Back to Journals » Drug Design, Development and Therapy » Volume 11
Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach
Authors Tefas LR, Sylvester B, Tomuta I , Sesarman A, Licarete E , Banciu M, Porfire A
Received 29 November 2016
Accepted for publication 16 March 2017
Published 25 May 2017 Volume 2017:11 Pages 1605—1621
DOI https://doi.org/10.2147/DDDT.S129008
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Lucia Ruxandra Tefas,1 Bianca Sylvester,1 Ioan Tomuta,1 Alina Sesarman,2,3 Emilia Licarete,2,3 Manuela Banciu,2,3 Alina Porfire1
1Department of Pharmaceutical Technology and Biopharmaceutics, Faculty of Pharmacy, University of Medicine and Pharmacy “Iuliu Hatieganu”, 2Department of Molecular Biology and Biotechnology, Faculty of Biology and Geology, 3Molecular Biology Centre, Institute for Interdisciplinary Research in Bio-Nano-Sciences, Babeş-Bolyai University, Cluj-Napoca, Romania
Abstract: The aim of this work was to use the quality-by-design (QbD) approach in the development of long-circulating liposomes co-loaded with curcumin (CUR) and doxorubicin (DOX) and to evaluate the cytotoxic potential of these liposomes in vitro using C26 murine colon carcinoma cell line. Based on a risk assessment, six parameters, namely the phospholipid, CUR and DOX concentrations, the phospholipid:cholesterol molar ratio, the temperature during the evaporation and hydration steps and the pH of the phosphate buffer, were identified as potential risk factors for the quality of the final product. The influence of these variables on the critical quality attributes of the co-loaded liposomal CUR and DOX was investigated: particle size, zeta potential, drug loading and entrapment efficiency. For this, a 26-2 factorial design was employed to establish a proper regression model and to generate the contour plots for the responses. The obtained data served to establish the design space for which different combinations of variables yielded liposomes with characteristics within predefined specifications. The validation of the model was carried out by preparing two liposomal formulations corresponding to the robust set point from within the design space and one outside the design space and calculating the percentage bias between the predicted and actual experimental results. The in vitro antiproliferative test showed that at higher CUR concentrations, the liposomes co-encapsulating CUR and DOX had a greater cytotoxic effect than DOX-loaded liposomes. Overall, this study showed that QbD is a useful instrument for controlling and optimizing the manufacturing process of liposomes co-loaded with CUR and DOX and that this nanoparticulate system possesses a great potential for use in colon cancer therapy.
Keywords: doxorubicin, curcumin, co-loaded long-circulating liposomes, quality by design, design of experiments
Introduction
The World Health Organization has stated that cancer is one of the major leading causes of mortality around the world.1 However, in the past decades, advances in cancer treatment have led to an increase in the survival rate of cancer patients.2 In spite of the indisputable benefits of cancer treatment, and even with the development of new anticancerous agents, often cancer treatment regimens are unsuccessful.3 Failure in cancer treatment mostly occurs as a result of drug-induced toxicity and resistance of cancer cells.4 Because of these drawbacks, it is obvious that chemotherapy alone faces challenges, and a new approach is needed in order to achieve a satisfactory outcome in cancer patients. Therefore, combination therapy has emerged as a new and effective strategy capable of overcoming the limitations of chemotherapy.5 It is suggested that the association of common chemotherapeutic agents with existing anticancer agents, new ones or even natural products, could maximize the chemotherapy efficacy and reduce the toxic effects to normal cells. Recently, there has been growing interest in the scientific field toward the use of natural products.6 Natural products with potential anticancer activity are mostly dietary compounds which can be found in various foods like fruits, vegetables and other plants. They could be good candidates for combination therapy because aside from the fact that they have shown efficacy toward a wide range of cancerous diseases, their side effects and toxicity are low.7
Doxorubicin (DOX), an anthracycline antibiotic, is one of the efficient chemotherapeutic agents commonly used in the treatment of solid tumors, acute leukemia and malignant lymphoma.8,9 Its main mechanisms of action involve topoisomerase II inhibition, DNA intercalation and free radical formation.10 DOX can inhibit the replication of tumor cells by intercalating in the base pairs of DNA.9 Also, it induces apoptosis in cancerous cells as a result of oxidative damage to DNA.11 However, despite the fact that it is a very potent chemotherapeutic agent, its clinical use is limited by its induced toxicity and drug resistance.8 DOX can induce severe cytotoxic effects in normal cells, mostly leading to cardiotoxicity and myelosupression.9 Incorporating DOX into PEGylated liposomes reduces the toxicity associated with DOX use and prolongs the circulation time in the bloodstream, overall improving the accumulation at the tumor site through the enhanced permeability and retention effect.12 Liposomal DOX is currently marketed as various products, namely Doxil®/Caelyx® (Johnson & Johnson) and Lipo-Dox® (Taiwan Liposome), for the treatment of Kaposi’s sarcoma, ovarian cancer, breast cancer and multiple myeloma.13 Doxil (in the US) or Caelyx (in Europe) was the first DOX-loaded liposomal formulation to be approved in 1995 by the regulatory authorities.12,14 Preclinical and clinical studies have shown that liposomal DOX has a smaller distribution volume, an increased circulation time and a decreased clearance compared to free DOX. Also, due to the small size of the particles, liposomal DOX preferentially accumulates within the tumor tissue with altered vasculature, which accounts for the lower toxicity and side effects, such as cardiotoxicity and myelosupression.15
Curcumin (CUR; 1,7-bis(4-hydroxy 3-methoxy phenyl)-1,6-heptadiene-3,5-dione), also called diferuloylmethane, is a diphenolic compound extracted from the rhizome of the perennial herb Curcuma longa (turmeric), a plant which belongs to the Zingiberaceae family.16–18 CUR is a yellow pigment extensively used as a spice, coloring agent and flavoring agent, but also in traditional Asian medicine to treat different types of diseases.6 There are many reports which state that CUR has a plethora of biological activities, including antioxidant, anti-inflammatory, antimicrobial, anticarcinogenic, hepatoprotective and neuroprotective properties.17,19,20 Of late, it has attracted considerable attention in cancer prevention and therapeutics. The underlying molecular mechanisms of action through which CUR exerts its anticancerous effect are various and possibly involve the modulation of different intracellular signaling pathways, such as those involved in proliferation, inflammation and apoptosis.21 CUR has been shown to inhibit the activation of NF-κB6 and also to downregulate the intracellular levels of P-glycoprotein and MRP1, which play an important role in multidrug resistance, including chemotherapy.22 Moreover, in vivo studies have demonstrated that CUR possesses potential cardioprotective effects.23 Besides its potential beneficial effects, the use of CUR in cancer chemoprevention and therapy is of interest due to its safety profile and lack of toxicity in animals or humans.16 CUR has been reported to be an ideal chemosensitizer capable of reversing multidrug resistance in cancer cells.24 Recent research suggests that the association of a chemotherapeutic agent with a chemosensitizer is much more efficient than a traditional combination of two or several cytotoxic drugs.8 Also, it has been demonstrated that CUR has synergistic effects with DOX when associated.24 In spite of its low toxicity and multiple biological properties, the clinical use of CUR is limited by its low solubility in water and poor bioavailability.25
The co-delivery of two or several drugs via nanoparticulate drug delivery systems, for chemotherapy, shows multiple advantages such as synergism of the pharmacological effect, decrease of the effective dose, reduction of side effects and overcoming of multidrug resistance.26 Up to the present, several studies have emphasized the superior anticancer efficacy of the DOX-CUR association co-delivered by various nanoparticulate systems, including lipid nanoparticles,8 polymeric nanoparticles,27 polymeric or lipidic micelles24,26,28–30 and liposomes,25 to hepatic, brain and breast cancer, leukemia and melanoma. Therefore, developing a drug delivery system such as liposomes can provide a solution to the aforementioned challenges, by decreasing the toxicity of DOX, enhancing the solubility of CUR and overall improving the stability of both the drugs. Even though the co-encapsulation of DOX and CUR in liposomes has been previously reported in another study, to the best of our knowledge, no study with liposomal DOX and CUR has been performed in colon cancer.
The current necessity in drug development is an approach like quality-by-design (QbD). QbD is a concept used to ensure a predefined quality of a product by assisting the design and development of the manufacturing process.31 As mentioned in the ICH guideline Q8(R2), a QbD study includes the following elements of the pharmaceutical development: 1) defining the quality target product profile (QTPP), 2) identifying the critical quality attributes (CQAs) of a drug product and the critical process parameters (CPPs), 3) performing a risk assessment in order to identify which formulation or process parameters can potentially influence the product’s CQAs and 4) establishing the design space that ensures desired product specifications.32,33 In order to study the relation between the CPPs and the CQAs, often the design of experiments (DOE) strategy is used in the formulation and process design.34
The present study was aimed at developing liposomes co-encapsulated with CUR and DOX with long-circulating properties (CUR-DOX-LCL) and evaluating their antiproliferative activity in vitro using C26 murine colon carcinoma cells. The QbD approach used for liposomal development allowed us to evaluate the influence of several critical formulation and process parameters on the CUR-DOX-LCL characteristics and to establish the design space, such as the combination of process parameters and material attributes providing the assurance of quality.
Materials and methods
Materials
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), N-(carbonyl-methoxypolyethyleneglycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (PEG-2000-DSPE) and sodium salt were purchased from Lipoid GmbH (Ludwigshafen, Germany). Cholesterol, CUR and DOX hydrochloride were purchased from Sigma-Aldrich (St Louis, MO, USA). Potassium dihydrogen phosphate was purchased from Chemical Company (Iasi, Romania). Sodium hydroxide was purchased from EMD Millipore (Billerica, MA, USA). Ethanol was purchased from Chemical Company, and methanol and acetonitrile were purchased from LGC Standards GmbH (Wesel, Germany). Murine colon carcinoma cells, C26, were obtained from Cell Line Services (Baden-Eppelheim, Germany). RPMI 1640 cell-culturing media (Lonza) containing L-glutamine, HEPES, antibiotics and 10% fetal calf serum was purchased from Sigma-Aldrich. The Cell Proliferation ELISA kit, BrdU, was obtained from Roche Applied Science (Penzberg, Germany).
Methods
Preparation of CUR-DOX-LCL and DOX-LCL
CUR-DOX-LCL were prepared by the film hydration method.35 According to this method, DPPC, PEG-2000-DSPE, cholesterol and CUR were dissolved in 10 mL ethanol. The obtained solution was subjected to rotary evaporation (Heidolph, Schwabach, Germany) at a specific temperature and 80 rpm, for 30 minutes. After the organic solvent had evaporated, the remaining lipid film was hydrated with 5 mL of DOX solution in phosphate buffer saline, in a water bath, at the same temperature as for the evaporation step, at 100 rpm for 30 minutes. DOX-loaded liposomes (DOX-LCL) were prepared according to the same method, but without the addition of CUR in the ethanolic solution of lipids.
In both the cases, the obtained liposomal dispersion was extruded through polycarbonate membranes (Whatman International Ltd, Maidstone, UK) using a LiposoFast LF-50 extruder (Avestin Europe GmbH, Mannheim, Germany). Each sample was passed thrice through membranes with a specific porosity of 0.8, 0.6, 0.4 and 0.2 μm, respectively. After size reduction, non-entrapped CUR and DOX were removed by the dialysis method using Silde-A-Lyzer® Dialysis Cassettes (Thermo Fisher Scientific, Waltham, MA, USA) with a 10 kDa molecular weight cut-off (MWCO), in phosphate buffer saline, for 24 hours. The study was carried out on mouse cells, and did not require ethical permission.
Particle size analysis
The particle size and particle size distribution of liposomes were determined by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) apparatus. After dilution with distilled water (1:100), the samples were analyzed at an angle of 90°, at 25°C. All measurements were carried out in triplicate and are expressed as mean ± standard deviation.
Zeta potential analysis
The zeta potential of liposomal samples was measured by laser Doppler electrophoresis, with the Zetasizer Nano ZS, after appropriately diluting the samples with distilled water. The determination was performed at 25°C, and results are expressed as an average of three consecutive measurements ± standard deviation.
EE of CUR and DOX
The amounts of CUR and DOX entrapped in the liposomes were determined by high-performance liquid chromatography (HPLC) analysis with fluorescence detection. Briefly, the liposomal samples were dissolved in methanol, and the resulting solution was further diluted with a mixture of acetonitrile and water (20:80, v/v), and then 2 μL of sample was injected into the HPLC system. The chromatographic separation was conducted on an Agilent 1100 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a Zorbax C18 column (3.5 μm) and a fluorescence detector. The mobile phase was composed of 0.2% formic acid and acetonitrile and had a flow rate of 1 mL/min. A gradient elution was used so that after the first minute of analysis, the percentage of organic solvent increased abruptly from 18% to 50% which was held over a period of 5 minutes, and then decreased back to 18% which remained until the end of the run. The excitation and emission wavelengths were set at 490 and 560 nm for DOX and 425 and 533 nm for CUR, respectively. All measurements were performed at 30°C and in triplicate. The entrapment efficiency (EE) was calculated according to the following equation:
|
QbD approach
Identifying the CQAs of liposomal CUR and DOX by risk assessment
A risk assessment was carried out for CUR-DOX-LCL in order to define the CQAs of the product and identify the potential risk factors with the highest influence on the quality of the final product (or QTPP). Based on scientific literature information and on preliminary formulation studies, the size, surface charge, drug loading and EE were chosen as CQAs of our liposomal product. Potential risk factors which are likely to influence the CQAs have been identified through risk analysis. At the end of this step, six risk variables were selected to be further investigated by means of experimental design.
DOE
A screening study was conducted in order to find the factors with the most relevant influence on CUR-DOX-LCL characteristics (or CQAs). The experiment was designed and the data were statistically analyzed using Modde 11 Pro software (Umetrics, Malmö, Sweden). Six variables were established as independent factors to be investigated, namely the phospholipid concentration, the phospholipid:cholesterol molar ratio, the CUR concentration, the DOX concentration, the working temperature and the pH of the buffer. Each independent factor was assigned two levels, low and high, which were represented by the values of −1 and +1, respectively (Table 1). Taking into account the number of experimental factors and their levels, a 26-2 fractional factorial design resolution IV was generated, resulting in 19 experiments. The experimental setup is given in Table 2. The investigated responses (dependent variables) were the CQAs of the CUR-DOX-LCL, such as the encapsulated CUR concentration (Y1), the encapsulated DOX concentration (Y2), the EE for CUR (Y3) and DOX (Y4) and the size (Y5) and zeta potential (Y6) of the liposomes. The data were fitted by means of partial least squares using the statistical module of Modde 11 Pro software. The experiments were performed in a random order to reduce the experimental variability.
  | Table 1 Independent variables and their levels |
Establishment of the design space
The design space was established with Modde 11 Pro software, as the combination of factors for which the target specifications of the CUR-DOX-LCL were met at specific risk levels. The models developed in the experimental design were validated by performing the preparation and determination of CQAs of one formulation within the design space and one formulation outside the design space. The formulations were characterized, and experimental results, such as the determined CQAs, were compared to the predicted ones, for all six investigated responses, and percentage bias was calculated.
In vitro release study
The in vitro release of CUR and DOX from liposomes was assessed by the dialysis method, according to a modified technique by Li et al.36 The Silde-A-Lyzer® Dialysis Cassettes (Thermo Scientific) with 10 kDa MWCO were hydrated in the release medium prior to the test. Three milliliters of CUR-DOX-LCL was introduced in the dialysis cassette which was immersed in 100 mL release medium. The release medium consisted of a mixture of phosphate buffer saline (pH 5) and absolute ethanol in a ratio of 65:35 (v/v). The samples were maintained at 37°C under continuous stirring at 100 rpm. At different time points, namely 0.5, 1, 3, 6, 16, 24, 48 and 72 hours, 1 mL of release medium was withdrawn and replaced with an equal volume of fresh release medium in order to maintain a constant volume during the whole test. The CUR and DOX concentrations in the samples were determined by HPLC analysis. All experiments were carried out in triplicate and are expressed as mean ± standard deviation.
Culturing conditions and treatments
The C26 murine colon carcinoma cells were cultured in complete RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, at 37°C in a humidified atmosphere containing 5% CO2.37
DOX, in free form (dissolved in phosphate buffer saline) as well as in DOX-LCL form, was tested at various concentrations (0.05, 0.10, 0.15 and 0.25 μM) to investigate its cytotoxic effect on C26 murine colon cancer cells. Moreover, the same DOX concentrations were tested for cytotoxicity as liposomal formulations co-encapsulated with CUR and DOX, in two different molar ratios, such as DOX:CUR =1:9 and DOX:CUR =1:167.
Cell proliferation assay
C26 murine colon cancer cells were seeded at a density of 5×103 cells/well into 96-well plates. After 12 hours of incubation, the cells were treated with DOX, DOX-LCL and the two DOX-CUR-LCL formulations, for an additional 48 hours. The in vitro cytotoxicity of different treatments applied on C26 cells was evaluated by using the ELISA BrdU-colorimetric immunoassay according to the manufacturer’s instructions, as previously described.37 Results are expressed as percentage of inhibition of cell proliferation compared to that of untreated C26 cells and are presented as the mean ± standard deviation of triplicate measurements.
Statistical analysis
All data are expressed as mean ± standard deviation (n=3). For statistical analysis of the effects of different treatments with cytotoxic agents on C26 cell proliferation, a one-way analysis of variance (ANOVA) with Bonferroni correction was used. Correlations between different pharmacological agent concentrations and their cytotoxic effects were evaluated by using Pearson correlation coefficient, r. All statistical analyses were performed by using GraphPad Prism version 6 for Windows (GraphPad Software Inc., La Jolla, CA, USA). Significance was considered at values of P<0.05 (ns, P>0.05; *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001).
Results and discussion
Physicochemical characterization of CUR-DOX-LCL
According to the experimental design, 19 experiments were carried out, and the results obtained for the evaluated responses are given in Table 3.
QbD approach
CQAs of liposomal CUR and DOX identified by risk assessment
In the QbD approach, in order to achieve a desired QTPP, the CQAs of the product have to be established first. A CQA according to ICH Q8(R2) is “a physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality”.33 According to the current scientific knowledge and our previous formulation studies, the size, size distribution, zeta potential, drug loading and encapsulation efficiency are the most important attributes of nanoparticulate systems, including liposomes.
The potential risk factors affecting the CQAs of the liposomes co-loaded with CUR and DOX were identified based on the literature and preliminary formulation studies. The factors were divided into two categories: formulation factors and process parameters. The formulation factors chosen for our study were the phospholipid concentration, phospholipid:cholesterol molar ratio, CUR concentration and DOX concentration. The ratio between the main lipid (DPPC) and the PEGylated lipid (PEG-DSPE) was kept constant throughout the experimental plan (5% PEGylated lipid of the total phospholipid concentration) and was not considered a formulation factor. The reason behind PEGylation is to improve the stability and biological performance of liposomes in vivo by extending their circulation time in the bloodstream.38 Since the proposed liposomal system was not yet assessed in vivo, it was not considered necessary to study the influence of the degree of PEGylation on the characteristics of the liposomes. Also, usually, the proportion of PEGylated phospholipids which confers stealth properties is quite low, approximately 5–7 mol%, but enough to prevent fast elimination of liposomes from the bloodstream.39 Among the process parameters, the temperature and the pH of the buffer were evaluated. The temperature maintained during the extrusion and hydration steps was above the transition temperature of the phospholipids. Also, the pH of the phosphate buffer was set such that the stability of the drugs was ensured.
The size and polydispersity are critical physicochemical properties which determine the in vivo uptake and fate of the liposomes.40 These parameters can be modulated in order to specifically direct the distribution of liposomes in vivo. Generally, nanoparticulate systems of approximately 100 nm have longer circulation half-lives. Also, nanoparticles with a size of 100–200 nm have been shown to be able to extravasate through the fenestrations of the endothelial lining of blood vessels and leak into tumors to a greater extent than in normal tissues due to the enhanced permeation and retention effect.41 However, by ensuring a small size for the liposomes, often the necessary dose of active substance to be delivered is reduced. Thus, the most appropriate size for nanoparticles, including liposomes, which can deliver a corresponding dose of therapeutic agent, is usually in the range of 10–200 nm.42 A size within the previously mentioned range can be achieved by extruding the liposomes through membranes with a specific porosity at a temperature above the phase transition temperature of the lipids. According to the risk analysis carried out, the phospholipid concentration, the ratio of phospholipids to cholesterol and the extrusion temperature are the most influential parameters which affect the liposome size.
The zeta potential is an important parameter which can directly influence the stability of a liposomal dispersion. It is agreed that particles can aggregate due to insufficient electric repulsion between them when the zeta potential values range between −30 and +30 mV.43,44 Thus, the zeta potential was chosen as a CQA of CUR-DOX-LCL. The most important factors influencing zeta potential, identified by the risk assessment, are the type and concentration of lipids and the physicochemical properties of the active substance. Liposome stability is reportedly related to the rigidity of the lipid bilayer, and thus, an important requirement is selecting the appropriate lipids in terms of chain length, degree of unsaturation and phase transition temperature.45 We have chosen DPPC and DSPE (PEGylated) as phospholipids for our study due to their suitable properties: long acyl chain (16C for DPPC and 18C for DSPE, respectively), saturated nature and high transition temperature. Also, cholesterol has been shown to have an impact on the rigidity of the lipid membrane. Studies investigating the role of cholesterol in liposome formulation have revealed that it increases the packing of phospholipids, tempers the particle aggregation and reduces the membrane permeability.45
Designing liposomes with optimum therapeutic payload is an essential prerequisite to ensure the delivery of an adequate dose of active substance to the site of action. The phospholipid concentration, content of cholesterol and drug concentrations have been previously shown to influence the drug loading and EE. Therefore, we have selected these two latter properties as CQAs of liposomal CUR and DOX.
After the risk analysis, our next focus was to investigate the impact of the risk factors, both formulation and process variables, on the CQAs of CUR-DOX-LCL, by an experimental design.
DOE
It is ideal to develop a pharmaceutical product having desirable quality characteristics by running a minimum number of experiments, in the shortest time possible. The traditional approach which involves changing one variable at a time, while keeping the others constant, requires a lot of time, energy and resources. As opposed to this classical technique, experimental design allows to simultaneously modulate several input variables.46 DOE is a powerful and useful tool for evaluating the relation between a set of input variables and the investigated experimental responses.
The first step in conducting an experimental design is choosing the appropriate type of design, which depends on the number of investigated variables. In this study, six factors were evaluated, among which four, namely the phospholipid, CUR and DOX concentrations and the phospholipid:cholesterol molar ratio, were quantitative-type factors, while the remaining two factors, the temperature and the pH of the buffer, were qualitative-type variables. Another important aspect in planning an experimental design is to select an adequate level range for each formulation factor. All chosen independent variables were evaluated at two different levels, with the purpose of identifying the factors with a significant influence on the output variables. Hence, for the reasons of saving time and resources, a two-level fractioned factorial design was proposed as the most suitable one.
Summary of fit, fitting the model to the experimental data and regression coefficient analysis
The most important statistical parameters to be calculated when fitting a regression model to the experimental data are the coefficient of determination or R2 and the coefficient of prediction or Q2. R2 is known as “goodness of fit”, and it shows the extent to which the data fit the model. On the other hand, Q2, called “goodness of prediction”, reflects the model’s power of prediction. What is more, model validity indicates whether the appropriate model was chosen, while reproducibility denotes a summary of variability.47
According to Figure 1, the values of R2 and Q2 were greater than 0.5 indicating that the chosen model fitted well to the set of experimental data and showing a sufficiently good predictive power. In addition, the model validity and the reproducibility were greater than 0.25 and 0.5, respectively, thus suggesting a reduced experimental error.
Also, the ANOVA test was performed to assess the significance of the regression model and to confirm the model accuracy (Table 4). The low probability values (P<0.05) obtained for the regression model indicated the significance of the model. The validity of the model was also supported by the absence of lack of fit (P>0.05). Overall, the results confirm that the chosen model was adequate and reliable and had a good predictive power.
Model regression coefficients were estimated and are presented in Figure 2. The coefficient plots illustrate the influence of formulation and process variables on the studied response.48
Contour plots were generated for a better understanding of the main effects and the interactions between the investigated variables and are illustrated in Figure 3.
Influence of variables on the encapsulated drug concentration
The encapsulated CUR concentrations ranged between 321.32±20.41 and 3,508.59±87.16 μg/mL, while entrapped DOX concentrations varied from 5.07±0.04 to 191.45±2.14 μg/mL. The results from the ANOVA test (Table 4) indicated a significant influence of the independent variables on the response (P=0.000) and absence of lack of fit (P=0.212 for CUR and P=0.449 for DOX).
According to Figure 2Aand B, the concentrations of both encapsulated CUR and DOX were influenced by the phospholipid concentration. An increase in the amount of phospholipid led to an increase in the amount of CUR and DOX entrapped in the liposomes. The positive influence that the phospholipid concentration had on these responses could be explained by the fact that a larger amount of phospholipids could accommodate a larger amount of drug. Our results are in accordance with those published by Sailor et al.49
Both drugs positively influenced their own concentrations, but had no effect whatsoever on each other’s concentration. By increasing the amount of drug during the preparation process, the concentration of entrapped drug increased, both for CUR and for DOX. This shows that the saturation concentration of liposomes was not reached, and more drug could be incorporated into the particles.50
No effect on the encapsulated drug concentration was observed when varying the phospholipid:cholesterol molar ratio, the temperature or the pH of the buffer solution.
The interactions between the variables for encapsulated drug concentration were also investigated and are depicted by the contour plots in Figure 3A. Results showed a synergistic effect of the phospholipid and CUR concentrations on the entrapped CUR concentration. It appears that entrapped CUR concentration increases when simultaneously increasing the phospholipid and CUR concentrations, while keeping the temperature at 55°C and the pH at 5. However, for phospholipid concentrations below 40 mM, the increase of CUR concentration over 6 mM does not result in a further increase of encapsulated CUR, so the saturation concentration is reached in these conditions. Regarding the entrapped DOX concentration, the contour plot seen in Figure 3B reveals that on the one hand at low phospholipid concentration, the CUR concentration had a somewhat positive effect, but on the other hand at high concentrations of phospholipids, the entrapped DOX concentration decreased when increasing CUR concentration.
Influence of variables on the encapsulation efficiency
The EE was found to be between 18.68%±0.08% and 98.26%±3.44% for CUR and from 1.83%±0.01% to 73.49%±1.95% for DOX. According to the results of the ANOVA test shown in Table 4, the investigated variables exhibited a significant effect on the entrapment efficiencies of CUR and DOX (P=0.000). Also, the chosen model did not show a significant lack of fit (P=0.118 for Y3 response and P=0.346 for Y4 response).
As shown in Figure 2C and D, increasing the phospholipid concentration significantly increased the percentage of encapsulated CUR and DOX, respectively.
It appears that both the drugs negatively influenced their own entrapment efficiencies, as indicated by the negative values of the regression coefficients. The EE is dependent on the initial amount of drug and the entrapped drug concentration, but it appears that it is a nonlinear relation, according to the EE equation. Therefore, it is possible for high initial amounts of drugs to translate to reduced EE values.
While DOX had no effect on the CUR EE, CUR led to a decrease in DOX EE. Due to the lipophilic nature of the molecule, CUR is incorporated into the liposomes by insertion and entrapment in the lipid bilayer. On the other hand, DOX is readily soluble in water, and thus should be incorporated in the hydrophilic interior of the liposomes. However, it has been shown that due to its amphiphilic structure, DOX can interact with the lipid membrane and possibly be incorporated in the bilayer of the liposomes.51 Our results suggested that CUR, at higher concentrations, hindered the incorporation of DOX into the liposomes.
The EE was also affected by the working conditions so that CUR EE was influenced by temperature, and DOX EE was influenced by the pH of the buffer.
Concerning CUR, the EE was higher when working at 55°C and lower at 45°C. Only CUR EE was influenced by the temperature possibly because of its hydrophobic nature and its ability to interact and mix with the melted lipids. On the other hand, DOX EE was increased when the pH of the buffer was 5, but decreased at pH 4.5.
Although we could not highlight the influence of the phospholipid:cholesterol molar ratio on drug encapsulation efficiency in this study, other reports state that drug loading was highly influenced by cholesterol concentrations. Generally, a larger proportion of cholesterol results in increased EE. Due to greater rigidity, the permeability of the lipid bilayer is reduced and drug leakage is prevented, which translates into enhanced drug encapsulation.52
The contour plots in Figure 3C–E show a strong interaction between the phospholipid concentration and the drug concentration on CUR encapsulation efficiency and DOX encapsulation efficiency. It can be deducted from the graphical representation that the phospholipid and CUR concentrations had antagonistic effects on CUR encapsulation efficiency. As discussed earlier, increasing the phospholipid concentration led to higher CUR EE values, and the effect was more important at lower CUR concentrations. At CUR concentrations over 3 mM, the increase of EE with phospholipid concentration was not so important. Figure 3D and E illustrates the interaction between the phospholipid concentration and DOX concentration with regard to DOX EE, at different pH values of the phosphate buffer. At pH 4.5 (Figure 3D), the phospholipid and DOX concentrations showed a synergistic effect, while at pH 5 (Figure 3E), the two variables had an antagonistic effect on DOX EE. The DOX EE varied dramatically when increasing the phospholipid concentration, but the variation was rather moderate by modifying the DOX concentration, regardless of the pH. Hence, it can be concluded that the phospholipid concentration had the most important impact on the interaction regarding the effect on DOX EE.
Influence of variables on the size of the liposomes
The mean size, polydispersity and zeta potential are important physicochemical parameters which can influence the release of the entrapped drug and the therapeutic outcome.43,53
The mean particle size varied from 160.30±0.95 to 200.80±2.86 nm. Regarding the particle size distribution, the polydispersity index of the liposomal formulations had values from 0.028±0.02 to 0.120±0.03 (data not shown). The statistical analysis of the data for particle size (Table 4) indicated the significant influence which the independent variables exhibited (P=0.000) and the absence of lack of fit (P=0.659).
Figure 2E reveals that the composition of the lipid bilayer influenced the size of the liposomes. Increasing the phospholipid concentration resulted in the formation of larger liposomes, since phospholipids are the main component of the liposomal lipid bilayer.
However, the phospholipid:cholesterol molar ratio had an opposite effect on the response. Cholesterol is frequently used in the preparation of liposomes and plays a key role in the formation and stability of these nanoparticulate systems. It has a direct influence on the fluidity and permeability of the liposomes.52 The decrease in size observed with an increase in phospholipid:cholesterol molar ratio is the result of a higher flexibility of the lipid bilayer. A higher concentration of cholesterol renders a more rigid structure of the lipid membrane, and thus a more stable formulation.54
Neither the concentration of drugs nor the working conditions influenced the size of the liposomes. The temperature was adequately selected for this study to be over the transition temperature of DPPC (41°C).55 During melting, the phospholipids undergo transformation from a gel to a fluid phase. According to some research groups, this phenomenon is accompanied by reduction in liposome size due to a more compact packing of the lipids.56 Although unobservable in the present study, it is possible that the preparation at 45°C, which was closer to the transition temperature of the phospholipids, resulted in smaller-size liposomes. On the other hand, it was reported by Roy et al that above the transition temperature of the lipid, the size of the liposomes increased as a result of an increase in volume.56
Influence of variables on the zeta potential
All liposomal formulations showed a negative surface charge as zeta potential ranged from −54.70±2.08 to −32.20±0.90 mV. According to the ANOVA results from Table 4, the zeta potential response can be well described by the chosen model (P=0.000), and the validity of the model can be confirmed by the absence of lack of fit (P=0.583).
The formulation factors with the most impact on zeta potential were the phospholipid concentration and the phospholipid:cholesterol molar ratio, but the observed effects were opposite (Figure 2F). By increasing the phospholipid concentration, the surface charge of the particles decreased. The negative surface charge which the liposomes exhibited is due to the type of structural phospholipids and, according to the literature, is consistent with the liposomes prepared with phosphatidylcholine.43 These findings are in agreement with those reported by others.55,57 DPPC is a zwitterionic-type phospholipid which can change the orientation of its polar groups depending on the ionic strength of the medium. In low-ionic strength conditions, the net surface charge of the particles is negative, while the opposite can be observed with high ionic strength.55 Other research groups reported that by adding a PEGylated lipid, the zeta potential would shift toward more negative values.52 The magnitude of the zeta potential depends on the charge at the surface of the particles. The decrease in zeta potential with the increase in phospholipid concentration could be due to the presence of more phosphocholine moieties on the unit surface of the liposomes.
In contrast to these results, the zeta potential increased when increasing the phospholipid:cholesterol molar ratio. We observed that the higher the ratio, the lower the zeta potential absolute values. The bigger the concentration of phospholipids in the case of an increased phospholipid:cholesterol molar ratio, the higher the zeta potential value.
Although the drug concentrations had no influence on the response, the contrary was observed for the process parameters. We observed an increase in zeta potential values when the liposome preparation was carried out at 45°C, using phosphate buffer with pH 4.5, but the surface charge was lower when working at 55°C and pH 5. The shift in zeta potential could be related to the ionic strength of the buffer solution. The phosphate buffer solutions had different molarities, namely 0.05 M for the buffer with pH 4.5 and 0.02 M for the buffer with pH 5, and thus different ionic strengths. The underlying explanation would be that by increasing the ionic strength, a more compact layer of ions formed around the particles which consequently led to shielding the surface charge to a greater extent.58
Several interactions between variables were identified and showed significant effects on zeta potential (Figure 3F–H). As with the DOX EE discussed in the previous section, there was an interaction between phospholipid and DOX concentrations which influenced the zeta potential. It is clear from the contour plots that this interaction exhibited a different effect on zeta potential depending on the pH of the phosphate buffer. In both cases, an increase in phospholipid concentration resulted in decrease in zeta potential, but at different pH values, the DOX concentration had antagonistic effects on the zeta potential. At pH 4.5 (Figure 3F), by increasing the DOX concentration, the zeta potential absolute value decreased, while the opposite was observed at pH 5 (Figure 3G). Furthermore, the analysis showed a significant negative interaction between the phospholipid:cholesterol molar ratio and the CUR concentration on the zeta potential of the liposomes, at 55°C and pH 5. zeta potential increased when increasing the phospholipid:cholesterol molar ratio, but for CUR concentrations over 5 mM, the increase was not so important.
Establishment and evaluation of the design space
The design space is a region in the experimental domain in which any possible combination of formulation variables and process parameters assures obtaining formulations with desired characteristics.31 For CUR-DOX-LCL, the design space is the green area of the plot shown in Figure 4, each point of this area being a possible formulation for which the prediction of the CQAs is made with a probability of failure of less than 1%. Moreover, the design space hypercube is shown in the same plot as the dotted frame inside, where factor values can vary independent from each other without influencing the obtaining of a product with the quality within specifications.
Based on the obtained results, four variables, namely the phospholipid concentration, the phospholipid:cholesterol molar ratio, the CUR concentration and DOX concentration, were selected as key parameters with the greatest impact on the CQAs of CUR-DOX-LCL. Therefore, the design space was constructed using these four variables, by setting the acceptance criteria for the investigated responses. In this sense, the size of the liposomes was minimized, while the entrapment efficiencies for both CUR and DOX were maximized. The zeta potential and the encapsulated drug concentrations were excluded from this evaluation. As long as the independent variables are kept within the limits of the hypercube (Table 5), the target quality of the liposomal formulations can be assured and controlled. The combination of variables within the design space hypercube located at the intersection of the two perpendicular black lines corresponds to the robust set point which has the lowest prediction error.
  | Table 5 Design space hypercube limits for independent variables, at 55°C and pH 5 of phosphate buffer |
The validation was carried out by performing the preparation and evaluation of one formulation within the defined design space hypercube (F1, corresponding to the robust set point) and one outside the established design space (F2). According to data, robustness of the manufacturing process was assured by using a higher phospholipid concentration (62 mM), a higher phospholipid:cholesterol molar ratio (13.67) and a higher DOX concentration (0.65 mM), but a lower CUR concentration (2.07 mM), and also by operating in conditions of 55°C and pH 5 of the phosphate buffer. Working in the previously described optimum conditions would ensure obtaining liposomal dispersions with desired CQAs (Table 6) and minimizing process variability. The formulation corresponding to the robust set point was prepared in triplicate, and the obtained experimental results were compared to the predicted ones. Except for the CUR EE, experimental values of CQAs were in the predicted range, and bias had low values, thus suggesting that the proposed model had a good ability of predicting the manufacturing process of liposomes. By operating in conditions outside the design space, namely 50 mM phospholipid concentration, 5:1 phospholipid:cholesterol molar ratio, 5 mM CUR concentration and 0.5 mM DOX concentration, at 55°C and using phosphate buffer of pH 5, we obtained CQAs outside the target range and high percentage biases, indicating a poor correlation between the experimental results and the predicted ones.
In vitro release study
In this paper, a dialysis bag method was employed to study the release of CUR and DOX from PEGylated liposomes. Prior to performing this assay, various release mediums were tested to find the best medium in which DOX and CUR show adequate solubility (data not shown). The most suitable release medium was found to be a mixture of phosphate buffer saline of pH 5 and ethanol (65:35, v/v). According to our previous solubility studies, 100 mL of this release medium provided sink conditions. The release test was carried out on the formulation corresponding to the robust set point. The release profile of CUR and DOX from liposomes is illustrated in Figure 5. The results indicated that both CUR and DOX show a biphasic release from the liposomes, with rapid release in the first hours followed by sustained release up to 72 hours. The initial burst effect seen with both drugs could be attributed to the CUR and DOX incorporated at the surface of the liposomes.8 The further sustained-release pattern might be due to the drugs encapsulated more deeply in the liposome structure. The accumulated drug release percentage at 72 hours was 84.34% and 33.50% for DOX and CUR, respectively. The relatively low percentage of released CUR might be owed to the slow diffusion through the lipid bilayer. These findings are in agreement with those from other studies which reported that liposomes could provide a depot effect, especially in PEGylated formulations.59,60
  | Figure 5 In vitro release profile of CUR and DOX from liposomes. |
In vitro cytotoxic effects of DOX and CUR co-encapsulated in CUR-DOX-LCL
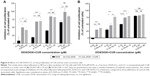
Several studies have demonstrated the ability of CUR to enhance the therapeutic potential of cytotoxic agents against various types of cancers including colon cancer.61,62 Particularly, the coadministration of CUR has been ascribed to boost DOX, 5-fluorouracil, oxaliplatin, gemcitabine or camptothecin cytotoxic actions.25,63,64 In this experiment, we aimed to evaluate the cytotoxic effects of LCL formulations based on the co-encapsulation of pharmacological agents, CUR and DOX, in C26 murine colon cancer cells. As the therapeutic potential of DOX is limited by the adverse side effects65 and in order to maximize its cytotoxic actions in in vivo experiments, we encapsulated this agent in LCL and tested its cytotoxicity toward C26 cell proliferation. We reported herein that upon 48 hours of treatment of C26 murine colon cancer cells, liposomal DOX (DOX-LCL) was able to significantly reduce cell proliferation compared to its free form (DOX), at all tested concentrations (Figure 6A and B, P<0.05). Moreover, this effect was visible at concentrations approximately two- to threefold lower than that of the free drug. This is in agreement with previous studies that demonstrated a remarkable enhancement of the cytotoxic actions of DOX when included in liposomes, nanoparticles or other lipid-based formulations.66–70 To enhance the efficacy of DOX for further in vivo studies, we designed two liposomal formulations based on the co-encapsulation of DOX and CUR. The composition of these formulations was established using the Optimizer function in Modde 11 Pro software. Thus, the co-encapsulated liposomal formulations were designed such as to get two different DOX:CUR molar ratios, that is, 1:9 and 1:167. The results related to the cytotoxic effects of CUR-DOX-LCL at DOX:CUR =1:9 molar ratio are presented in Figure 6A and demonstrated a strong DOX concentration-dependent cytotoxic effect in murine C26 colon carcinoma cells (r=0.981, P=0.018 for free DOX; r=0.954, P=0.046 for DOX-LCL; r=0.7954, P=0.204 for CUR-DOX-LCL). Irrespective of the concentration, the antiproliferative effects of CUR-DOX-LCL (DOX:CUR =1:9) on C26 cells could not exceed that of DOX-LCL, at all concentrations tested (P>0.05). On the other hand, CUR-DOX-LCL at DOX:CUR =1:167 molar ratio was able to inhibit cell proliferation with more than 75% compared to the proliferation of control cells at the highest concentrations tested (0.25 μM DOX +41.85 μM CUR) but to a similar extent to that noted after C26 cell incubation with 0.25 μM DOX administered as free as well as LCL form (Figure 6B). Interestingly, a synergistic cytotoxicity on cancer cells (about 75%) was assessed, when CUR-DOX-LCL was administered at the following concentrations of active agents: 0.10 μM DOX+16.74 μM CUR. The administration of 0.10 μM DOX as free and LCL form exerted only moderate antiproliferative effects (29% and 45%, respectively) on C26 cells. Our results showed that the co-incorporation of DOX and CUR in LCL at a ratio of 1:9 did not improve the cytotoxicity of DOX-LCL. Moreover, by increasing the liposomal content of CUR (DOX:CUR =1:167), an additional inhibitory effect by the DOX cytotoxic action on C26 cells was seen. These data imply that the cytotoxic synergistic effects of DOX and CUR encapsulated in LCL, on C26 cells, are dependent on the concentration of encapsulated CUR. Our work is in accordance with previously published data describing concentration-dependent modulatory effects of CUR when coadministrated with DOX or other chemotherapeutic agents.71 Further studies regarding the elucidation of the synergistic cytotoxicity of DOX and CUR might be a promise for future colon cancer-targeted therapies based on liposomal formulations.
Conclusion
In summary, we successfully formulated, prepared, characterized and optimized liposomes co-loaded with CUR and DOX by applying DOE as a tool of the QbD approach. This methodology helped establish the effects of phospholipid concentration, drug concentration, phospholipid:cholesterol molar ratio and working conditions on the physicochemical characteristics of CUR-DOX-LCL such as size, zeta potential, encapsulated drug concentration and EE. Phospholipid concentration was found to influence all the investigated responses. Increase in lipid concentrations resulted in larger particles, but with a higher stability due to lower zeta potential values. On the other hand, phospholipid:cholesterol molar ratios had opposite effects on these responses. Drug loading and encapsulation efficiency depended on the concentrations of CUR and DOX, but also varied with temperature and buffer pH. Results indicated that CUR-DOX-LCL showed higher stability and percentage of encapsulated CUR and DOX when the preparation was performed at 55°C using phosphate buffer of pH 5. Based on these findings, the experimental design helped establish the design space for formulating liposomes with desired characteristics. The developed mathematical model was applied to determine the optimum manufacturing conditions for CUR-DOX-LCL, which would render a final product with optimum specifications. The developed CUR-DOX-LCL were evaluated for their cytotoxicity in vitro on C26 murine colon carcinoma cells. Our results showed that LCL formulations co-encapsulating DOX and CUR have greater antiproliferative effects on C26 colon carcinoma cells compared to those induced by free DOX and that cytotoxic synergistic effects of DOX and CUR encapsulated in LCL, on C26 cells, are dependent on the concentration of encapsulated CUR.
The present study showed the importance of and usefulness in using QbD to identify the critical variables influencing a manufacturing process, understand the relation between formulation and process variables and the CQAs and determine the optimum conditions for developing CUR-DOX-LCL. Since the proposed liposomal formulation shows great potential in vitro, further studies will be focusing on establishing the pharmacokinetic profile and the cytotoxic effect of CUR-DOX-LCL in vivo, in animal models.
Acknowledgment
This work was supported by a grant from the Romanian National Authority for Scientific Research and Innovation, CNCS-UEFISCDI (project number PN-II-RU-TE-2014-4-0220).
Disclosure
The authors report no conflicts of interest in this work.
References
Cruz M, Duarte-Rodrigues J, Campelo M. Cardiotoxicity in anthracycline therapy: prevention strategies. Rev Port Cardiol. 2016;35(6):359–371. | ||
Svilaas T, Lefrandt JD, Gietema JA, Kamphuisen PW. Long-term arterial complications of chemotherapy in patients with cancer. Thromb Res. 2016;140 (Suppl 1):S109–S118. | ||
Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59(6):491–504. | ||
Roy NK, Deka A, Bordoloi D, et al. The potential role of boswellic acids in cancer prevention and treatment. Cancer Lett. 2016;377(1):74–86. | ||
Li W, Peng J, Tan L, et al. Mild photothermal therapy/photodynamic therapy/chemotherapy of breast cancer by Lyp-1 modified docetaxel/IR820 co-loaded micelles. Biomaterials. 2016;106:119–133. | ||
Kwon Y. Curcumin as a cancer chemotherapy sensitizing agent. J Korean Soc Appl Biol Chem. 2014;57(2):273–280. | ||
Kallifatidis G, Hoy JJ, Lokeshwar BL. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin Cancer Biol. 2016;40–41:160–169. | ||
Zhao X, Chen Q, Liu W, et al. Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer. Int J Nanomedicine. 2014;10:257–270. | ||
Elbialy NS, Mady MM. Ehrlich tumor inhibition using doxorubicin containing liposomes. Saudi Pharm J. 2015;23(2):182–187. | ||
Sun J, Song Y, Lu M, et al. Evaluation of the antitumor effect of dexamethasone palmitate and doxorubicin co-loaded liposomes modified with a sialic acid–octadecylamine conjugate. Eur J Pharm Sci. 2016;93:177–183. | ||
Sriraman SK, Salzano G, Sarisozen C, Torchilin V. Anti-cancer activity of doxorubicin-loaded liposomes co-modified with transferrin and folic acid. Eur J Pharm Biopharm. 2016;105:40–49. | ||
Seynhaeve AL, Dicheva BM, Hoving S, Koning GA, ten Hagen TL. Intact Doxil is taken up intracellularly and released doxorubicin sequesters in the lysosome: evaluated by in vitro/in vivo live cell imaging. J Control Release. 2013;172(1):330–340. | ||
Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–157. | ||
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. | ||
Tejada-Berges T, Granai CO, Gordinier M, Gajewski W. Caelyx/Doxil for the treatment of metastatic ovarian and breast cancer. Expert Rev Anticancer Ther. 2002;2(2):143–150. | ||
Goel A, Aggarwal BB. Curcumin, the golden spice from Indian Saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62(7):919–930. | ||
Li J, Hwang IC, Chen X, Park HJ. Effects of chitosan coating on curcumin loaded nano-emulsion: study on stability and in vitro digestibility. Food Hydrocoll. 2016;60:138–147. | ||
Yue GG, Kwok HF, Lee JK, et al. Combined therapy using bevacizumab and turmeric ethanolic extract (with absorbable curcumin) exhibited beneficial efficacy in colon cancer mice. Pharmacol Res. 2016;111:43–57. | ||
Derosa G, Maffioli P, Simental-Mendía LE, Bo S, Sahebkar A. Effect of curcumin on circulating interleukin-6 concentrations: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2016;111:394–404. | ||
Jourghanian P, Ghaffari S, Ardjmand M, Haghighat S, Mohammadnejad M. Sustained release curcumin loaded solid lipid nanoparticles. Adv Pharm Bull. 2016;6(1):17–21. | ||
Sharma V, Kumar L, Mohanty SK, Maikhuri JP, Rajender S, Gupta G. Sensitization of androgen refractory prostate cancer cells to anti-androgens through re-expression of epigenetically repressed androgen receptor – synergistic action of quercetin and curcumin. Mol Cell Endocrinol. 2016;431:12–23. | ||
Ma W, Wang J, Guo Q, Tu P. Simultaneous determination of doxorubicin and curcumin in rat plasma by LC–MS/MS and its application to pharmacokinetic study. J Pharm Biomed Anal. 2015;111:215–221. | ||
Chen TH, Yang YC, Wang JC, Wang JJ. Curcumin treatment protects against renal ischemia and reperfusion injury-induced cardiac dysfunction and myocardial injury. Transplant Proc. 2013;45(10):3546–3549. | ||
Ma W, Guo Q, Li Y, Wang X, Wang J, Tu P. Co-assembly of doxorubicin and curcumin targeted micelles for synergistic delivery and improving anti-tumor efficacy. Eur J Pharm Biopharm. 2017;112: 209–223. | ||
Barui S, Saha S, Mondal G, Haseena S, Chaudhuri A. Simultaneous delivery of doxorubicin and curcumin encapsulated in liposomes of pegylated RGDK-lipopeptide to tumor vasculature. Biomaterials. 2014;35(5):1643–1656. | ||
Li H, Li M, Chen C, et al. On-demand combinational delivery of curcumin and doxorubicin via a pH-labile micellar nanocarrier. Int J Pharm. 2015;495(1):572–578. | ||
Misra R, Sahoo SK. Coformulation of doxorubicin and curcumin in poly(D,L-lactide-co-glycolide) nanoparticles suppresses the development of multidrug resistance in K562 cells. Mol Pharm. 2011;8(3):852–866. | ||
Yan T, Li D, Li J, et al. Effective co-delivery of doxorubicin and curcumin using a glycyrrhetinic acid-modified chitosan-cystamine-poly(ε-caprolactone) copolymer micelle for combination cancer chemotherapy. Colloids Surf B Biointerfaces. 2016;145:526–538. | ||
Wang J, Ma W, Tu P. Synergistically improved anti-tumor efficacy by co-delivery doxorubicin and curcumin polymeric micelles. Macromol Biosci. 2015;15(9):1252–1261. | ||
Sarisozen C, Dhokai S, Tsikudo EG, Luther E, Rachman IM, Torchilin VP. Nanomedicine based curcumin and doxorubicin combination treatment of glioblastoma with scFv-targeted micelles: in vitro evaluation on 2D and 3D tumor models. Eur J Pharm Biopharm. 2016;108:54–67. | ||
Amasya G, Badilli U, Aksu B, Tarimci N. Quality by design case study 1: design of 5-fluorouracil loaded lipid nanoparticles by the W/O/W double emulsion – solvent evaporation method. Eur J Pharm Sci. 2016;84:92–102. | ||
Xu X, Khan MA, Burgess DJ. A quality by design (QbD) case study on liposomes containing hydrophilic API: II. Screening of critical variables, and establishment of design space at laboratory scale. Int J Pharm. 2012;423(2):543–553. | ||
ich.org. ICH Harmonised Tripartite Guideline. Pharmaceutical Development Q8(R2) [updated August 2009; cited November 18, 2016]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf. Accessed November 28, 2016. | ||
Sainz V, Peres C, Ciman T, et al. Optimization of protein loaded PLGA nanoparticle manufacturing parameters following a quality-by-design approach. RSC Adv. 2016;6(106):104502–104512. | ||
Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238–252. | ||
Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133(3):238–244. | ||
Patras L, Sesarman A, Licarete E, et al. Dual role of macrophages in the response of C26 colon carcinoma cells to 5-fluorouracil administration. Oncol Lett. 2016;12(2):1183–1191. | ||
Bunker A, Magarkar A, Viitala T. Rational design of liposomal drug delivery systems, a review: combined experimental and computational studies of lipid membranes, liposomes and their PEGylation. Biochim Biophys Acta. 2016;1858(10):2334–2352. | ||
Rabanel JM, Aoun V, Elkin I, Mokhtar M, Hildgen P. Drug-loaded nanocarriers: passive targeting and crossing of biological barriers. Curr Med Chem. 2012;19(19):3070–3102. | ||
Ghasemian E, Vatanara A, Najafabadi AR, Rouini MR, Gilani K, Darabi M. Preparation, characterization and optimization of sildenafil citrate loaded PLGA nanoparticles by statistical factorial design. Daru. 2013;21(1):68. | ||
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. | ||
Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2014;4(1):81–89. | ||
Balanč B, Trifković K, Ðorđević V, et al. Novel resveratrol delivery systems based on alginate-sucrose and alginate-chitosan microbeads containing liposomes. Food Hydrocoll. 2016;61:832–842. | ||
Zhao L, Du J, Duan Y, et al. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: preparation, optimization and in vitro characterization. Colloids Surf B Biointerfaces. 2012;97:101–108. | ||
Briuglia ML, Rotella C, McFarlane A, Lamprou DA. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv Transl Res. 2015;5(3):231–242. | ||
Curić A, Reul R, Möschwitzer J, Fricker G. Formulation optimization of itraconazole loaded PEGylated liposomes for parenteral administration by using design of experiments. Int J Pharm. 2013;448(1): 189–197. | ||
Eriksson L, Johansson E, Kettaneh-Wold N, Wikström C, Wold S. Design of Experiments: Principles and Applications. 3rd ed. Umeå: MKS Umetrics AB; 2008. | ||
Soema PC, Willems GJ, Jiskoot W, Amorij JP, Kersten GF. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur J Pharm Biopharm. 2015;94:427–435. | ||
Sailor G, Seth AK, Parmar G, Chauhan S, Javia A. Formulation and in vitro evaluation of berberine containing liposome optimized by 32 full factorial designs. J Appl Pharm Sci. 2015;5(7):23–28. | ||
Missirlis D, Kawamura R, Tirelli N, Hubbell JA. Doxorubicin encapsulation and diffusional release from stable, polymeric, hydrogel nanoparticles. Eur J Pharm Sci. 2006;29(2):120–129. | ||
Fritze A, Hens F, Kimpfler A, Schubert R, Peschka-Süss R. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. Biochim Biophys Acta. 2006;1758(10):1633–1640. | ||
Haeri A, Alinaghian B, Daeihamed M, Dadashzadeh S. Preparation and characterization of stable nanoliposomal formulation of fluoxetine as a potential adjuvant therapy for drug-resistant tumors. Iran J Pharm Res. 2014;13(Suppl):3–14. | ||
Ghanbarzadeh S, Valizadeh H, Zakeri-Milani P. Application of response surface methodology in development of sirolimus liposomes prepared by thin film hydration technique. Bioimpacts. 2013;3(2):75–81. | ||
Hathout RM, Mansour S, Mortada ND, Guinedi AS. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS PharmSciTech. 2007;8(1):E1–E12. | ||
Chibowski E, Szcześ A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption. 2016;22(4):755–765. | ||
Roy B, Guha P, Bhattarai R, et al. Influence of lipid composition, pH, and temperature on physicochemical properties of liposomes with curcumin as model drug. J Oleo Sci. 2016;65(5):399–411. | ||
Mady MM, Darwish MM. Effect of chitosan coating on the characteristics of DPPC liposomes. J Adv Res. 2010;1(3):187–191. | ||
Mozuraityte R, Rustad T, Storrø I. Oxidation of cod phospholipids in liposomes: effects of salts, pH and zeta potential. Eur J Lipid Sci Technol. 2006;108(11):944–950. | ||
Yang T, Cui FD, Choi MK, et al. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm. 2007;338(1–2):317–326. | ||
Panwar P, Pandey B, Lakhera PC, Singh KP. Preparation, characterization, and in vitro release study of albendazole-encapsulated nanosize liposomes. Int J Nanomedicine. 2010;5:101–108. | ||
Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des. 2002;8(19):1695–1706. | ||
Patel BB, Majumdar AP. Synergistic role of curcumin with current therapeutics in colorectal cancer: minireview. Nutr Cancer. 2009;61(6):842–846. | ||
Xiao B, Si X, Han MK, Viennois E, Zhang M, Merlin D. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J Mater Chem B Mater Biol Med. 2015;3(39):7724–7733. | ||
Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS One. 2013;8(2):e57218. | ||
Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–1642. | ||
Lin J, Shigdar S, Fang DZ, et al. Improved efficacy and reduced toxicity of doxorubicin encapsulated in sulfatide-containing nanoliposome in a glioma model. PLoS One. 2014;9(7):e103736. | ||
Zhang Y, Yang C, Wang W, et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci Rep. 2016;6:21225. | ||
Huang SK, Mayhew E, Gilani S, Lasic DD, Martin FJ, Papahadjopoulos D. Pharmacokinetics and therapeutics of sterically stabilized liposomes in mice bearing C-26 colon carcinoma. Cancer Res. 1992;52(24):6774–6781. | ||
Riganti C, Voena C, Kopecka J, et al. Liposome-encapsulated doxorubicin reverses drug resistance by inhibiting P-glycoprotein in human cancer cells. Mol Pharm. 2011;8(3):683–700. | ||
Lin J, Yu Y, Shigdar S, et al. Enhanced antitumor efficacy and reduced systemic toxicity of sulfatide-containing nanoliposomal doxorubicin in a xenograft model of colorectal cancer. PLoS One. 2012;7(11):e49277. | ||
Sadzuka Y, Nagamine M, Toyooka T, Ibuki Y, Sonobe T. Beneficial effects of curcumin on antitumor activity and adverse reactions of doxorubicin. Int J Pharm. 2012;432(1–2):42–49. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.