Back to Journals » Drug Design, Development and Therapy » Volume 13
Developmental Pharmacogenetics of SLCO2B1 on Montelukast Pharmacokinetics in Chinese Children
Authors Li Q, Wang K, Shi HY, Wu YE, Zhou Y, Kan M, Zheng Y, Hao GX, Yang XM, Yang YL, Su LQ, Wang XL, Jacqz-Aigrain E, Zhou J, Zhao W
Received 12 August 2019
Accepted for publication 12 November 2019
Published 27 December 2019 Volume 2019:13 Pages 4405—4411
DOI https://doi.org/10.2147/DDDT.S226913
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Qian Li,1,2,* Kai Wang,3,* Hai-Yan Shi,2 Yue-E Wu,1 Yue Zhou,1 Min Kan,1 Yi Zheng,1 Guo-Xiang Hao,1 Xin-Mei Yang,2 Yi-Lei Yang,2 Le-Qun Su,2 Xiao-Ling Wang,4 Evelyne Jacqz-Aigrain,5,6 Jun Zhou,7,* Wei Zhao1,2,*
1Department of Clinical Pharmacy, School of Pharmaceutical Sciences, Shandong University, Jinan, People’s Republic of China; 2Department of Pharmacy, Shandong Provincial Qianfoshan Hospital, The First Affiliated Hospital of Shandong First Medical University, Jinan, People’s Republic of China; 3Department of Respiratory Disease, Shandong Provincial Qianfoshan Hospital, The First Affiliated Hospital of Shandong First Medical University, Jinan, People’s Republic of China; 4Clinical Research Center, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China; 5Department of Pediatric Pharmacology and Pharmacogenetics, Hôpital Robert Debré, AP-HP, Paris, France; 6University Paris Diderot, Sorbonne Paris-Cité, Paris, France; 7Clinical Training Center, Shandong Provincial Qianfoshan Hospital, The First Affiliated Hospital of Shandong First Medical University, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Zhou
Clinical Training Center, Shandong Provincial Qianfoshan Hospital, The First Affiliated Hospital of Shandong First Medical University, 16766 Jing Shi Street, Jinan 250014, People’s Republic of China
Tel/Fax +86 531 8237 3200
Email [email protected]
Wei Zhao
Department of Clinical Pharmacy, School of Pharmaceutical Sciences, Shandong University, 44 Culture West Road, Jinan 250012, People’s Republic of China
Tel/Fax +86 531 8838 3308
Email [email protected]
Background: Montelukast, a potent oral selective leukotriene-receptor antagonist, inhibits the action of cysteinyl-leukotriene in patients with asthma. Although pharmacokinetic studies of montelukast have been reported in Caucasian adults and children, and showed large inter-individual variability on pharmacokinetics, none of these studies has been explored in Chinese children. Given the potential inter-ethnic difference, the purpose of the present study was to evaluate the effects of developmental factors and pharmacogenetics of CYP2C8 and SLCO2B1 on montelukast clearance in Chinese pediatric patients.
Methods: After the administration of montelukast, blood samples were collected from children and plasma concentrations were determined using an adapted micro high-performance liquid chromatography coupled with the fluorescence detection (HPLC-FLD) method. A previously published pharmacokinetic model was validated using the opportunistic pharmacokinetic samples, and individual patient’s clearance was calculated using the validated model. Population pharmacokinetic analysis was performed using a nonlinear mixed-effects model approach (NONMEM V 7.2.0) and variants of CYP2C8 and SLCO2B1 were genotyped.
Results: Fifty patients (age range: 0.7–10.0 years) with asthma were enrolled in this study. The clearance of montelukast was significantly higher in children with the SLCO2B1 c.935GA and c.935AA genotypes compared with that of children with the SLCO2B1 c.935GG genotype (0.94 ± 0.26 versus 0.77 ± 0.21, p = 0.020). The patient’s weight was also found to be significantly corrected with montelukast clearance (p <0.0001).
Conclusion: The developmental pharmacology of montelukast in Chinese children was evaluated. Weight and SLCO2B1 genotype were found to have independent significant impacts on the clearance of montelukast.
Keywords: montelukast, ontogeny, pharmacogenetics, children
Introduction
Montelukast is a selective leukotriene-receptor antagonist (LTRA) that blocks the action of cysteinyl leukotrienes, and its efficacy in the control of symptoms associated with asthma has been previously demonstrated in adults1 and children.2–5 Convenience in administration (once-daily), good tolerance and improved adherence make montelukast a good option for monotherapy or as an add-on therapy of asthma in pediatric patients.6 Currently, the 4 mg (granules or chewable tablet) and 5 mg (chewable tablets) dose formulations have been identified as a prophylactic or long-term treatment option for asthma in children.
Montelukast is absorbed well after oral administration, and oral bioavailability (chewable tablets) is approximately 73%.7 Moreover, montelukast undergoes extensive metabolism and a majority of the metabolites are excreted through bile. Previous studies have shown that the enzymes of cytochrome P450 (CYP) 2C8, 2C9, 3A4 and uridine diphosphate-glucuronosyltransferase (UGT) 1A3 are involved in the metabolism of montelukast.8–12 Meanwhile, some reports have suggested that the organic anion transporting polypeptide (OATP) 1B1, 1B3, and 2B1 transporters play a part in the permeability of montelukast.13,14
In clinical practice, the effectiveness of montelukast shows large inter-individual variability.15,16 Variations among genes encoding the metabolizing enzymes or membrane transporters8–11,13,14,17 were correlated with the clinical response to montelukast.18,19 For infants and children, the disposition of the drug is influenced by developmental changes in the body, such as organ maturation and function, as well as composition and amount of plasma proteins.20 Meanwhile, age-dependent changes in gastric emptying, intestinal enzyme activity and transporters in infants and children may also alter the bioavailability of a drug.21 In addition, some studies have suggested that CYP2C8 played an important role in the metabolism of montelukast;8–10 several other studies have demonstrated that a common polymorphism of SLCO2B1, the gene coding for the OATP2B1 transport protein, exhibited a substantial effect on the pharmacokinetics and pharmacodynamics of montelukast.13,17 Recently, a study reported that the pharmacokinetics of montelukast was determined by OATPs-mediated uptake, along with CYP2C8 metabolism.14 It should be noted that all these pharmacogenetic studies have been conducted in Caucasian adults. Given the impact of ontogeny and potential inter-ethnic difference, the objective of the present study was to evaluate the ontogeny and pharmacogenetic effects of CYP2C8 and SLCO2B1 on montelukast clearance in Chinese pediatric patients.
Methods
Subjects
The study was designed as a prospective, open-label pharmacokinetic-pharmacogenetic trial and was carried out at Shandong Provincial Qianfoshan Hospital. Patients aged between 6 months and 12 years, with a history of asthma or asthma-like symptoms (including but not limited to wheezing, cough and shortness of breath) who underwent routine treatment with montelukast were chosen for the study. Patients were excluded if they had been enrolled in another clinical trial, had other serious diseases or had been co-administrated with known inhibitors or inducers of CYP2C8 and SLCO2B1. The study protocol was approved by the ethics committees of the clinical institution (Shandong Provincial Qianfoshan Hospital). A written informed consent was obtained from the parents or guardians of the patients, prior to the initiation of the study. This trial was conducted in accordance with the Declaration of Helsinki. The Clinical Trial registration number is NCT03238560.
Dosage Regimen and Blood Sampling
Four milligram (0.5–5years) and 5 mg (6–12years) doses of chewable montelukast tablets (Singulair™, Merck Sharp & Dohme Limited, USA) were administered once-daily at bedtime without food. Since the volume of blood that could be obtained from individual participants was limited, an opportunistic sampling design was chosen for the collection of pharmacokinetic samples.22 Precise drug administration time and sampling time were recorded. A blood volume of 200 μL per sample was obtained at the steady-state condition from each patient for concentration determination. After centrifugation (4000 rpm, 10 min), the plasma samples were stored at −80°C and protected from light until analysis.
Determination of Montelukast
Montelukast plasma concentration was measured using a micro high-performance liquid chromatography with fluorescence detention (HPLC-FLD). Mefenamic acid was used as an internal standard. The standard of montelukast (lot numbers M568000) was purchased from the Toronto Research Chemicals (North York, Canada). The internal standard of mefenamic acid (lot number 100190–200903) was purchased from National Institutes for Food and Drug Control (Beijing, China). HPLC‐grade acetonitrile was obtained from Sigma‐Aldrich (St Louis, MO, USA). The sodium acetate trihydrate, triethylamine and glacial acetic acid were obtained from Sinopharm Chemical Reagent (Shanghai, China). HPLC separation is achieved on C18 column connected to a fluorescent detector with an excitation wavelength of 350nm and emission wavelength of 400nm, using acetonitrile and water (3.4g/L sodium acetate trihydrate, 0.005% triethylamine, pH 4.0) in a gradient mode of elution at a flow rate of 1.0 mL/min at 40℃. Acetonitrile was used to precipitate protein during the plasma extraction procedures. The recovery is 87.2%. The lower limit of quantification (LLOQ) of montelukast was 1.0 ng/mL. The calibration curve used in this study stretched over a range of 1.0–2000 ng/mL. The intra- and inter-day coefficients of variation (CVs) were assessed using quality control (QC) samples and were found to be 3.58% and 4.02%, respectively.
Genotyping
Isolation of DNA from blood was performed through standard methods using the TIANamp Blood DNA Kit (TIANGEN Biotech, Beijing, China). The single nucleotide polymorphisms (SNPs) of CYP2C8*1B (rs7909236) and SLCO2B1 c.935G>A (rs12422149) were selected for genotyping. Polymerase chain reaction (PCR) was performed on a Fluorescence Quantitative PCR (Bio-Rad, Hercules, CA, USA) using TaqMan® Universal PCR Master Mix and probes (Thermo Fisher Scientific, MA, USA).
Pharmacokinetic Analysis
Pharmacokinetic parameters were calculated using NONMEM software (ver. 7.2.0, Icon Development Solutions, Ellicott City, MD, USA). An external validation of the previously published model in children was performed at the first step; then, the validated model was used to derive individual clearance of montelukast for the following pharmacogenetic analysis. A linear three-compartment pharmacokinetic model23 with 73% bioavailability of the chewable tablets7 and a constant absorption rate (Ka) of 0.5L/h24 was evaluated. The following modeling information was extracted from the published article: interindividual variability for clearance (CL), central compartment volume of distribution (Vc) and peripheral volume of distribution (Vp) (% coefficient of variation [%CV], 24.2%, 14.0% and 45.4%, respectively) by exponential error model, residual variability using a combined additive and proportional model (%CV, 3.7 ng/mL and 10.4%) and a significant influence of current weight (CW) on CL and Vc, VP1, VP2, Q1, Q2 with the following regression equations (equations 1–6):
(1) (2) (3) (4) (5) (6)
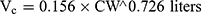
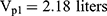

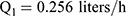

The individual concentrations were predicted by Bayesian estimation (MAXEVAL = 0 in the estimation step, where MAXEVAL is the maximum number of model evaluations that can be used) with NONMEM using the population pharmacokinetic parameters.
Statistical Analysis
Statistical analyses were performed using SPSS statistical software version 16.0 (IBM SPSS Statistics, IBM Inc., NY, USA). The Hardy–Weinberg equilibrium was verified for CYP2C8*1B (rs7909236) and SLCO2B1 c.935G>A (rs12422149) genotypes. The medians and ranges of continuous variables were summarized, and linear regression analysis was carried out. Frequencies and proportions were used to summarize categorical variables, while the Mann–Whitney U-test was used to compare the variance between groups (patients with homozygous wild-type alleles and patients with heterozygotes and homozygous alleles). Multiple linear regression analysis with entry criteria of 0.20 and exit criteria of 0.05 in a stepwise selection method was performed to determine the association between multiple factors and montelukast clearance.
Results
Fifty patients were enrolled in this study. All patients met the inclusion and exclusion criteria and written informed consent for their participation was obtained. Eight patients use montelukast as monotherapy and 42 patients with co-medication of loratadine. No drug-related adverse events were shown to have a causal association with Montelukast therapy. The mean (standard deviation) age was 4.4 (2.4) (range 0.7–10.0) years and the mean (standard deviation) weight was 19.9 (8.4) (range 7.0–49.0) kilograms. The characteristics of patients are summarized and presented in Table 1. The opportunistic sampling time ranged from 0.5 to 24.8 hrs post-dose. The external validation of the developed model showed a good predictive performance (Figure 1). The average prediction bias was 7.1%, and 74.1% of the measurements were within ±50% of the prediction error.
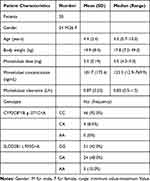 |
Table 1 Characteristics of the 50 Children |
 |
Figure 1 External validation of the published model. X-axis: DV observed concentrations (µg/mL). Y-axis: IPRED individual predicted concentrations (µg/mL). |
All 50 patients who participated in the study were genotyped for CYP2C8*1B g.-271C A (rs7909236) and SLCO2B1 c.935G
A (rs7909236) and SLCO2B1 c.935G A (rs12422149) polymorphism. The frequencies of CYP2C8*1B and SLCO2B1 c.935G
A (rs12422149) polymorphism. The frequencies of CYP2C8*1B and SLCO2B1 c.935G A were 8.0% and 58.0%, respectively. For CYP2C8*1B, the frequency is comparable to that of published data in Chinese populations.25 All genotypes were in Hardy–Weinberg equilibrium. The results are summarized in Table 1.
A were 8.0% and 58.0%, respectively. For CYP2C8*1B, the frequency is comparable to that of published data in Chinese populations.25 All genotypes were in Hardy–Weinberg equilibrium. The results are summarized in Table 1.
The pharmacokinetics of montelukast was found to be significantly correlated with SLCO2B1 genotype. Montelukast clearance was significantly higher in SLCO2B1 c.935GA and c.935AA subjects compared with that of SLCO2B1 c.935GG subjects (0.94 ± 0.26 versus 0.77 ± 0.21, Mann–Whitney U-test, p = 0.020) (Figure 2). Weight was also found to be significantly correlated with montelukast clearance (p 0.0001) (Figure 3). In final, multiple linear regression analyses revealed that the distinctive factors that are associated with montelukast clearance include weight (p = 0.035) and SLCO2B1 c.935G>A genotype (p = 0.045). These two factors could explain about 44.1% of the variability in montelukast clearance (R2 = 0.464). Weight and SLCO2B1 genotype explained 60.2% and 24.9% strength of association, respectively. The two SLCO2B1 genotype groups are comparable in terms of age, weight, dose and co-treatment (Table 2).
0.0001) (Figure 3). In final, multiple linear regression analyses revealed that the distinctive factors that are associated with montelukast clearance include weight (p = 0.035) and SLCO2B1 c.935G>A genotype (p = 0.045). These two factors could explain about 44.1% of the variability in montelukast clearance (R2 = 0.464). Weight and SLCO2B1 genotype explained 60.2% and 24.9% strength of association, respectively. The two SLCO2B1 genotype groups are comparable in terms of age, weight, dose and co-treatment (Table 2).
 |
Figure 2 Relationship between SLCO2B1 genotype and Montelukast clearance (n = 50, p = 0.020). X-axis: SLCO2B1 genotype (c.935GG vs c.935GA and c.935AA). Y-axis: Montelukast clearance (L/h). |
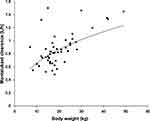 |
Figure 3 Relationship between body weight and Montelukast clearance (n = 50, p |
 |
Table 2 Comparison Between SLCO2B1 Genotypes Groups |
Discussion
The developmental pharmacogenetics of montelukast was evaluated for the first time in Chinese children. The independent impacts of weight and SLCO2B1 genotype on montelukast clearance were demonstrated.
In our present study, montelukast clearance showed considerable inter-individual variability that may partly be due to the variants of SLCO2B1 c.935G>A genotypes among patients. Our findings suggest that SLCO2B1 c.935G>A genetic polymorphism has a significant influence on montelukast clearance, and that the mean clearance increased by 22.1% in c.935GA and c.935AA groups compared with that of the c.935GG group. The increase in montelukast clearance is reflected through lower concentrations of montelukast, resulting in decreased AUC. This result is consistent with previous studies on adults.13,17 In addition, previous data indicated that subjects with the c. 935GG genotype had a better clinical response in montelukast add-on therapy compared with those with the c.935GA and c.935AA genotypes, after one and six months of therapy, respectively.13 The present results showed that subjects with c.935GA and c.935AA genotypes had lower plasma levels of montelukast, which may result in poor efficacy, and this factor should be considered in order to optimize the dosing of montelukast during therapy. Some previous studies showed a negative impact of SLCO2B1 polymorphisms on the pharmacokinetics of montelukast.26,27 The reasons for this discrepancy can be speculated as follows. As shown in Table 1, the allele frequency of c.935G>A in the Chinese population is 58.0%, which is higher than that in other ethnic groups: 8.18% for Caucasians, 13.6% for African-Americans,17 13.2% for Finnish26 and 42.7% for Koreans.27 Furthermore, the number of participants in previous studies was between 24 and 33 individuals. Therefore, it can be ruled out that a limited number of participants may have yielded false-negative results.
In addition to the SLCO2B1 c.935G>A polymorphism, we also evaluated CYP2C8 polymorphism. The results showed no effect of CYP2C8 polymorphism on Montelukast clearance was detected, even though it was estimated that 80% of the montelukast metabolism is mediated by CYP2C8.9 Previous studies have suggested that CYP2C8, especially CYP2C8*3 and CYP2C8*4 alleles, play an important role in the inter-individual variability of many drugs.25,28,29 However, the approximate allele frequencies of CYP2C8*3 and CYP2C8*4 in Han Chinese are nearly zero. For CYP2C8*1B (rs7909236), the allele frequency in our study was found to be only 8%, which is lower than that in Caucasians (24%).30 Moreover, it has been suggested that except for an increase in promoter activity and transcription factor binding, there was no difference in protein expression between CYP2C8*1B variant allele and wild-type allele.25,30 These factors may explain why CYP2C8 variants were not found to be significantly associated with montelukast clearance.
The impact of weight on montelukast clearance is in agreement with the results of a previous pharmacokinetic study in children.23 The clearance of montelukast increased allometrically along with body weight.
However, there are some limitations in our present study. Since the primary objective was to evaluate the ontogeny and pharmacogenetic effects of CYP2C8 and SLCO2B1 on montelukast pharmacokinetics in Chinese pediatric patients, the effectiveness was not evaluated. In addition, the unexplained variability in our study is still large, indicating that other covariates still contribute to the pharmacokinetics of montelukast. These missing covariates also require further evaluation.
In conclusion, the developmental pharmacogenetics of montelukast in Chinese children was evaluated. Weight and SLCO2B1 genotype were found to have independent significant impacts on the montelukast clearance. Our results emphasize the importance of evaluating the developmental pharmacology of montelukast in inter-ethnic populations.
Data Sharing Statement
The pharmacogenetic data can be shared by emailing requests to the corresponding author.
Acknowledgments
The abstract of this paper was presented at the European Society for Developmental Perinatal and Paediatric Pharmacology Congress as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Archives of Disease in Childhood.
Disclosure
WZ reports grants from the National Science and Technology Major Project for Major New Drugs Innovation and Development, the Young Taishan Scholars Program of Shandong Province, and the Qilu Young Scholars Program of Shandong University, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Reiss TF, Chervinsky P, Dockhorn RJ, et al. Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch Intern Med. 1998;158:1213–1220. doi:10.1001/archinte.158.11.1213
2. Knorr B, Larson P, Nguyen HH, et al. Montelukast dose selection in 6- to 14-year-olds: comparison of single-dose pharmacokinetics in children and adults. J Clin Pharmacol. 1999;39:786–793. doi:10.1177/00912709922008434
3. Knorr B, Nguyen HH, Kearns GL, et al. Montelukast dose selection in children ages 2 to 5 years: comparison of population pharmacokinetics between children and adults. J Clin Pharmacol. 2001;41:612–619. doi:10.1177/00912700122010492
4. Migoya E, Kearns GL, Hartford A, et al. Pharmacokinetics of montelukast in asthmatic patients 6 to 24 months old. J Clin Pharmacol. 2004;44:487–494. doi:10.1177/0091270004264970
5. Knorr B, Maganti L, Ramakrishnan R, et al. Pharmacokinetics and safety of montelukast in children aged 3 to 6 months. J Clin Pharmacol. 2006;46:620–627. doi:10.1177/0091270006288324
6. Muijsers RB, Noble S. Montelukast: a review of its therapeutic potential in asthma in children 2 to 14 years of age. Paediatr Drugs. 2002;4:123–139. doi:10.2165/00128072-200204020-00005
7. Knorr B, Holland J, Schwartz J. Clinical pharmacology of montelukast. Clin Exp Allergy Rev. 2001;1:254–260. doi:10.1046/j.1472-9725.2001.t01-1-00011.x
8. Filppula AM, Laitila J, Neuvonen PJ, et al. Reevaluation of the microsomal metabolism of montelukast: major contribution by CYP2C8 at clinically relevant concentrations. Drug Metab Dispos. 2011;39:904–911. doi:10.1124/dmd.110.037689
9. Karonen T, Neuvonen PJ, Backman JT. CYP2C8 but not CYP3A4 is important in the pharmacokinetics of montelukast. Br J Clin Pharmacol. 2012;73:257–267. doi:10.1111/j.1365-2125.2011.04086.x
10. Cardoso Jde O, Oliveira RV, Lu JB, et al. In vitro metabolism of montelukast by cytochrome P450s and UDP-glucuronosyltransferases. Drug Metab Dispos. 2015;43:1905–1916. doi:10.1124/dmd.115.065763
11. Chiba M, Xu X, Nishime JA, et al. Hepatic microsomal metabolism of montelukast, a potent leukotriene D4 receptor antagonist, in humans. Drug Metab Dispos. 1997;25:1022–1031.
12. Hirvensalo P, Tornio A, Neuvonen M, et al. Comprehensive pharmacogenomic study reveals an important role of UGT1A3 in montelukast pharmacokinetics. Clin Pharmacol Ther. 2018;104:158–168. doi:10.1002/cpt.v104.1
13. Mougey EB, Feng H, Castro M, et al. Absorption of montelukast is transporter mediated: a common variant of OATP2B1 is associated with reduced plasma concentrations and poor response. Pharmacogenet Genomics. 2009;19:129–138. doi:10.1097/FPC.0b013e32831bd98c
14. Varma MV, Kimoto E, Scialis R, et al. Transporter-mediated hepatic uptake plays an important role in the pharmacokinetics and drug-drug interactions of montelukast. Clin Pharmacol Ther. 2017;101:406–415. doi:10.1002/cpt.v101.3
15. Lima JJ. Treatment heterogeneity in asthma: genetics of response to leukotriene modifiers. Mol Diagn Ther. 2007;11:97–104. doi:10.1007/BF03256228
16. Weiss ST, Litonjua AA, Lange C, et al. Overview of the pharmacogenetics of asthma treatment. Pharmacogenomics J. 2006;6:311–326. doi:10.1038/sj.tpj.6500387
17. Mougey EB, Lang JE, Wen X, et al. Effect of citrus juice and SLCO2B1 genotype on the pharmacokinetics of montelukast. J Clin Pharmacol. 2011;51:751–760. doi:10.1177/0091270010374472
18. Telleria JJ, Blanco-Quiros A, Varillas D, et al. ALOX5 promoter genotype and response to montelukast in moderate persistent asthma. Respir Med. 2008;102:857–861. doi:10.1016/j.rmed.2008.01.011
19. Tantisira KG, Lima J, Sylvia J, et al. 5-lipoxygenase pharmacogenetics in asthma: overlap with Cys-leukotriene receptor antagonist loci. Pharmacogenet Genomics. 2009;19:244–247. doi:10.1097/FPC.0b013e328326e0b1
20. Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi:10.1056/NEJMra035092
21. Kearns GL, Lu S, Maganti L, et al. Pharmacokinetics and safety of montelukast oral granules in children 1 to 3 months of age with bronchiolitis. J Clin Pharmacol. 2008;48:502–511. doi:10.1177/0091270008314251
22. Leroux S, Turner MA, Guellec CB, et al. Pharmacokinetic studies in neonates: the utility of an opportunistic sampling design. Clin Pharmacokinet. 2015;54:1273–1285. doi:10.1007/s40262-015-0291-1
23. Ramakrishnan R, Migoya E, Knorr B. A population pharmacokinetic model for montelukast disposition in adults and children. Pharm Res. 2005;22:532–540. doi:10.1007/s11095-005-2493-y
24. Zhou W, Johnson TN, Bui KH, et al. Predictive Performance of Physiologically Based Pharmacokinetic (PBPK) modeling of drugs extensively metabolized by major cytochrome P450s in children. Clin Pharmacol Ther. 2018;104:188–200. doi:10.1002/cpt.v104.1
25. Aquilante CL, Niemi M, Gong L, et al. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 8. Pharmacogenet Genomics. 2013;23:721–728. doi:10.1097/FPC.0b013e3283653b27
26. Tapaninen T, Karonen T, Backman JT, et al. SLCO2B1 c.935G>A single nucleotide polymorphism has no effect on the pharmacokinetics of montelukast and aliskiren. Pharmacogenet Genomics. 2013;23:19–24. doi:10.1097/FPC.0b013e32835bac90
27. Kim KA, Lee HM, Joo HJ, et al. Effects of polymorphisms of the SLCO2B1 transporter gene on the pharmacokinetics of montelukast in humans. J Clin Pharmacol. 2013;53:1186–1193. doi:10.1002/jcph.144
28. Bahadur N, Leathart JB, Mutch E, et al. CYP2C8 polymorphisms in Caucasians and their relationship with paclitaxel 6alpha-hydroxylase activity in human liver microsomes. Biochem Pharmacol. 2002;64:1579–1589. doi:10.1016/S0006-2952(02)01354-0
29. Tornio A, Niemi M, Neuvonen PJ, et al. Trimethoprim and the CYP2C8*3 allele have opposite effects on the pharmacokinetics of pioglitazone. Drug Metab Dispos. 2008;36:73–80. doi:10.1124/dmd.107.018010
30. Rodriguez-Antona C, Niemi M, Backman JT, et al. Characterization of novel CYP2C8 haplotypes and their contribution to paclitaxel and repaglinide metabolism. Pharmacogenomics J. 2008;8:268–277. doi:10.1038/sj.tpj.6500482
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

