Back to Journals » Infection and Drug Resistance » Volume 15
Development of Rheumatoid Arthritis in Cavitary Mycobacterium avium Pulmonary Disease: A Case Report of Successful Treatment with CTLA4-Ig (Abatacept)
Authors Tanaka H , Asakura T , Kikuchi J , Ishii M , Namkoong H, Kaneko Y, Fukunaga K, Hasegawa N
Received 17 October 2021
Accepted for publication 14 December 2021
Published 11 January 2022 Volume 2022:15 Pages 91—97
DOI https://doi.org/10.2147/IDR.S343763
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Hiromu Tanaka,1 Takanori Asakura,1 Jun Kikuchi,2 Makoto Ishii,1 Ho Namkoong,3 Yuko Kaneko,2 Koichi Fukunaga,1 Naoki Hasegawa3
1Division of Pulmonary Medicine, Department of Medicine, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan; 2Division of Rheumatology, Department of Medicine, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan; 3Department of Infectious Diseases, Keio University School of Medicine, Shinjuku-ku, Tokyo, Japan
Correspondence: Takanori Asakura
Division of Pulmonary Medicine, Department of Medicine, Keio University School of Medicine, 35 Shinanomachi, Shinjuku, Tokyo, 160-8582, Japan
Tel +81-3-3353-1211
Fax +81-3-3353-2502
Email [email protected]
Background: Nontuberculous mycobacterial pulmonary disease (NTM-PD) often develops in patients with rheumatoid arthritis (RA), especially during immunosuppressive treatment, including biological disease-modifying antirheumatic drugs. NTM-PD is associated with airway lesions such as bronchiectasis, which is frequently seen in RA patients. Distinguishing which diseases cause the pulmonary lesion is difficult. However, there are limited reports of the development of RA during the follow-up of NTM-PD and how biological agents should be administered in these conditions, especially with cavitary lesions.
Case Presentation: A 62-year-old woman with hemosputum was referred to our hospital, where she was diagnosed with Mycobacterium avium pulmonary disease. She began treatment with several antibiotics, including clarithromycin, ethambutol, rifampicin, and amikacin. In the course of treatment, M. avium became macrolide-resistant. Five years after beginning antibiotic treatment, she felt arthralgia in the fingers and wrists and had a high titer of rheumatoid factor and anticitrullinated peptide antibody, with which we diagnosed RA. Methotrexate, prednisolone, and iguratimod were subsequently administered, but the activity of RA gradually worsened. Meanwhile, M. avium changed to a macrolide-susceptible strain, her sputum smear results remained almost negative, and the NTM-PD disease was well controlled with antimicrobial therapy, despite her having cavitary lesions. Therefore, we started using CTLA4-Ig (abatacept). RA symptoms were substantially ameliorated. The pulmonary lesions and NTM-PD worsened mildly, but her pulmonary symptoms were stable.
Conclusion: Physicians should be mindful of the etiologies of bronchiectasis, including RA, even in patients with a long-term history of treatment for bronchiectasis and NTM-PD. When NTM-PD is well controlled, even with remaining cavitary lesions, abatacept may be an option for patients with RA based on a comprehensive assessment of disease progression using NTM sputum smear/culture, computed tomography findings, and treatment response.
Keywords: rheumatoid arthritis, nontuberculous mycobacterial infection, cavitary lesion, biological agent, bronchiectasis
Background
The incidence rate of nontuberculous mycobacterial (NTM) pulmonary disease (NTM-PD) is increasing.1 The most common species is the Mycobacterium avium complex (MAC), which includes M. avium and M. intracellulare.2 MAC pulmonary disease (MAC-PD) usually worsens slowly. Some patients present with chronic progressive disease with respiratory failure, affecting health-related quality of life and survival.3,4
NTM-PD typically presents as bronchiectasis on computed tomography images. The US Bronchiectasis Registry (BRR) reports that 63% of patients with non-cystic fibrosis bronchiectasis (NCFB) have had a history of NTM-PD or NTM isolation, indicating a close association between NCFB and NTM-PD.5 NCFB has various etiologies such as airway obstruction, cigarette smoking, pulmonary infection, allergic bronchopulmonary aspergillosis (ABPA), and connective tissue diseases such as rheumatoid arthritis (RA).6 RA is a progressive and systemic autoimmune disease characterized by chronic symmetrical erosive synovitis. Pleuropulmonary manifestations of RA include interstitial lung disease, pleural effusions, rheumatoid nodules, and airway complications, including bronchiolitis and bronchiectasis.7 Among these etiologies, bronchiectasis, in particular, has high disease activity and severity and poor prognosis in patients with RA.8,9 NTM-PD is also often complicated with RA.10 However, it is difficult to distinguish whether bronchiectasis is caused by RA, NTM-PD, or sometimes both.
Physicians often attempt to investigate the complication of NTM-PD in RA patients because an underlying infection can influence the decision against the use of immunosuppressive treatment, including biological disease-modifying antirheumatic drugs (bDMARDs). There are several reports of NTM-PD being diagnosed after initiating treatment for RA,10,11 including the use of bDMARDs.12 However, there are few reports of RA being newly diagnosed in NTM-PD patients. Furthermore, little is known about the feasibility of using biological agents against RA complicated with NTM-PD, especially in severe phenotypes showing cavitary lesions.13,14 In this paper, we report a patient diagnosed with RA after long-term treatment for cavitary NTM-PD due to M. avium. The patient had cavitary lesions, although the administration of abatacept for RA, along with multiple antimicrobial therapies against M. avium pulmonary disease, achieved disease control.
Case Presentation
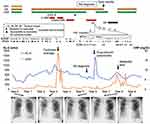
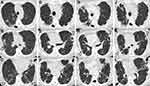
Figure 1 shows the overall course summary. A 62-year-old woman was referred to our hospital for refractory MAC-PD diagnosed 3 years before the referral, which had been treated with several antibiotics (details unknown) for a few months. However, she complained of hemosputum. She had no other pulmonary diseases and had never smoked cigarettes. Chest radiography showed some cavitary lesions in the right lung field and consolidation in both lower lung fields (Figure 1A). Chest computed tomography (CT) imaging revealed multiple cavitary lesions in the bilateral lower lobes and consolidation in the lingula with bronchiectasis (Figure 2A–C). The laboratory examination revealed elevated serum Krebs von den Lungen-6 (KL-6) levels of 608 U/mL (reference range, <500 U/mL), which is useful to monitor disease activity in MAC-PD and interstitial lung disease.15,16 Her sputum culture grew M. avium with resistance to clarithromycin twice immediately after the referral.
We administered antibiotic therapy with clarithromycin (800 mg/day), ethambutol (750 mg/day), and rifampicin (600 mg/day). After 8 months of this therapy, her hemosputum improved, but liver function impairment gradually developed, which might have been caused by the antibiotics. The three-drug therapy was interrupted. However, consolidation with bronchiectasis in the bilateral lower lung fields had worsened approximately 1 year after the interruption of antibiotics. We resumed clarithromycin and ethambutol, followed by intravenous amikacin (600 mg, three times weekly).
Four months after the treatment, she developed secondary pyothorax due to M. avium (Figure 1B), which was successfully treated with drainage for several weeks. After the treatment, she was treated with multiple antibiotic therapies, including clarithromycin, ethambutol, inhaling amikacin (600 mg/day), or sitafloxacin (200 mg/day). These antibiotics maintained an improvement in her symptoms, including hemosputum and cough.
Six years after the referral, the patient began to feel arthralgia in her fingers that gradually worsened. At that time, she had multiple swollen finger joints, and her blood test showed a high titer of rheumatoid factor (RF) (437 IU/mL) and anticitrullinated peptide antibody (ACPA) (442.0 U/mL). Ultrasound examination indicated multiple and bisymmetrical synovitis of her joints with a clinical disease activity index (CDAI) as high as 21.3, suggesting RA. Prior to the RA diagnosis, RF had been measured and was increasing: 59 IU/mL two years ago, 248 IU/mL a year and a half ago, and 311 IU/mL a year ago. At the diagnosis of RA, chest radiography showed worsening bilateral consolidation in both lower lung fields (Figure 1C), and her chest CT images still showed several cavitary lesions and bronchiectasis (Figure 2D–F). She was administered methotrexate (8 mg/week) and prednisolone (5 mg/day). After several months of using these drugs, drug-induced pneumonitis developed, which might have been caused by methotrexate. We changed methotrexate to iguratimod (25 mg/day) and temporarily added a high prednisolone dose (maximum 40 mg/day, gradually decreased to 10 mg/day as the maintenance dose). For a while, iguratimod with prednisolone therapy was effective for RA, with low CDAI.
Regarding the therapy for macrolide-resistant M. avium, combination therapy that included sitafloxacin, inhaled amikacin, and ethambutol improved lung lesions and sputum culture conversion. The regimen was changed to clarithromycin and ethambutol therapy for macrolide-susceptible M. avium, followed by erythromycin (200 mg/day), owing to fasciculitis optica and a stable disease state. One year later, joint pain in her fingers deteriorated with an elevated C-reactive protein level and active synovitis, as revealed by ultrasound, with CDAI reaching 9.8.
The patient began using abatacept (500 mg every 4 weeks) for RA because her pulmonary symptoms and lesions had not changed, despite the presence of cavitary lesions (Figures 1D and 2G–I). Most of the sputum smear test results had been negative for approximately 3 years, and the bacterial burden in sputum was apparently lower than before (Figure 1). Clarithromycin (800 mg/day) and ethambutol (250 mg/day) were administered simultaneously. Several cavitary lesions and consolidation in the bilateral lung lobes had worsened mildly (Figures 1E and 2J–L), although her pulmonary symptoms were stable, with low CDAI, and the sputum smear results remained negative.
Discussion and Conclusions
This case report describes the successful treatment of emerging RA with abatacept in a patient with cavitary M. avium pulmonary disease. On referral, the patient had bronchiectasis with cavitary lesions, which was refractory to clarithromycin-containing therapy, and had developed macrolide resistance. She developed pyothorax complications during the follow-up, which suggested a severe phenotype of NTM-PD.17,18 She developed RA complicated by drug-induced pneumonitis; however, abatacept therapy for RA combined with multiple microbial therapies for M. avium resulted in disease control, despite the patient having a cavitary lesion. This case highlights the development of RA in patients with bronchiectasis, including NTM-PD. Abatacept therapy with antimicrobial therapy may be a treatment option for RA patients with severe NTM-PD complicated with cavitary lesions.
The etiologies of bronchiectasis are sometimes identified during the clinical course. Among the background diseases, NTM-PD can be the cause of and the result of bronchiectasis because airway clearance is impaired, and established organisms such as NTM are more easily detected in the presence of bronchiectasis.19 Thus, in cases of definite NTM-PD or in cases of bronchiectasis in which NTMs are isolated, it is important to consider the possibility that there may be hidden background diseases other than NTM-PD that cause bronchiectasis. Previous reports show that antineutrophil cytoplasmic antibody-associated vasculitis and ABPA developed during progressive impairment by bronchiectasis with NTM-PD.20,21,22 In our patient, CT imaging on referral showed bronchiectasis, several cavitary lesions, bronchiolitis, nodular lesions, and consolidation, all of which may have been formed by NTM at that time, although these features could be complications of RA (early extra-articular manifestations). However, positive sputum culture, along with cavitary lesions and infiltrative shadows, which are not typically seen in RA,23 were seen at the time of referral. These findings suggest that NTM-PD was the main source of these lesions. Therefore, in cases of bronchiectasis, it is easy to assume that NTM-PD is the cause of the disease because of NTM isolation. However, it is important to monitor the disease course while paying attention to the background.
Our case showed high RF (59 IU/mL) two years before RA diagnosis, followed by a gradual increase (248 and 311 IU/mL 1.5 and 1 year before the diagnosis, respectively). Pulmonary diseases associated with RA, including bronchiectasis, may precede joint symptoms. RA-related autoantibodies were detected in the sputum samples of patients with early RA, suggesting that lung inflammation plays an important role in the pathogenesis of RA.24 Additionally, serum ACPA levels were higher in bronchiectasis patients without RA compared to those in healthy individuals, suggesting that chronic airway disease may be sufficient for the generation of ACPA, even in the absence of a systemic immune response.25 Taken together, RA-related autoimmune reactions may be initiated by airway and alveolar epithelial cell injury triggered by exposure to environmental factors, including NTM, via the airway mucosa. Clinically, RF measurement is useful for diagnosing RA, and high serum RF levels are associated with a higher long-term risk for RA.26 A previous study showed that RF positivity is high in the bronchiectasis population, and 50% of both RF and ACPA-positive patients later develop RA,27 suggesting that screening for RF and ACPA may identify high-risk patients likely to develop RA subsequently.
The use of bDMARDs to treat RA patients with NTM-PD is clinically important. The TNF response, one of the major targets by bDMARDs, is important in suppressing the activity of NTM organisms.28 NTM-PD occurs in patients using anti-TNFα antibodies, especially in patients with RA.12 A previous report from the United States indicated that the incidence rate of NTM-PD in RA patients is increased with the use of bDMARDs.10 The most common cause of death in RA patients with NTM-PD is the exacerbation of NTM-PD;29 therefore, bDMARDs such as anti-TNFα therapy are not recommended for use in RA patients with NTM-PD in the United States.30 Japanese postmarketing studies have reported a lower incidence of NTM-PD with abatacept (CTLA4-Ig fusion protein) (0.05%) than with etanercept (a TNFR2 dimeric fusion protein) (0.12%), adalimumab (a fully human monoclonal antibody) (0.1%), and tocilizumab (anti-IL-6R monoclonal antibody) (0.2%).31 However, there are few reports on the development of NTM-PD during the use of these biological agents, other than anti-TNFα antibodies.
bDMARDs may affect the development of NTM-PD in RA patients, although several reports suggest that bDMARDs can be resumed in RA patients with adequate control of NTM-PD. In two RA patients who developed MAC-PD with cavitary lesions after treatment with tocilizumab, the appropriate antimicrobial therapy and/or surgical resections, followed by the resumption of tocilizumab, controlled both diseases properly.32,33 In another case of MAC-PD with cavitary lesions that developed while the patient was receiving etanercept, MAC-PD was controlled by introducing antimicrobial therapy with continuing etanercept.34 Mori et al35 additionally reported that tocilizumab was newly administered in two cases of RA complicated by MAC-PD. Both patients did not have cavitary lesions, and the sputum NTM culture results were negative after antimicrobial therapy. MAC-PD notably worsened after tocilizumab administration, but MAC-PD was controlled by the discontinuation of tocilizumab and the introduction of antimicrobial therapy. Taken together with our case, we believe that concomitant antimicrobial therapy is important when introducing biological agents for RA in patients with NTM-PD. Furthermore, a recent single-center study showed that bDMARDs were not significantly associated with a greater exacerbation of NTM-PD in RA patients with bDMARDs compared to those without it.36 Thus, these case reports suggested that the temporary discontinuation of biological agents and appropriate antimicrobial therapy can control NTM-PD and provide room for the reintroduction of biological agents. In our patient, NTM-PD was controlled by long-term antimicrobial therapy. Therefore, we introduced abatacept without causing NTM-PD exacerbation, even though the patient had MAC-PD complicated by cavitary lesions. Important findings at the time of abatacept administration were that cavitary lesions were present but did not increase and that sputum smear results revealed apparent decrease in the amount of bacteria. In addition, at that time, the use of multiple antibiotics to control MAC-PD was also terminated and the patient was ultimately prescribed erythromycin only (Figure 1). The use of biological agents can be considered for RA patients when cavitary NTM-PD is well controlled by comprehensively considering the sputum culture, history of antimicrobial therapy, and the type of biological agents to be used.
In conclusion, our case suggests that physicians should consider the etiologies of bronchiectasis, even in patients with a long history of treatment for bronchiectasis and NTM-PD. When severe NTM-PD is well controlled under antimicrobial therapy, even with remaining cavitary lesions, abatacept may be an option for patients with RA, based on a comprehensive assessment of disease progression, as revealed by NTM sputum smear/culture findings, CT findings, and treatment response.
Abbreviation
ABPA, allergic bronchopulmonary aspergillosis; ACPA, anticitrullinated peptide antibody; CT, computed tomography; ILD, interstitial lung disease; KL-6, Krebs von den Lungen-6; MAC, Mycobacterium avium complex; MAC-PD, MAC pulmonary disease; NCFB, non-cystic fibrosis bronchiectasis; NTM, nontuberculous mycobacteria; NTM-PD, NTM pulmonary disease; RA, rheumatoid arthritis; RF, rheumatoid factor; TNF, tumor necrosis factor.
Consent to Publish
The patient provided written informed consent to publish the case details and any accompanying images.
Author Contributions
HT and TA drafted the manuscript. JK and NH provided patent care and supervised the manuscript revision. All authors contributed to data analysis and drafting and revision of the article. All authors also agreed on the journal to which the article will be submitted, approved the final version to be published, and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
Dr Jun Kikuchi reports personal fees from Bristol-Myers Squibb, Abbie GK, Chugai Pharmaceutical Co. Ltd., Eisai Co. Ltd, Asahikasei Pharma Corp, Mitsubishi Tanabe Pharma Co, Eli Lilly Japan KK, Janssen Pharmaceutical KK, and Sanofi KK, outside the submitted work. The authors declare that they have no other competing interests.
References
1. Namkoong H, Kurashima A, Morimoto K, et al. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan. Emerg Infect Dis. 2016;22(6):1116–1117. doi:10.3201/eid2206.151086
2. Morimoto K, Hasegawa N, Izumi K, et al. A laboratory-based analysis of nontuberculous mycobacterial lung disease in Japan from 2012 to 2013. Ann Am Thorac Soc. 2017;14(1):49–56. doi:10.1513/AnnalsATS.201607-573OC
3. Asakura T, Funatsu Y, Ishii M, et al. Health-related quality of life is inversely correlated with C-reactive protein and age in Mycobacterium avium complex lung disease: a cross-sectional analysis of 235 patients. Respir Res. 2015;16:145. doi:10.1186/s12931-015-0304-5
4. Vinnard C, Longworth S, Mezochow A, Patrawalla A, Kreiswirth BN, Hamilton K. Deaths related to nontuberculous mycobacterial infections in the United States, 1999–2014. Ann Am Thorac Soc. 2016;13(11):1951–1955. doi:10.1513/AnnalsATS.201606-474BC
5. Aksamit TR, O’Donnell AE, Barker A, et al. Adult patients with bronchiectasis: a first look at the US Bronchiectasis Research Registry. Chest. 2017;151(5):982–992. doi:10.1016/j.chest.2016.10.055
6. Gao YH, Guan WJ, Liu SX, et al. Aetiology of bronchiectasis in adults: a systematic literature review. Respirology. 2016;21(8):1376–1383. doi:10.1111/resp.12832
7. Amital A, Shitrit D, Adir Y. The lung in rheumatoid arthritis. Presse Med. 2011;40(1 Pt 2):e31–48. doi:10.1016/j.lpm.2010.11.003
8. Perry E, Eggleton P, De Soyza A, Hutchinson D, Kelly C. Increased disease activity, severity and autoantibody positivity in rheumatoid arthritis patients with co-existent bronchiectasis. Int J Rheum Dis. 2017;20(12):2003–2011. doi:10.1111/1756-185X.12702
9. Swinson DR, Symmons D, Suresh U, Jones M, Booth J. Decreased survival in patients with co-existent rheumatoid arthritis and bronchiectasis. Br J Rheumatol. 1997;36(6):689–691. doi:10.1093/rheumatology/36.6.689
10. Winthrop KL, Baxter R, Liu L, et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis. 2013;72(1):37–42. doi:10.1136/annrheumdis-2011-200690
11. Brode SK, Jamieson FB, Ng R, et al. Increased risk of mycobacterial infections associated with anti-rheumatic medications. Thorax. 2015;70(7):677–682. doi:10.1136/thoraxjnl-2014-206470
12. Yoo JW, Jo KW, Kang BH, et al. Mycobacterial diseases developed during anti-tumour necrosis factor-alpha therapy. Eur Respir J. 2014;44(5):1289–1295. doi:10.1183/09031936.00063514
13. Tokuda H, Harigai M, Kameda H, et al. Consensus statements for medical practice: biological agents and lung disease [Abridged English translation by the Japanese Respiratory Society]. Respir Investig. 2017;55(3):229–251. doi:10.1016/j.resinv.2017.01.002
14. Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med. 2014;108(3):417–425. doi:10.1016/j.rmed.2013.09.014
15. Asakura T, Kimizuka Y, Nishimura T, et al. Serum Krebs von den Lungen-6 level in the disease progression and treatment of Mycobacterium avium complex lung disease. Respirology. 2021;26(1):112–119. doi:10.1111/resp.13886
16. Ohnishi H, Yokoyama A, Kondo K, et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165(3):378–381. doi:10.1164/ajrccm.165.3.2107134
17. Morimoto K, Namkoong H, Hasegawa N, et al. Macrolide-Resistant Mycobacterium avium Complex Lung Disease: analysis of 102 Consecutive Cases. Ann Am Thorac Soc. 2016;13(11):1904–1911. doi:10.1513/AnnalsATS.201604-246OC
18. Yagi K, Ito A, Fujiwara K, et al. Clinical features and prognosis of nontuberculous mycobacterial pleuritis: a Multicenter Retrospective Study. Ann Am Thorac Soc. 2021;18(9):1490–1497. doi:10.1513/AnnalsATS.202008-938OC
19. Szymanski EP, Leung JM, Fowler CJ, et al. Pulmonary nontuberculous mycobacterial infection. a multisystem, multigenic disease. Am J Respir Crit Care Med. 2015;192(5):618–628. doi:10.1164/rccm.201502-0387OC
20. Asano S, Mizuno S, Okachi S, et al. Antineutrophil cytoplasmic antibody-associated vasculitis superimposed on infection-related glomerulonephritis secondary to pulmonary mycobacterium avium complex infection. Intern Med. 2016;55(17):2439–2445. doi:10.2169/internalmedicine.55.6588
21. Addy C, Doran G, Jones AL, Wright G, Caskey S, Downey DG. Microscopic polyangiitis secondary to Mycobacterium abscessus in a patient with bronchiectasis: a case report. BMC Pulm Med. 2018;18(1):170. doi:10.1186/s12890-018-0732-3
22. Ishiguro T, Takayanagi N, Takaku Y, et al. Allergic bronchopulmonary aspergillosis with repeated isolation of nontuberculous mycobacteria. Intern Med. 2013;52(15):1721–1726. doi:10.2169/internalmedicine.52.9537
23. Mohd Noor N, Mohd Shahrir MS, Shahid MS, Abdul Manap R, Shahizon Azura AM, Azhar Shah S. Clinical and high resolution computed tomography characteristics of patients with rheumatoid arthritis lung disease. Int J Rheum Dis. 2009;12(2):136–144. doi:10.1111/j.1756-185X.2009.01376.x
24. Willis VC, Demoruelle MK, Derber LA, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013;65(10):2545–2554. doi:10.1002/art.38066
25. Quirke AM, Perry E, Cartwright A, et al. Bronchiectasis is a Model for Chronic Bacterial Infection Inducing Autoimmunity in Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67(9):2335–2342. doi:10.1002/art.39226
26. Nielsen SF, Bojesen SE, Schnohr P, Nordestgaard BG. Elevated rheumatoid factor and long term risk of rheumatoid arthritis: a prospective cohort study. BMJ. 2012;345:e5244. doi:10.1136/bmj.e5244
27. Perry E, Stenton C, Kelly C, Eggleton P, Hutchinson D, De Soyza A. RA autoantibodies as predictors of rheumatoid arthritis in non-cystic fibrosis bronchiectasis patients. Eur Respir J. 2014;44(4):1082–1085. doi:10.1183/09031936.00064014
28. Greinert U, Schlaak M, Rusch-Gerdes S, Flad HD, Ernst M. Low in vitro production of interferon-gamma and tumor necrosis factor-alpha in HIV-seronegative patients with pulmonary disease caused by nontuberculous mycobacteria. J Clin Immunol. 2000;20(6):445–452. doi:10.1023/A:1026407815946
29. Mori S, Koga Y, Nakamura K, et al. Mortality in rheumatoid arthritis patients with pulmonary nontuberculous mycobacterial disease: a retrospective cohort study. PLoS One. 2020;15(12):e0243110. doi:10.1371/journal.pone.0243110
30. Winthrop KL, Iseman M. Bedfellows: mycobacteria and rheumatoid arthritis in the era of biologic therapy. Nat Rev Rheumatol. 2013;9(9):524–531. doi:10.1038/nrrheum.2013.82
31. Gono T. Overview: clinical Significance of Lung Disease Associated with Rheumatoid Arthritis. In: Lung Disease Associated with Rheumatoid Arthritis. Springer Nature Singapore Pte Ltd; 2018.
32. Nakahara H, Kamide Y, Hamano Y, et al. A case report of a patient with rheumatoid arthritis complicated with Mycobacterium avium during tocilizumab treatment. Mod Rheumatol. 2011;21(6):655–659. doi:10.3109/s10165-011-0448-1
33. Namkoong H, Tasaka S, Akiyama M, et al. Successful resumption of tocilizumab for rheumatoid arthritis after resection of a pulmonary Mycobacterium avium complex lesion: a case report. BMC Pulm Med. 2015;15:126. doi:10.1186/s12890-015-0130-z
34. Mori S, Sugimoto M. Is continuation of anti-tumor necrosis factor-alpha therapy a safe option for patients who have developed pulmonary mycobacterial infection?: case presentation and literature review. Clin Rheumatol. 2012;31(2):203–210. doi:10.1007/s10067-011-1902-3
35. Mori S, Tokuda H, Sakai F, et al. Radiological features and therapeutic responses of pulmonary nontuberculous mycobacterial disease in rheumatoid arthritis patients receiving biological agents: a retrospective multicenter study in Japan. Mod Rheumatol. 2012;22(5):727–737. doi:10.3109/s10165-011-0577-6
36. Takei H, Nishina N, Namkoong H, et al. Rheumatoid Arthritis with Nontuberculous Mycobacterial Pulmonary Disease: a Retrospective, Single-center Cohort Study. Modern Rheumatol. 2021. doi:10.1093/mr/roab032
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.