-severe postoperative pain,1,2 which in turn may impair mobility, slow rehabilitation,3 and reduce the quality of life. Therefore, optimal anesthesia and analgesic methods are vital for patients undergoing TKA.
Although neuraxial anesthesia was historically a popular analgesia approach for TKA, an increasing number of patients are willing to receive general anesthesia (GA) for nervousness, complications (ie, urinary retention requiring indwelling catheters and hypotension), limitations (ie, anticoagulation), or the possibility of puncture difficulty. An analysis of population-based administrative data including more than a quarter of a million TKA patients showed approximated only one-fifth of patients received a neuraxial anesthetic.4 With the popularity of ultrasound, the technique of peripheral nerve block (PNB), a less invasive, more convenient analgesic method than epidural anesthesia, is considered a feasible choice in major orthopedic surgery (such as hip or knee replacement).5,6 The femoral nerve combined with the sciatic nerve innervates most of the sensation in the lower limbs and their block is widely used in TKA. However, sciatic nerve block (SNB) has high technical requirements for operators, especially in obese and elderly patients for deep location. A case of permanent injury to the sciatic nerve after SNB has been reported,7 and whether the analgesic value of SNB outweighs concerns of concomitant motor and sensory loss of the lower leg is a perplexing question.8 In addition, the reported incidence of common peroneal nerve palsy after TKA ranged from 0.3% to 4%,9 SNB may disguise perioperative nerve injury of the lower leg and may cause a delay in early detection and treatment of surgically induced nerve injury.8 The sensation in the surgical area of TKA is mainly innervated by the femoral nerve. Therefore, we speculated whether femoral nerve block (FNB) alone combined with other analgesics could provide effective analgesia to an extent comparable to FNB combined SNB.
Recently in clinical practice, dexmedetomidine (DEX), a highly selective alpha-2 receptor agonist, is increasingly used for inducing sedation, analgesia, anxiolysis, and sympathetic tone inhibition.10 The analgesic efficacy of DEX has been stated explicitly in several studies.11–13 Specifically, Li et al14 suggested that DEX combined with general anesthesia provides similar intraoperative stress response reduction when compared with a combined general and epidural anesthetic technique in open gastrectomy. However, the relevant studies of DEX in TKA are limited and incomplete.
We performed the trial to determine the analgesic effect of DEX combined with FNB, and hypothesized that it could provide adequate pain similar to SNB combined with FNB. The primary outcome was the pain visual analog scale (VAS) score during activities at postoperative 24 hours, and the secondary outcomes were the total dose of sufentanil, the maintenance dose of propofol during anesthesia maintenance, postoperative pain VAS scores, postoperative remedial analgesia (time of first remedial analgesia and dose of remedial analgesics), and the incidence of adverse events.
Methods
This randomized controlled trial (RCT) was approved by the clinical medical research ethics committee of the first affiliated hospital of Anhui Medical University (PJ2019-16-08) and registered with the clinical trials registry on November 17, 2019 (identifier: ChiCTR1900027552). Patients were informed about the study during the preoperative screening visit and signed the informed consent once enrolled, and patients were recruited from December 2019 to September 2020.
Inclusion and Exclusion Criteria
Adults aged 50–80 years were recruited with the American Society of Anesthesiologists (ASA) physical status class II–III and had TKA the following day. All patients had normal communication and learning skills. Exclusion criteria included the following: TKA resulting from trauma; allergy to DEX or medicines used in the research; contraindications for PNB; severe sinus bradycardia (heart rate <50 bpm), sick sinus node syndrome, arrhythmias that affect hemodynamic stability, or history of heart failure; neurological or psychiatric diseases (ie, cerebral infarction associated with sequelae or dementia); failed preoperative screening of Montreal cognitive assessment (MoCA); a history of opioid use; or failed cooperation with researchers. Participants meeting the following conditions were removed from the study: serious complications (ie, cerebral infarction and heart failure) or sent to intensive care unit (ICU); or incomplete data.
Randomization and Blinding
Patients were randomized into one of the two treatment groups using a computer-generated randomization sequence. The random sequence was generated with sequentially numbered, opaque, and sealed envelopes by anesthesiologists. Before the performance of anesthesia, the specialist nurse opened a consecutively numbered envelope and prepared the drug solution. Anesthesiologists, surgeons, and other nurses were unaware of group assignment, while patients and researchers who assessed postoperative outcomes were unaware of that.
The interventions are as follows:
DF group: FNB with 20mL ropivacaine 0.375% and SNB with 20mL saline, and i.v. a loading dose of 0.6µg /kg DEX (which was diluted to 4 µg/mL; Jiangsu Pharmaceutical Co., Ltd, China) for 15 minutes before the skin, followed by 0.2–0.4µg/ (kg. h) maintenance to articular closure.
SF group: FNB and SNB with 20mL ropivacaine 0.375%, respectively, and i.v. the same volume of saline in the same way.
Anesthesia Protocol and Surgical Procedure
Once in the operating room, all patients were routinely monitored by electrocardiogram (ECG), pulse, pulse oxygen saturation (SpO2), and blood pressure (BP). Patients were also opened for peripheral venous access for the delivery of sodium lactate ringer solution 5mL/kg/h and received masked oxygen. A bispectral index monitor (BIS) VISTA Monitoring System (Aspect Medical Systems, Inc., USA) was also used to adjust the appropriate depth of anesthesia.
Before the operation of PNB, 5µg sufentanil was given intravenously to relieve anxiety and pain. All operations of PNB were performed with the aid of ultrasound (FUJIFILM Sonosite Inc., USA) and neurostimulator (Stimuplex HNS12, B. Braun Medical Inc., Germany). The patient was placed in the supine position and a high-frequency ultrasound probe was placed in the groin of the operative limb. On ultrasound, the femoral nerve is seen in the inverted triangle formed by the femoral artery and iliopsoas muscle. The patient was placed in the supine position, the hip joint is in external rotation and a low-frequency ultrasound probe was placed in the middle and upper third of the inner thigh. On ultrasound, the adductor longus, the adductor brevis, the adductor magnus, and the hyperechoic sciatic nerve are seen on the medial side of the femur from top to bottom. The needle was guided by ultrasound to the corresponding position, avoiding damage to blood vessels and nerves during the process of inserting. The current of the neurostimulator was adjusted at 1–1.2mA, and lowered the current when the muscle innervated by blocked nerve contracted. The position of the needle tip was optimal to inject local anesthetics when the corresponding muscles stopped contracting at a current of 0.3–0.4mA. Each nerve was blocked with 0.375% ropivacaine 20mL or equal volume saline. Local anesthetics were observed spreading around the nerves under ultrasound to ensure the safety of nerve blocking.
For anesthesia induction, etomidate at 0.2–0.3mg/kg, sufentanil at 0.2–0.5µg/kg, and cis-atracurium at 0.15–0.3mg/kg were injected, followed by laryngeal mask airway insertion and mechanical ventilation. Mechanical ventilation parameters are set to maintain the pressure of end-tidal carbon dioxide (PETCO2) at 35–45mmHg and SpO2 at 99–100%. Intravenous-inhalation balanced anesthesia (propofol and 0.5–0.7 MAC sevoflurane for avoiding intraoperative awareness) was used to maintain BIS at 40–60 during the whole operation. According to multimodal analgesia protocol, they were treated with 50mg flurbiprofen for preemptive analgesia 10 minutes before surgery as well as 50mg flurbiprofen for preventive analgesia when articular closure. To prevent postoperative nausea and vomiting (PONV), 10mg metoclopramide was administered intravenously 30 minutes before the end of surgery.
In addition, a loading dose of 0.6µg/kg DEX (4µg/mL) was pumped intravenously for 15 minutes before the skin was incised, followed by 0.2–0.4µg/(kg. h) maintenance until articular closure in DF group and i.v. equal volume saline in the SF group.
Intraoperative hypertension was defined as an increase in systolic blood pressure (SBP) >20% from preoperative values and/or SBP>160mmHg and intraoperative hypotension was defined as a decrease in SBP>20% from preoperative values and/or SBP<90mmHg. Patients with intraoperative hypertension were treated with sufentanil 5–10µg and the adjustment of propofol dosage to target BIS, then given nicardipine 0.2–0.3mg in a single static push as needed; patients with intraoperative hypotension were treated with rapid fluid infusion and immediately within an infusion of ephedrine or phenylephrine by bolus. When patients’ heart rate (HR) <50 bpm, 0.2–0.5mg atropine was given by intravenous bolus and repeated if necessary.
Three experienced orthopedic surgeons performed all surgeries using a standardized medial parapatellar approach in a bloodless field using a thigh tourniquet. All patients were given antibiotics intravenously 30 minutes before surgery and given 1.0 g tranexamic acid intravenously intraoperatively. There was no local infiltration analgesia. All patients received a subcutaneous injection of 4100 IU low molecular heparin every day from the day after surgery.
Analgesia Scheme
All patients in both groups were treated with 50mg flurbiprofen for preemptive analgesia before the surgery, as well as 50mg flurbiprofen for preventive analgesia when articular closure. In the post-anesthesia care unit (PACU), 5–10µg sufentanil was given intravenously as rescue analgesia. In the ward, postoperative analgesia was achieved with scheduled oral celecoxib 200mg every 12 hours. Patients received additional oral oxycodone (10mg), intramuscular injection of tramadol (100mg), or diclofenac sodium and lidocaine (2mL) as rescue analgesia when pain VAS scores >3/10. The nurse assessed the intensity of pain every 6 hours. The type of remedial analgesics is based on the request of patients.
Primary and Secondary Outcomes
The primary outcome was the pain VAS score during activities at postoperative 24 hours. The dose of sufentanil; the proportion of patients treated with vasoactive drug; the maintenance dose of propofol; the duration of analgesia (the duration from the completion of PNB to the first postoperative rescue analgesia); the dose of rescue analgesia up to 48 hours; pain VAS scores during rest at postoperative 6h, 12h, 24h, 48h; pain VAS scores during activities at postoperative 12h, 24h, 48h; the maximum pain VAS score; the proportion of patients without postoperative additional analgesia in the first 48 hours; the occurrence rate of PONV and postoperative delirium (POD); the number of tourniquet-induced hypertension (TIH); the first time to walk with a walker; and the length of hospital stay (LOHS) were the secondary outcomes. TIH was defined as SBP higher than 160mmHg lasting for 3 min at 20 min after tourniquet inflation.
Statistical Analysis
Statistical analysis of the data was performed using the Statistical Package for the Social Sciences (SPSS), V 25.0. All quantitative data were tested for normality by Shapiro–Wilk test of SPSS software. The quantitative data were expressed as mean ± standard deviation (SD) or median (interquartile range, IQR) according to their distribution. The data, normally distributed, were assessed by an independent two-sample t-test while other quantitative data were analyzed by the Mann–Whitney U-test. The qualitative data were analyzed using Chi-square (χ2) tests. The time of the first rescue analgesia was analyzed by Kaplan–Meier survival analysis and compared between groups with the Log rank test. All tests were performed at the bilateral 5% level.
In the pre-experiment, the mean of pain VAS score during activities at postoperative 24 hours is 4.3 in the DF group and 4.0 in the SF group, and the SD is 1.34 in the DF group and 1.25 in the SF group. When α is 0.05, 1-β is 0.8, and the non-inferiority margin is 4 * 30%, the sample size calculated by PASS 11 software was 27 patients in each group. A 30% difference was selected as this would certainly represent the clinical significance and potentially demonstrate equivalence between the two types of anesthesia regime. Considering accident factors, a total of 88 participants were included.
Results
We screened 108 patients initially and a total of 88 patients were enrolled in the study (Figure 1). One patient developed cerebral infarction after surgery and was sent to the ICU. Finally, data from 87 patients were analyzed.
 |
Figure 1 The flowchart. Abbreviations: DF, dexmedetomidine combined with femoral nerve block; SF, sciatic nerve combined with femoral nerve block. |
Demographic characteristics were similar between the two groups (Table 1). The duration of tourniquet time between groups was 57.5 (42.8–71.0) min vs 55.0 (42.0–71.0) min (P=0.878), and the duration of surgery was 72.0 (63.0–78.8) min vs 70.0 (63.0–76.0) min (P=0.414).
 |
Table 1 Demographic Characteristics and Operation Details |
As shown in Table 2, postoperative pain VAS scores during activities in postoperative 24 hours were comparable between the two groups. Further, pain VAS scores in other time points and the maximum pain VAS score during the first 48 hours were similar between treatment groups.
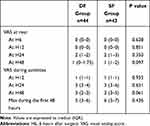 |
Table 2 Postoperative VAS Pain Scores |
The mean duration of analgesia for patients with rescue analgesic requests was similar between the two groups: 25.4 ± 6.3 hours in the DF group vs 24.8 ± 6.4 hours in the SF group (two-sample t-test, P=0.738). Using Kaplan–Meier survival analysis, the duration of analgesia for patients without remedial analgesia was calculated as 48 hours and the duration of analgesia was also comparable between groups (Figure 2, Log rank test: P=0.844).
The total dose of intraoperative sufentanil (P=0.355) and the amount of propofol during anesthesia maintenance (P=0.741) was similar between groups (Table 3). The proportion of patients without rescue analgesic requests up to postoperative 48 hours was similar (P=0.896). Moreover, the dosage of rescue analgesics was comparable (P>0.05). The proportion of nicardipine in the DF group was higher than that in the SF group, but there was no statistically significant difference (31.8% vs 18.6%, respectively; P=0.156). While patients in the SF group were more likely to receive treatment with ephedrine (P=0.001). The likelihood of treatment with atropine was similar in both groups.
 |
Table 3 The Details of Perioperative Medication |
The time to extubate in the DF group was longer than in the SF group (P<0.05), though the duration of PACU was comparable (Table 4). The incidence rate of POD, PONV, and TIH were comparable (P>0.05 for all comparisons). The first time to get out of bed and walk with the help of a walker and the LOHS were similar between groups.
 |
Table 4 Incidence of Adverse Events and Rehabilitation |
Discussion
The administration of DEX combined with FNB provided analgesia equivalent to SNB combined with FNB in patients undergoing TKA during the first 24 hours after surgery. The results show that the total dose of intraoperative sufentanil, the first time to rescue analgesia, the proportion of patients without rescue analgesic request, and pain VAS scores were similar between the treatment groups. These results were supported by the previous studies that have demonstrated the analgesic effect of DEX.11–13,15–17 However, many previous studies compared DEX to placebo or propofol. Chan et al15 have illustrated that an intraoperative infusion of DEX for sedation in patients receiving spinal anesthesia can decrease the use of morphine in the first 24 hours and produce postoperative analgesic effects following TKA. Therefore, the extent to which DEX alleviates pain is not clear.
Despite a functioning FNB, TKA continues to be associated with moderate-to-severe postoperative pain in the majority of patients.1 A meta-analysis of 170 RCTs suggests that the combination of FNB and SNB appears to be the overall best approach in TKA,18 and available evidence supports the analgesic effect of adding SNB to FNB following TKA.1,19,20 However, the anterior sciatic nerve is more prone to damage because of its deep location and unclear muscular layers in elderly obese patients, and permanent sciatic nerve injury after sciatic nerve block has been previously reported.7 Meanwhile, it has also been reported that the incidence of common peroneal nerve injury for its anatomical location ranges from 0.3% to 4% after TKA.9 This situation often leads to unnecessary disputes with surgeons because the common peroneal nerve could be easily damaged by surgical procedures for TKA. Based on the above, we compared the analgesic effect between DEX and SNB.
Our results show that the administration of DEX combined with FNB blunts the intensity of postoperative pain and the doses of postoperative remedial analgesic to an extent comparable to combined SNB with FNB. DEX, as an important component of multimodal analgesia, can attenuate perioperative stress and inflammation induced by surgical trauma, protect the immune function, exhibit multifaceted protective effects, and may improve the clinical outcomes of surgical patients.10 It has been suggested that DEX could also reduce ischemia-reperfusion injury markers21 and the incidence of TIH.22 A meta-analysis reported DEX administration leads to lower postoperative pain and reduced opioid consumption.16 In clinical, DEX was administrated in a wide range of doses and the analgesic effect of DEX may vary with the dose of DEX. The loading dose of DEX in our study was the same as Li et al14 which suggests that intraoperative DEX in conjunction with general anesthesia could decrease stress responses similar to epidural combined with general anesthesia. We assumed that favorable demulcent results in the DF group were due to inhibition of stress, anti-inflammatory effects, and a decrease of ischemia-reperfusion injury of DEX. It has also been reported that systemic DEX could prolong the duration of analgesia.23,24 Additional sciatic nerve block could prolong the duration of analgesia for relieving postoperative pain. In our study, the duration of analgesia between the two groups was similar. However, the time range of remedial analgesia is wide, and a considerable proportion of patients did not require any rescue analgesia. Thus, Kaplan–Meier survival analysis was used on account of the censored values. In general, the analgesic effect was similar between the two groups.
Further, the total dose of sufentanil during surgery and the amount of propofol during anesthesia maintenance were similar between groups. Previous RCTs suggested that DEX could decrease the dose of sedatives and analgesics,25 as well as SNB. Thus, we suggest that DEX could decrease the dose of narcotics to the same extent as SNB. Our data showed that patients receiving the infusion of DEX were less likely to need treatment of ephedrine. Theoretically, it may due to transient hypertension of DEX administration26 especially during skin preparation, which is extremely beneficial to the elderly with fragile heart and brain functions.
Our data showed that the time to extubate in the DF group was prolonged compared to that in the SF group, which was not seen in Li et al’s study.14 However, the average age of participants in the current study compared to the previous study was 66.9 years versus 56.5 years. Five participants developing POD were observed in both groups, similar to Wang et al’s study,27 which recorded observing 6–41% of total joint arthroplasty patients showing delirium. The incidence rate of POD, PONV, and TIH was comparable between the two treatment groups in our study. A meta-analysis shows that DEX may reduce the incidence of POD,28 and PNB may lower the incidence of postoperative acute confusional syndrome.29 Our results indicate additional DEX or SNB lower similar incidence of POD.
According to an analysis of population-based data, approximately only one-fifth of patients undergoing TKA received a neuraxial anesthetic.4 Based on the available evidence, a multimodal approach is recommended in TKA.30 Considering the anesthesia method commonly used in our hospital, we used GA combined with multimodal analgesia in this study.
Compared with total intravenous anesthesia (TIVA), anesthesia methods combined with intravenous and inhaled anesthesia (CIIA) can significantly lower the risk of intraoperative awareness and postoperative cognitive dysfunction.31 Previous studies suggested that maintaining the sevoflurane concentration (0.7MAC) using end-tidal sevoflurane could lower the possibility of awareness.32,33 Thus, we chose the combination of intravenous and inhaled anesthesia intraoperatively.
There are some limitations to the study. First, the dosage of DEX in the current study is certain and it is not shown how the different doses of DEX affect the results of the study. Second, our study lacks the details of functional recovery after surgery and the long-term consequences, which is a defect. Overall, further studies are needed to address these issues.
Conclusion
In summary, the administration of DEX could relieve postoperative pain to an extent comparable to SNB when combined with FNB in TKA under GA, without the potential risk of injury to the sciatic nerve. Further studies are required to see whether DEX combined with FNB influences the long-term quality of life.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the privacy policy but are available from the corresponding author (E-mail: [email protected]) on reasonable requests.
Ethical Approval
Ethical approval was provided by the Ethical Committee of the first affiliated hospital of Anhui Medical University, Hefei, Anhui, China. All patients provided informed consent and all procedures were conducted according to the Declaration of Helsinki.
Acknowledgments
We thank the support of the National Nature Science Foundation of China (Grant No. 81870837). Meanwhile, we thank the nurses involved in the trial for their invaluable support. We also thank the surgeons for the trust they have in our anesthetics team.
Funding
This work was supported by the National Nature Science Foundation of China (Grant No. 81870837).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Abdallah FW, Madjdpour C, Brull R. Is sciatic nerve block advantageous when combined with femoral nerve block for postoperative analgesia following total knee arthroplasty? A meta-analysis. Can J Anaesth. 2016;63(5):552–568. doi:10.1007/s12630-016-0613-2
2. Grosu I, Lavand’homme P, Thienpont E. Pain after knee arthroplasty: an unresolved issue. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1744–1758. doi:10.1007/s00167-013-2750-2
3. Li S, Zhou J, Li X, et al. Analgesic impact of single-shot versus continuous femoral nerve block after total knee arthroplasty: a systematic review and meta-analysis. Adv Ther. 2020;37(2):671–685. doi:10.1007/s12325-019-01194-z
4. Memtsoudis SG, Sun X, Chiu YL, et al. Perioperative comparative effectiveness of anesthetic technique in orthopedic patients. Anesthesiology. 2013;118:1046–1058. doi:10.1097/ALN.0b013e318286061d
5. Fowler SJ, Symons J, Sabato S, Myles PS. Epidural analgesia compared with peripheral nerve blockade after major knee surgery: a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2008;100(2):154–164. doi:10.1093/bja/aem373
6. Rawal N. Current issues in postoperative pain management. Eur J Anaesthesiol. 2016;33(3):160–171. doi:10.1097/EJA.0000000000000366
7. Shah S, Hadzic A, Vloka JD, Cafferty MS, Moucha CS, Santos AC. Neurologic complication after anterior sciatic nerve block. Anesth Analg. 2005;100(5):1515–1517. doi:10.1213/01.ANE.0000150613.23987.92
8. Kopp SL, Borglum J, Buvanendran A, et al. Anesthesia and analgesia practice pathway options for total knee arthroplasty: an evidence-based review by the American and European Societies of Regional Anesthesia and Pain Medicine. Reg Anesth Pain Med. 2017;42(6):683–697. doi:10.1097/AAP.0000000000000673
9. Park JH, Restrepo C, Norton R, Mandel S, Sharkey PF, Parvizi J. Common peroneal nerve palsy following total knee arthroplasty: prognostic factors and course of recovery. J Arthroplasty. 2013;28(9):1538–1542. doi:10.1016/j.arth.2013.02.025
10. Wang K, Wu M, Xu J, et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br J Anaesth. 2019;123(6):777–794. doi:10.1016/j.bja.2019.07.027
11. Li C, Qu J. Efficacy of dexmedetomidine for pain management in knee arthroscopy: a systematic review and meta-analysis. Medicine. 2017;96(43):e7938. doi:10.1097/MD.0000000000007938
12. Yang Q, Ren Y, Feng B, Weng X. Pain relieving effect of dexmedetomidine in patients undergoing total knee or hip arthroplasty: a meta-analysis. Medicine. 2020;99(1):e18538. doi:10.1097/MD.0000000000018538
13. Shin H-J, Do S-H, Lee J-S, Kim T-K, Na H-S. Comparison of intraoperative sedation with dexmedetomidine versus propofol on acute postoperative pain in total knee arthroplasty under spinal anesthesia. Anesth Analg. 2019;129(6):1512–1518. doi:10.1213/ANE.0000000000003315
14. Li Y, Wang B, Zhang LL, et al. Dexmedetomidine combined with general anesthesia provides similar intraoperative stress response reduction when compared with a combined general and epidural anesthetic technique. Anesth Analg. 2016;122(4):1202–1210. doi:10.1213/ANE.0000000000001165
15. Chan IA, Maslany JG, Gorman KJ, O’Brien JM, McKay WP. Dexmedetomidine during total knee arthroplasty performed under spinal anesthesia decreases opioid use: a randomized-controlled trial. Can J Anaesth. 2016;63(5):569–576. doi:10.1007/s12630-016-0597-y
16. Schnabel A, Meyer-Friessem CH, Reichl SU, Zahn PK, Pogatzki-Zahn EM. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trials. Pain. 2013;154(7):1140–1149. doi:10.1016/j.pain.2013.03.029
17. Blaudszum G, Lysakowski C, Elia N. Effect of perioperative systemic 2 agonists on postoperative morphine consumption and pain intensity systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–1322. doi:10.1097/ALN.0b013e31825681cb
18. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923–937. doi:10.1097/ALN.0000000000001607
19. Grape S, Kirkham KR, Baeriswyl M, Albrecht E. The analgesic efficacy of sciatic nerve block in addition to femoral nerve block in patients undergoing total knee arthroplasty: a systematic review and meta-analysis. Anaesthesia. 2016;71(10):1198–1209. doi:10.1111/anae.13568
20. Zorrilla-Vaca A, Li J. The role of sciatic nerve block to complement femoral nerve block in total knee arthroplasty: a meta-analysis of randomized controlled trials. J Anesth. 2018;32(3):341–350. doi:10.1007/s00540-018-2480-1
21. Yagmurdur H, Ozcan N, Dokumaci F, Kilinc K, Yilmaz F, Basar H. Dexmedetomidine reduces the ischemia-reperfusion injury markers during upper extremity surgery with tourniquet. J Hand Surg Am. 2008;33(6):941–947. doi:10.1016/j.jhsa.2008.01.014
22. Li YH, Wang YQ, Zhang YJ, Zheng DY, Hu L, Tian ML. Influence of dexmedetomidine on the tourniquet related responses in hypertension patients receiving unilateral knee arthroplasty under general anesthesia. J Arthroplasty. 2015;30(8):1359–1363. doi:10.1016/j.arth.2015.02.034
23. Tseng WC, Lin WL, Lai HC, et al. Adjunctive dexmedetomidine infusion in open living donor hepatectomy: a way to enhance postoperative analgesia and recovery. Int J Clin Pract Jan. 2021;75:e14002.
24. Rutkowska K, Knapik P, Misiolek H. The effect of dexmedetomidine sedation on brachial plexus block in patients with end-stage renal disease. Eur J Anaesthesiol. 2009;26(10):851–855. doi:10.1097/EJA.0b013e32832a2244
25. Le Guen M, Liu N, Tounou F, et al. Dexmedetomidine reduces propofol and remifentanil requirements during bispectral index-guided closed-loop anesthesia: a double-blind, placebo-controlled trial. Anesth Analg. 2014;118(5):946–955. doi:10.1213/ANE.0000000000000185
26. Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin CP. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi:10.1007/s40262-017-0507-7
27. Wang L, Seok S, Kim S, Kim K, Lee S, Lee K. The risk factors of postoperative delirium after total knee arthroplasty. J Knee Surg. 2017;30(6):600–605. doi:10.1055/s-0036-1593872
28. Duan X, Coburn M, Rossaint R, Sanders RD, Waesberghe JV, Kowark A. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth. 2018;121(2):384–397. doi:10.1016/j.bja.2018.04.046
29. Roche-Albero A, Cassinello-Ogea C, Martin-Hernandez C. Factors of presenting an acute confusional syndrome after a hip fracture. Injury. 2021;52:S54–S60. doi:10.1016/j.injury.2021.04.065
30. Chassery C, Marty P, Rontes O, et al. Total knee arthroplasty under quadruple nerve block with ropivacaine 0.32%: effect of addition of intravenous dexmedetomidine to intravenous dexamethasone on analgesic duration. Reg Anesth Pain Med. 2021;46(2):104–110. doi:10.1136/rapm-2020-101749
31. Yu H, Wu D. Effects of different methods of general anesthesia on intraoperative awareness in surgical patients. Medicine. 2017;96(42):e6428. doi:10.1097/MD.0000000000006428
32. Avidan MS, Jacobsohn E, Glick D, et al. Prevention of intraoperative awareness in a high-risk surgical population. N Engl J Med. 2011;365(7):591–600. doi:10.1056/NEJMoa1100403
33. Wang J, Zhang L, Huang Q, et al. Monitoring the end-tidal concentration of sevoflurane for preventing awareness during anesthesia (MEETS-PANDA): a prospective clinical trial. Int J Surg. 2017;41:44–49. doi:10.1016/j.ijsu.2017.03.015
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

