Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Dexmedetomidine Alleviates LPS-Induced Neuronal Dysfunction by Modulating the AKT/GSK-3β/CRMP-2 Pathway in Hippocampal Neurons
Authors Zeng W, Zhang C, Long Q, Li Y
Received 14 December 2020
Accepted for publication 5 February 2021
Published 9 March 2021 Volume 2021:17 Pages 671—680
DOI https://doi.org/10.2147/NDT.S297365
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Wei Zeng,1,2 Chunyuan Zhang,2 Qingshan Long,3 Yalan Li1
1Department of Anesthesiology, The First Affiliated Hospital of Jinan University, Guangzhou, 510630, Guangdong, People’s Republic of China; 2Department of Anesthesiology, Affiliated Boai Hospital of Zhongshan, Southern Medical University, Zhongshan, 528400, Guangdong, People’s Republic of China; 3Department of Neurosurgery, Huizhou Third People’s Hospital, Guangzhou Medical University, Huizhou, 516002, Guangdong, People’s Republic of China
Correspondence: Yalan Li
Department of Anesthesiology, The First Affiliated Hospital of Jinan University, Guangzhou, 510630, Guangdong, People’s Republic of China
Email [email protected]
Objective: Dexmedetomidine, an α 2-adrenergic receptor agonist, mitigates cognitive dysfunction in elderly patients after surgery with general anesthesia. However, the underlying mechanism by which dexmedetomidine reduces cognitive dysfunction remains to be fully elucidated. The aim of this study was to investigate the effects of dexmedetomidine on lipopolysaccharide (LPS)-induced neuronal dysfunction in cultured hippocampal neurons.
Methods: LPS, in the presence and absence of dexmedetomidine, was applied to cultured hippocampal neurons to mimic post-surgical inflammation. Neuronal morphology, including neurite outgrowth and synaptic transmission, was observed, and miniature excitatory postsynaptic currents were recorded by electrophysiological patch-clamp.
Results: LPS significantly impaired neurite outgrowth in hippocampal neurons in a concentration- and time-dependent manner, which was reversed by dexmedetomidine treatment. Electrophysiological patch-clamp results showed that LPS induced synaptic transmission dysfunction, which was restored after dexmedetomidine addition. Furthermore, Western blotting assays showed that LPS suppressed the AKT/GSK-3β/CRMP-2 signaling pathway and dexmedetomidine countered the inhibitory effect of LPS by re-activating this pathway.
Conclusion: In general, dexmedetomidine protected against the effects of LPS-induced hippocampal neuron damage, including neurite outgrowth and synaptic transmission. Overall, dexmedetomidine modulated the AKT/GSK-3β/CRMP-2 signaling pathway to alleviate LPS-induced neurological dysfunction.
Keywords: dexmedetomidine, postoperative cognitive dysfunction, neurological impairment, AKT, GSK-3β, CRMP-2
Background
Elderly patients are susceptible to brain dysfunction after surgery, especially after general anesthesia; the symptoms of this dysfunction are collectively referred to as postoperative cognitive dysfunction (POCD).1 POCD was first observed in elderly patients after cardiac surgery, but more recently has been found in patients undergoing other surgical procedures.2–4 The total incidence of POCD after cardiac surgery can reach 51%.5 POCD is reversible for most patients, but a few patients suffer long-term or even permanent cognitive impairment. POCD leads to delayed rehabilitation, increased complications and even loss of self-care ability, prolongs the length of hospital stays, increases medical costs, and causes a series of medical, social, and economic problems. The incidence of POCD is closely related to neurodegenerative diseases in the elderly such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and multiple sclerosis.6,7 Ways of reducing the occurrence of cognitive dysfunction after general anesthesia in the elderly remain largely obscure.
The inflammatory response can affect the function of the central nervous system (CNS), in particular CNS inflammation can lead to changes in cognitive function,8 which leads to the occurrence of POCD. Both anesthesia and surgery can induce inflammatory responses in the CNS.9,10 Thus, the occurrence and development of POCD are closely related to the inflammatory response. Lipopolysaccharide (LPS; an immunostimulatory component of the cell wall of Gram-negative bacteria) was originally identified as a Toll-like receptor 4 (TLR-4) ligand.11 Once microglia are activated by LPS, they produce pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin-1β, prostaglandin E2, and nitric oxide.12,13 These cytokines are key mediators that mediate the neuroinflammatory process. Inhibition of TLR-4 abolishes LPS-induced inflammatory response.14 Administration of LPS to animals induces cognitive impairment15,16 and other complex dysfunctions, including anorexia, decreased exercise, weight loss, reduced exploratory behavior, increased anxiety, lethargy, and behavioral depression. These symptoms are very similar to the clinical symptoms of human neurodegenerative diseases. Thus, LPS-induced inflammation is a commonly used model for the study of POCD.17–19 However, the specific mechanism of LPS-induced cognitive impairment remains to be elucidated.
Dexmedetomidine is a highly selective α2 adrenergic receptor agonist, which is used in a similar manner to remifentanil during anesthesia20 and has anti-inflammatory activity.21–23 In recent years, dexmedetomidine has been shown to have certain interventional effects on cognitive dysfunction in elderly patients after general anesthesia. Data show that dexmedetomidine extends patient survival, and that only 6% of patients undergoing general anesthesia who receive dexmedetomidine have postoperative delirium,24,25 compared with 45% of patients receiving general drugs such as propofol or midazolam.26 However, the detailed mechanism of dexmedetomidine in reducing POCD remains unknown.
The main purpose of this study is to determine whether dexmedetomidine regulates LPS-induced neurological damage and to elucidate the underlying mechanisms in order to provide new approaches and intervention targets for the clinical prevention and treatment of POCD.
Methods
Neuron Culture and Transfection
Experiments were conducted using newborn 1-day-old Sprague-Dawley rats (male and female) provided by the Animal Center of Jinan University, as previously described (Zhang et al, 2007). Related animal procedures were strictly conducted in accordance with the guidelines by China Animal Protection Association. Briefly, rats were sacrificed using CO2 anesthesia, hippocampi were dissected from the brain, and then gently homogenized hippocampal tissue was placed in a 60 mm tissue culture dish. The hippocampal tissue was incubated with 0.25% trypsin (Gibco, MD, USA) at 37°C for 5 min. Neurons were cultured in Dulbecco’s modified Eagle’s medium (Gibco), supplemented with 10% fetal bovine serum (Gibco), and plated in a 24-well plate with coverslips at a density of 6 × 104 cells/well. Neurons were placed in a 37°C, 5% CO2 incubator. After overnight incubation, the medium was replaced with neurobasal feeding media (neurobasal medium containing 2% B27 supplement and 0.5 mM glutamine solution; Gibco). Half the volume of media was replaced with the same volume of fresh neurobasal feeding media every 3 days. Transient transfections of neurons were performed with Lipofectamine 2000 (Invitrogen Corporation, Carlsbad, CA, USA) according to the manufacturer’s instructions. The protocol was approved by the Institutional Animal Care and Use Committee at Jinan University.
Western Blotting
Protein samples were extracted from rat hippocampal neurons, and protein concentrations were quantified using the BCA assay (Sigma, MO, USA). The extracted protein samples were separated by 10% SDS-PAGE with ~30 µg protein loaded per lane and transferred onto a PVDF membrane (EMD Millipore, MA, USA). The membrane was blocked with 5% non-fat milk in TBS containing 0.1% tween-20 (TBST) at room temperature for 1 h and then incubated with antibodies against p-AKT, AKT, p-GSK-3β, GSK-3β, p-CRMP2, or CRMP2 (all from Abcam, MA, USA) in TBS buffer containing 3% BSA at 4°C overnight. GAPDH was used as a loading control. After incubation with secondary antibodies at room temperature for 1 h, the blots were visualized using enhanced chemiluminescence reagents. The intensity of the bands was analyzed and quantified by densitometric analysis using Image-Pro Plus 7.0 (Media Cybernetics, Inc., Silver Spring, MD, USA). The Western blotting results are representative of three independent experiments.
Immunocytochemistry
Immunofluorescence assays were performed as previously described.27 Briefly, neurons were fixed in 4% paraformaldehyde supplemented with 4% sucrose for 40 min at 4°C and blocked with blocking buffer (3% BSA in TBS). Sections were then incubated with rabbit anti-GFP (1:1000; cat no. Ab290, Abcam) and mouse anti-tau-1 antibody (1:500; cat no. MAB3420, EMD Millipore) overnight at 4°C. After washing three times with TBST (0.1% Triton X-100 in TBS), sections were labelled with appropriate fluorescent-tagged secondary antibodies (goat anti-mouse/rabbit IgG FITC, 1:1000; cat no. ab150115, Abcam) for 1 h at room temperature, and then neurons were mounted on glass slides using Fluoro Gel II containing DAPI for confocal microscopy studies (LSM 700; Zeiss GmbH, Germany).
Neuronal Morphology Analysis
The total length of neurites and Sholl analysis were performed to reveal neuronal development. All images were obtained using an ordinary fluorescence microscope or confocal microscope. Image-Pro Plus software with a neuronal tracer plug-in was used to determine neurite length, and ImageJ software with a Sholl plug-in was used for Sholl analysis of morphometric protrusions as previously described.28 Briefly, images were randomly captured from over 40 neurons per group; each image was processed with the neuronal tracer plug-in; and a trace of all neurites (based on immunocytochemical analysis of GFP and tau-1 staining) on a confocal image was drawn manually.
Whole-Cell Patch-Clamp
The whole-cell patch-clamp technique was used27 to record miniature excitatory postsynaptic currents (mEPSCs) from hippocampal neurons cultured for 11–12 days and treated with LPS and dexmedetomidine. For these experiments, the external solution contained (in mM) the following: 1 MgCl2, 5 KCl, 128 NaCl, 20 HEPES, 2 CaCl2, 15 glucose, 1 tetrodotoxin, and 100 μM picrotoxin. The pH of the external solution was adjusted to 7.27.3 with KOH. The internal solution of the recording electrodes (4- to 6-MΩ tip resistance) contained (in mM) the following: 2 MgATP, 5 Na2-phosphocreatine, 147 KCl, 2 EGTA, 10 HEPES, and 0.3 Na2GTP. The pH was adjusted to 7.2–7.3 with KOH, and the osmolarity was adjusted to 280 mmol/kg with sucrose. A multiclamp 700 B amplifier (Molecular Devices, Sunnyvale, CA, USA) and Clampex 10.5 software (Axon Instruments, Union City, CA, USA) were used.
Statistical Analysis
The experimental data were presented as mean ± SD from at least three experiments, and SPSS 19.0 software (SPSS Software, Chicago, IL, USA) was used for statistical analysis. A t-test was used for comparisons between two groups, and a one-way ANOVA was used for multiple comparisons; p < 0.05 was considered significantly different, * or # donates p < 0.05, ** donates p < 0.01, *** donates p < 0.001.
Results
LPS Administration Impairs Hippocampal Neurite Outgrowth
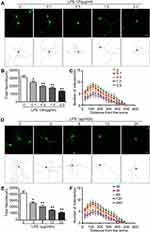
To clarify the effect of LPS on the morphology of hippocampal neurons, neurons were transfected with GFP-encoding plasmids and treated with different concentrations of LPS (0, 0.1, 0.5,1.0, and 2.0 μg/mL) for 12 h. LPS induced significant changes in the growth of neuronal processes in a concentration-dependent manner (Figure 1A). The protrusion length of LPS-treated groups was significantly smaller than that of the control group (p < 0.01) (Figure 1B). Sholl analysis, which counted the number of branch points in different radius ranges, showed that the complexity of neuronal morphology also decreased in a concentration-dependent manner (Figure 1C). A concentration of 1.0 μg/mL LPS was selected for further studies as this concentration produced the most significant effect and less toxicity. Neurons were treated with LPS for different times (0, 3, 6, 12, and 24 h; Figure 1D), and the total length (Figure 1E) and Sholl analysis (Figure 1F) of protrusions were calculated. The data showed that LPS induced morphological changes in neurons in a time-dependent manner. In general, these data suggest that LPS impairs neurite outgrowth.
Dexmedetomidine Antagonizes LPS-Induced Impairment of Neurite Outgrowth
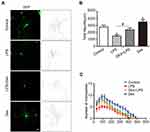
The effect of dexmedetomidine on hippocampal neuron development was observed in neurons treated with LPS in the absence or presence dexmedetomidine. As shown in Figure 2A, LPS significantly induced neuronal morphological changes, and this damage was rescued by the addition of dexmedetomidine (1 μM).29 The total length of all neurites (Figure 2B) and the number of intersection points (Figure 2C) in each group were calculated. The data suggest that dexmedetomidine treatment antagonizes the effects of LPS-induced damage on neuronal morphology.
Dexmedetomidine Rescues Synaptic Transmission Dysfunction Induced by LPS
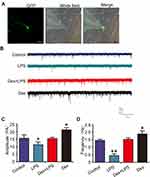
To verify whether dexmedetomidine and LPS modulated neuronal function, electrophysiological patch-clamp assays were applied to cultured hippocampal neurons to determine the mEPSCs (Figure 3A). As shown in Figure 3B, the results showed that LPS impaired the synaptic transmission of neurons; the amplitude (Figure 3C) and frequency (Figure 3D) of mEPSCs were significantly decreased, and these effects were restored after the addition of dexmedetomidine. Dexmedetomidine treatment alone increased mEPSC amplitude and frequency. These data suggest that dexmedetomidine reverses LPS-induced synaptic transmission dysfunction.
Dexmedetomidine Counters the Inhibitory Effect of LPS by Activating the AKT/GSK-3β/CRMP-2 Pathway
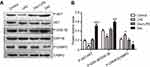
To further explore the molecular mechanism by which dexmedetomidine prevents LPS-induced neurodevelopmental impairment and defects in synaptic transmission, neuronal lysates were subjected to Western blotting. The results showed that LPS reduced the phosphorylation of AKT and GSK-3β, and increased CRMP-2 phosphorylation (Figure 4A). Application of dexmedetomidine significantly increased AKT and GSK-3β phosphorylation, and decreased CRMP-2 phosphorylation, thereby counteracting the inhibitory effect of LPS (Figure 4B). These results suggest that dexmedetomidine antagonizes LPS-induced neuronal damage by activating the AKT/GSK-3β/CRMP-2 pathway.
Inhibition of AKT/GSK-3 Abolishes the Effect of Dexmedetomidine
A pharmacological approach was used to confirm that dexmedetomidine prevents LPS-induced neural damage by activating the AKT/GSK-3β/CRMP-2 pathway. Insulin-like growth factor (IGF) was used to promote neuron development, and the AKT inhibitor LY294002 and GSK-3 inhibitor SB216763 were used to suppress the AKT/AKT/GSK-3 axis. As shown in Figure 5A, IGF activated the AKT pathway; AKT and GSK-3β phosphorylation was increased, and CRMP-2 phosphorylation was decreased, consistent with the phosphorylation pattern produced by dexmedetomidine. Addition of the AKT inhibitor or the GSK-3 inhibitor significantly abolished dexmedetomidine-mediated activation of this pathway; the phosphorylation of AKT, GSK-3β, and CRMP-2 was comparable to that observed in the LPS treatment group (Figure 5B). These data confirm that dexmedetomidine activates the AKT/GSK-3β/CRMP-2 pathway to antagonize the effects of LPS treatment in hippocampal neurons.
Discussion
In the current study, we demonstrated that LPS impaired hippocampal neuron development and disrupted synaptic transmission, and these effects were antagonized by dexmedetomidine. Dexmedetomidine antagonized LPS-induced nerve damage by re-activating the AKT/GSK-3β/CRMP-2 pathway.
POCD refers to the presence of mental disorders and abnormal brain function in elderly patients after surgery, which can be characterized by anxiety, personality changes, and memory impairment.30 General anesthesia combined with surgical shock can change the physiological function of elderly patients.31 Under the stimulation of a stress response, a series of adverse cardiovascular and cerebrovascular events can easily cause cognitive dysfunction in elderly patients.32 It is generally believed that three factors contribute to the pathogenesis of POCD: (1) The influence of surgical factors on the internal environment, such as the size of the surgical wound, the duration of the operation, and patient responses related to stress, micro thromboembolism, blood loss, and fluid loss33; (2) The effects of anesthetics on the patient, such as effects of general anesthetics on the CNS and effects of anesthetics on the homeostatic processes of the body, such as hypotension, hypertension, bradycardia, and hypothermia34; (3) The pathophysiological status and social factors of the patient undergoing surgery; for example, advanced age, combined diabetes, hypertension, and cognitive impairment before surgery correlate with POCD occurrence.35 Therefore, in-depth studies of the neuropathic mechanisms of POCD will be of important scientific value and significance to determine how POCD can be prevented.
Surgery can trigger neuroinflammation and induce POCD.36 Surgery-induced release of inflammatory factors or cells in the peripheral blood enter the brain to affect the CNS.37 These inflammatory factors activate microglia cells to produce an exaggerated immune response, leading to the release of a large number of inflammatory factors,38 including IL-1β and TNF-α. In addition, peripheral immune cells that enter the brain can amplify the inflammatory response.39 Accumulated inflammatory mediators cause reversible or irreversible damage to brain tissue, leading to the degeneration of neurite and cognitive dysfunction. As the memory center of the brain, the hippocampus is more sensitive to an overload of inflammatory cytokines because of widely expressed cytokine receptors.40 In this study, we applied LPS to induce an inflammatory response in cultured primary hippocampal neurons, and consistent with previous studies, we showed that LPS caused significant damage to neurite development and synaptic transmission.41
Dexmedetomidine is an α2-agonist that is widely used in clinical practice.42 Studies show that dexmedetomidine has anti-inflammatory actions,43 but the underlying mechanisms have not been fully described. In a rat model of cerebral ischemia, dexmedetomidine decreased blood catecholamine content and decreased sympathetic nerve activity.44 Dexmedetomidine inhibits systemic inflammatory responses and improves survival in a rat model of septic shock,45 and also has anti-inflammatory effects in a rat model of spinal cord injury.46 In clinical patients, dexmedetomidine markedly reduces the expression of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6.47 In a rat model of POCD, dexmedetomidine protects aged rats from cognitive dysfunction by decreasing hippocampal inflammation.48 In our study, we did not measure levels of inflammatory factors, but nevertheless our data showed that dexmedetomidine significantly alleviated LPS-induced neurite outgrowth and defects in synaptic transmission. Also, currently in this paper, we only used primary cultured hippocampal neurons to observe the effect, and an animal model should be applied to further evaluate of the role of dexmedetomidine in POCD and the detailed mechanism should be investigated.
The PI3K/AKT pathway is involved in cognitive function in POCD.49 Zhang et al discovered that ADAM2 activated the PI3K/AKT pathway to attenuate isoflurane-induced POCD50 and Zhou et al showed that dysfunction in gap junction communication during ischemia-reperfusion injury caused cognitive impairment via activation of the PI3K/AKT pathway.51 Furthermore, dexmedetomidine is reported to alleviate LPS-induced lung injury in Wistar rats via the Nrf2/Keap1 pathway.52 Whether or not dexmedetomidine functions via the Nrf2/Keap1 pathway in LPS-induced neuronal damage should be further clarified in our future study. Rui et al reported that miR-410 had neuroprotective effects against sevoflurane-induced cognitive dysfunction in rats via activation of the PI3K/AKT pathway.53 In the current study, LPS administration significantly suppressed activation of the AKT/GSK-3β/CRMP-2 pathway in cultured hippocampal neurons, and dexmedetomidine re-activated this pathway, rescuing neurite development and defects in synaptic transmission.
Conclusions
We demonstrated that LPS-induced inhibition of neuronal outgrowth and synaptic transmission dysfunction in hippocampal neurons was prevented by dexmedetomidine via the regulation of the PI3K/AKT/GSK-3β signaling pathway. This study provides a scientific basis for the clinical effects of dexmedetomidine, and could assist the clinical application of dexmedetomidine for the treatment of POCD.
Abbreviations
AD, Alzheimer’s disease; CNS, the central nervous system; LPS, Lipopolysaccharide; mEPSCs, miniature excitatory postsynaptic currents; PD, Parkinson’s disease; PGE2, prostaglandin E2; POCD, postoperative cognitive dysfunction; TLR-4, Toll-like receptor 4.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The protocol was approved by the Institutional Animal Care and Use Committee at Jinan University. Related animal procedures were strictly conducted in accordance with the guidelines by China Animal Protection Association.
Funding
This work was supported by Medical Scientific Research Foundation of Guangdong Province, China (Grant No. B2021190).
Disclosure
The authors declare no conflicts of interest.
References
1. Luyten FP, Tylzanowski P, Lories RJ. Wnt signaling and osteoarthritis. Bone. 2009;44:522–527. doi:10.1016/j.bone.2008.12.006
2. Hudetz JA, Patterson KM, Byrne AJ, Pagel PS, Warltier DC. Postoperative delirium is associated with postoperative cognitive dysfunction at one week after cardiac surgery with cardiopulmonary bypass. Psychol Rep. 2009;105:921–932. doi:10.2466/PR0.105.3.921-932
3. Gao L, Taha R, Gauvin D, Othmen LB, Wang Y, Blaise G. Postoperative cognitive dysfunction after cardiac surgery. Chest. 2005;128:3664–3670. doi:10.1378/chest.128.5.3664
4. Ida M, Kawaguchi M. Postoperative cognitive dysfunction after non-cardiac surgery. Masui. 2014;63:1228–1234.
5. Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103:i41–i46. doi:10.1093/bja/aep291
6. Allison DJ, Ditor DS. The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J Neuroinflammation. 2014;11. doi:10.1186/s12974-014-0151-1
7. Nguyen MD, Julien J-P, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nature Reviews. Neuroscience. 2002;3:216–227. doi:10.1038/nrn752
8. Combs CK. Inflammation and microglia actions in Alzheimer’s disease. J Neuroimmune Pharmacol. 2009;4:380–388. doi:10.1007/s11481-009-9165-3
9. Hudson AE, Hemmings HC. Are anaesthetics toxic to the brain? Br J Anaesth. 2011;107:30–37. doi:10.1093/bja/aer122
10. Vanderweyde T, Bednar MM, Forman SA, Wolozin B, Mandal PK, Fodale V. Iatrogenic risk factors for Alzheimer’s disease: surgery and anesthesia. J Alzheimers Dis. 2010;22(Suppl 3):91–104. doi:10.3233/JAD-2010-100843
11. Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi:10.1016/S0952-7915(99)00046-1
12. Janelidze S, Mattsson N, Stomrud E, et al. CSF biomarkers of neuroinflammation and cerebrovascular dysfunction in early Alzheimer disease. Neurology. 2018
13. Mrak RE, Griffin WST. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi:10.1016/j.neurobiolaging.2004.05.010
14. Lysakova-Devine T, Keogh B, Harrington B, et al. Viral inhibitory peptide of TLR4, a peptide derived from vaccinia protein A46, specifically inhibits TLR4 by directly targeting MyD88 adaptor-like and TRIF-related adaptor molecule. J Immunol. 2010;185:4261–4271. doi:10.4049/jimmunol.1002013
15. Choi DY, Lee JW, Lin G, Yong KL, Lee MS. Obovatol attenuates LPS-induced memory impairments in mice via inhibition of NF-κB signaling pathway. Neurochem Int. 2011;60:68–77. doi:10.1016/j.neuint.2011.11.005
16. Golestaneh AF, Atashi A, Langroudi L, Shafiee A, Ghaemi N, Soleimani M. miRNAs expressed differently in cancer stem cells and cancer cells of human gastric cancer cell line MKN-45. Cell Biochem Funct. 2012;30:411–418. doi:10.1002/cbf.2815
17. Chen L, Dong R, Lu Y, et al. MicroRNA-146a protects against cognitive decline induced by surgical trauma by suppressing hippocampal neuroinflammation in mice. Brain Behav Immun. 2019;78:188–201. doi:10.1016/j.bbi.2019.01.020
18. Zhao WX, Zhang JH, Cao JB, et al. Acetaminophen attenuates lipopolysaccharide-induced cognitive impairment through antioxidant activity. J Neuroinflammation. 2017;14:17. doi:10.1186/s12974-016-0781-6
19. Chen Y, Sun JX, Chen WK, et al. miR-124/VAMP3 is a novel therapeutic target for mitigation of surgical trauma-induced microglial activation. Signal Transduct Target Ther. 2019;4:27. doi:10.1038/s41392-019-0061-x
20. Schenarts CL, Burton JH, Riker RR. Adrenocortical dysfunction following etomidate induction in emergency department patients. Acad Emerg Med. 2001;8:1–7. doi:10.1111/j.1553-2712.2001.tb00537.x
21. Rohan D, Buggy DJ, Crowley S, Ling FKH, Moriarty DC. Increased incidence of postoperative cognitive dysfunction 24 hr after minor surgery in the elderly. Can J Anaesthes. 2005;52:137–142. doi:10.1007/BF03027718
22. Yamanaka D, Kawano T, Nishigaki A, Aoyama B, Yokoyama M, Yamamuro T. Preventive effects of dexmedetomidine on the development of cognitive dysfunction following systemic inflammation in aged rats. J Anesth. 2016;31:1–11. doi:10.1007/s00540-016-2262-6
23. Iliopoulos D, Malizos KN, Oikonomou P, Tsezou A, Koutsopoulos S. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS One. 2008;3:e3740. doi:10.1371/journal.pone.0003740
24. Han JH, Wilson A, Ely EW. Delirium in the older emergency department patient: a quiet epidemic. Emerg Med Clin North Am. 2010;28:611–631. doi:10.1016/j.emc.2010.03.005
25. Delaney M, Pepin J, Somes J. Emergency department delirium screening improves care and reduces revisits for the older adult patient. J Emerg Nurs. 2015;41:S0099176715004092.
26. Jie Y, Yongfang Z, Yan K, et al. Risk factors of delirium in sequential sedation patients in intensive care units. Biomed Res Int. 2017;1–9.
27. Zhang Z, Zhang J, Li J, et al. Ketamine regulates phosphorylation of CRMP2 to mediate dendritic spine plasticity. J Mol Neurosci. 2020;70:353–364. doi:10.1007/s12031-019-01419-4
28. Rojek KO, Krzemien J, Dolezyczek H, et al. Amot and Yap1 regulate neuronal dendritic tree complexity and locomotor coordination in mice. PLoS Biol. 2019;17:e3000253. doi:10.1371/journal.pbio.3000253
29. Jameson RR, Seidler FJ, Qiao D, Slotkin TA. Adverse neurodevelopmental effects of dexamethasone modeled in PC12 cells: identifying the critical stages and concentration thresholds for the targeting of cell acquisition, differentiation and viability. Neuropsychopharmacology. 2006;31:1647–1658. doi:10.1038/sj.npp.1300967
30. Czyz-Szypenbejl K, Medrzycka-Dabrowska W, Kwiecien-Jagus K, Lewandowska K. The occurrence of postoperative cognitive dysfunction (POCD) - systematic review. Psychiatr Pol. 2019;53:145–160. doi:10.12740/PP/90648
31. Ho YS, Zhao FY, Yeung WF, Wong GT, Zhang HQ, Chang RC. Application of acupuncture to attenuate immune responses and oxidative stress in postoperative cognitive dysfunction: what do we know so far? Oxid Med Cell Longev. 2020;2020:9641904. doi:10.1155/2020/9641904
32. Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth. 2017;119:i115–i125. doi:10.1093/bja/aex354
33. Bekker AY, Weeks EJ. Cognitive function after anaesthesia in the elderly. Clin Anaesthesiol. 2003;17:259–272. doi:10.1016/s1521-6896(03)00005-3
34. Mason SE, Noel-Storr A, Ritchie CW, Mandal PK, Fodale V. The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis. J Alzheimers Dis. 2010;22(Suppl 3):67–79. doi:10.3233/JAD-2010-101086
35. Xing L. Clinical study of postoperative cognitive dysfunction. Journal. 2009.
36. Chu JMT, Xiong W, Linghu KG, et al. Siegesbeckia orientalis L. extract attenuates postoperative cognitive dysfunction, systemic inflammation, and neuroinflammation. Exp Neurobiol. 2018;27:564–573. doi:10.5607/en.2018.27.6.564
37. Podjaski C, Alvarez JI, Bourbonniere L, et al. Netrin 1 regulates blood-brain barrier function and neuroinflammation. Brain. 2015;138:1598–1612. doi:10.1093/brain/awv092
38. Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi:10.1186/s12974-015-0332-6
39. Gyoneva S, Davalos D, Biswas D, et al. Systemic inflammation regulates microglial responses to tissue damage in vivo. Glia. 2014;62:1345–1360. doi:10.1002/glia.22686
40. Fakhoury M. Role of immunity and inflammation in the pathophysiology of neurodegenerative diseases. Neurodegener Dis. 2015;15:63–69. doi:10.1159/000369933
41. Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi:10.1016/j.bbi.2007.08.014
42. Keating GM. Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs. 2015;75:1119–1130. doi:10.1007/s40265-015-0419-5
43. Daskalopoulos EP, Malliou F, Rentesi G, Marselos M, Lang MA, Konstandi M. Stress is a critical player in CYP3A, CYP2C, and CYP2D regulation: role of adrenergic receptor signaling pathways. Am J Physiol Endocrinol Metab. 2012;303:E40–E54.
44. Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology. 1991;75:328–332. doi:10.1097/00000542-199108000-00022
45. Ma Y, Yu XY, Wang Y. Dose-related effects of dexmedetomidine on immunomodulation and mortality to septic shock in rats. World J Emerg Med. 2018;9:56–63. doi:10.5847/wjem.j.1920-8642.2018.01.009
46. Fang B, Li XQ, Bi B, et al. Dexmedetomidine attenuates blood-spinal cord barrier disruption induced by spinal cord ischemia reperfusion injury in rats. Cell Physiol Biochem. 2015;36:373–383. doi:10.1159/000430107
47. Yang D, Hong JH. Dexmedetomidine modulates histamine-induced Ca(2+) signaling and pro-inflammatory cytokine expression. Korean J Physiol Pharmacol. 2015;19:413–420. doi:10.4196/kjpp.2015.19.5.413
48. Chen N, Chen X, Xie J, Wu C, Qian J. Dexmedetomidine protects aged rats from postoperative cognitive dysfunction by alleviating hippocampal inflammation. Mol Med Rep. 2019;20:2119–2126. doi:10.3892/mmr.2019.10438
49. Small DH, Mok SS, Bornstein JC. Alzheimer’s disease and Abeta toxicity: from top to bottom. Nat Rev Neurosci. 2001;2:595–598. doi:10.1038/35086072
50. Zhang BJ, Yuan CX. Effects of ADAM2 silencing on isoflurane-induced cognitive dysfunction via the P13K/Akt signaling pathway in immature rats. Biomed Pharmacother. 2019;109:217–225. doi:10.1016/j.biopha.2018.10.020
51. Zhou S, Fang Z, Wang G, Wu S. Gap junctional intercellular communication dysfunction mediates the cognitive impairment induced by cerebral ischemia-reperfusion injury: PI3K/Akt pathway involved. Am J Transl Res. 2017;9:5442–5451.
52. Yan X, Cheng X, Zhou L, He X, Zheng W, Chen H. Dexmedetomidine alleviates lipopolysaccharide-induced lung injury in Wistar rats. Oncotarget. 2017;8:44410–44417. doi:10.18632/oncotarget.17899
53. Su R, Sun P, Zhang D, Xiao W, Feng C, Zhong L. Neuroprotective effect of miR-410-3p against sevoflurane anesthesia-induced cognitive dysfunction in rats through PI3K/Akt signaling pathway via targeting C-X-C motif chemokine receptor 5. Genes Genomics. 2019;41:1223–1231. doi:10.1007/s13258-019-00851-5
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.