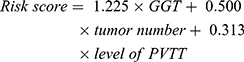Back to Journals » Cancer Management and Research » Volume 13
Development of a Prognostic Nomogram in Hepatocellular Carcinoma with Portal Vein Tumor Thrombus Following Trans-Arterial Chemoembolization with Drug-Eluting Beads
Authors Cheng S , Yu X, Liu S, Jin Z, Xue H, Wang Z, Xie P
Received 10 October 2021
Accepted for publication 10 December 2021
Published 24 December 2021 Volume 2021:13 Pages 9367—9377
DOI https://doi.org/10.2147/CMAR.S341672
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Professor Seema Singh
Sihang Cheng,1,2 Xiang Yu,2 Siyun Liu,3 Zhengyu Jin,1 Huadan Xue,1 Zhiwei Wang,1 Ping Xie2
1Department of Radiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 2Department of Radiology, Sichuan Provincial People’s Hospital, Sichuan Academy of Medical Sciences, Chengdu, People’s Republic of China; 3Pharmaceutical Diagnostics, GE Healthcare, Beijing, People’s Republic of China
Correspondence: Zhiwei Wang; Ping Xie Tel +8610 69159562
; +8628 87393976
Email [email protected]; [email protected]
Objective: To develop and validate a prognostic nomogram in eastern patients with hepatitis B virus (HBV)-associated hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT) receiving trans-arterial chemoembolization with drug-eluting beads (DEB-TACE).
Methods: This retrospective study included 200 patients with training cohort (n = 118) from institution 1 and test cohort (n = 82) from institution 2. All these patients received first-line DEB-TACE between October 2016 and October 2018. Multivariate Cox proportional hazard regression analysis was performed on the training cohort to reveal the independent prognostic factors, and then prognostic nomograms were developed. In order to evaluate the performance of the nomogram comprehensively in both the training and test cohorts, C-index, Kaplan–Meier curve with Log rank test, receiver operating characteristic curve (ROC), calibration plot, and decision curve analysis (DCA) were performed.
Results: Tumor number, serum γ-glutamyl transferase (GGT) level, and level of PVTT were independent risk factors of prognosis. A nomogram was constructed to predict 6-, 12- and 18-month overall survival (OS) based on these identified prognostic factors. C-indexes of the nomogram were 0.88 (95% confidence interval [CI], 0.79– 0.97) in the training cohort and 0.87 (95% CI, 0.75– 0.99) in the test cohort. The Kaplan–Meier curve analysis showed that the nomogram was able to separate patients into low- and high-risk subgroups. ROC curves for the nomogram at 6-, 12- and 18-month showed satisfied discrimination, with an AUC of 0.765, 0.803 and 0.809 in the training cohort, respectively, and 0.772, 0.724 and 0.746 in the test cohort, respectively. The calibration curve demonstrated good agreement between predicted and actual survival rates in the training and test cohorts. The decision curve showed good performance of the nomogram in terms of clinical application.
Conclusion: We developed and validated a nomogram that was accurate and clinically useful in eastern patients with HBV-associated HCC with PVTT who underwent DEB-TACE.
Keywords: hepatocellular carcinoma, portal vein tumor thrombus, trans-arterial chemoembolization, drug-eluting beads, nomogram
Introduction
Hepatocellular carcinoma (HCC) has been the sixth most common cancer and the third leading cause of cancer-related death in the world recently, with an increasing rate of 2% to 3% annually and a 5-year relative survival rate of 18%.1,2 Unfortunately, the opportunity for curative treatment is limited as approximate 10–40% of the patients are diagnosed at advanced stage with portal vein tumor thrombosis (PVTT).3,4 Recent preclinical studies and clinical trials evidence that combined treatments, involving alternative pathways, have an important role in therapy for advanced HCC, and they could bypass resistance to the tyrosine kinase inhibitors (TKIs).5 The advent of immune checkpoint inhibitors has also been reported to show unprecedented results in combination with bevacizumab.6,7 The combination therapy of trans-arterial chemoembolization (TACE) and sorafenib was confirmed to prolong survival for advanced HCC effectively.8 Among all these therapies, TACE was the most common treatment for HCC patients.
According to the Barcelona Clinic Liver Cancer (BCLC) staging system, TACE is the only recommended therapies for patients staged BCLC B.1 Different from western guidelines, most of the eastern guidelines, such as the Hong Kong Liver Cancer (HKLC) staging system, recommend that TACE could also be applied to patients with vascular invasion.9 In addition, several real-world studies reveal that TACE is widely performed not only in the intermediate stage but also in HCC with PVTT.10,11
With the development of embolization materials, drug-eluting beads (DEB)-TACE has become an alternative for the treatment of HCC in recent years. Compared with conventional TACE (cTACE), DEB-TACE has the advantage of stable drug releasing rate, long drug releasing time, and low systemic concentration of the chemotherapeutic agent.12 Theoretically, the prognosis between patients who undergo DEB-TACE and cTACE should be different. A recently published meta-analysis has demonstrated the superiority of DEB-TACE over cTACE in the aspect of tumor response rate and survival.13 Therefore, it is quite important to find significant prognostic factors, combined models or scoring systems that can accurately and individually predict the survival of patients who receive DEB-TACE. Thus, patients who may not benefit from this procedure can be identified preoperatively and transferred to alternative treatment plans promptly. Hepatoma arterial embolization prognostic (HAP) score, as the most commonly used and validated scoring system, consists of tumor size, alpha-fetoprotein (AFP), bilirubin, and albumin.14 Several improvements were made based on HAP, and modified HAP (mHAP)-II and mHAP-III were subsequently developed.15,16 Recently, Han et al11 have developed a new predictive model based on tumor number and size, AFP, albumin, bilirubin, vascular invasion, cause, and response, which showed higher predictive efficiency compared with the above-mentioned models. It is noteworthy that all these models are based on the results of cTACE with typical aetiologic pattern of western populations, whose predominant risk factors for HCC are hepatitis C virus (HCV) infection and alcohol abuse.17 However, for eastern populations, hepatitis B virus (HBV) infection is the main risk factor.18
Considering the fundamental differences in embolization materials and aetiology, we therefore aimed to establish a combined model for the precise and individualized prediction of overall survival (OS) based on clinical parameters in a real-life eastern hepatitis B virus (HBV)-associated HCC with PVTT cohort treated with DEB-TACE, and validate this model in an independent cohort.
Materials and Methods
Study Population
Approval and written informed consent for this study were waived by the Institutional Review Board (IRB) of Peking Union Medical College Hospital and Sichuan Provincial People’s Hospital due to its retrospective design. All the patients included in our study were histopathologically demonstrated to be HCC with PVTT or diagnosed by at least 2 senior radiologists according to Liver Imaging Reporting and Data System (LI-RADS) v2018. According to the Liver Cancer Study Group of Japan, the level of the PVTT (Vp) was classified into Vp1 (PVTT at distal to 2nd portal branch), Vp2 (PVTT at the 2nd portal branch), Vp3 (PVTT at the 1st portal branch), and Vp4 (PVTT at the main portal trunk).19 Patients were recruited from two institutions who underwent DEB-TACE between October 2016 and October 2018 and identified from institutional electronic databases. Patient consent to review their medical records was waived by the IRBs due to the retrospective nature of the study. We confirmed that patient data was maintained with confidentiality, and this study was in full compliance with the Declaration of Helsinki. Patients were included if they were treatment-naïve HBV-associated HCC with PVTT (BCLC stage C) before DEB-TACE, and had complete clinical and laboratory test data, imaging data and follow-up data. A total of 283 patients met the inclusion criteria. Patients were excluded if they had severe liver or kidney dysfunction, including hepatic encephalopathy, refractory ascites, Child-Pugh C, creatinine clearance <30 mL/min or creatinine level >2 mg/dl. The final study population included 200 patients.
DEB-TACE Treatment
Femoral artery puncture was performed using Seldinger technique. 5F-RH (Terumo, Tokyo, Japan) catheter was used for abdominal trunk and common hepatic artery angiography, combined with intraoperative cone-beam computed tomography (CBCT) and preoperative imaging data to reconfirm the location and number of tumors. If the tumor was found to be unclear, the phrenic artery, internal thoracic artery, and intercostal arteries would be added. A 2.5 F microcatheter (COOK, IN, USA) was used to superselect the tumor-feeding artery. Oxaliplatin and 5-FU were used for infusion chemotherapy. CalliSpheres® (Hengrui, Jiangsu, China) DEBs were loaded with epirubicin to embolize the tumor-feeding artery. After the treatment, CBCT was performed again to confirm the embolized area.
Follow-Up
After DEB-TACE treatment, patients were followed up every 2–3 months. Blood tests for the assessment of liver and kidney function, routine blood test, alpha-fetoprotein (AFP), and abdominal contrast-enhanced computed tomography (CECT) were recorded. Baseline CECT had to be within 1 month prior to treatment, and the first follow-up CECT should be within 1 month after treatment for patients included in this study. The range of follow-up time was from 16 to 26 months. OS was defined as the time from initial treatment using DEB-TACE to the date of death or the last follow-up.
Statistical Analysis
Continuous variables were expressed by mean ± standard deviation (SD) and were compared using the t-test; categorical variables were expressed as number and percentage and were compared using the Chi-square or Fisher’s exact test. All available clinical variables, including age, gender, history of cirrhosis, tumor number, tumor size, laboratory tests, and level of PVTT, were evaluated with a univariate Cox regression analysis on the training set. Variables with P-value <0.1 from univariate analysis were selected as candidates for further application in a multivariate Cox regression analysis. Likelihood ratio test based on the maximum partial likelihood estimates was applied to select the significant prognostic factors. The Schoenfeld residual test was used to test the proportional hazards (PH) assumption for each clinical factor.20 The clinical factors that satisfy the PH assumption enter into the Cox PH regression analysis to establish a prognostic model. In addition, a prognostic nomogram incorporating the selected predictors was constructed. The performance of the nomogram was measured by the concordance index (C-index). Survival curves were depicted using the Kaplan–Meier method, and statistical significance was determined using the Log rank test. A two-tailed P-value <0.05 was considered to be a statistically significant difference. Statistical analysis was done using SPSS 17.0 (IBM, Chicago, IL, USA) and R software (version 3.5.1; http://www.Rproject.org).
Development and Evaluation of Nomogram
Discrimination, calibration and clinical usefulness were evaluated for the prognostic nomogram. Receiver-operating characteristic (ROC) curve was performed to evaluate the discrimination performance of the nomogram based on area under the curve (AUC). Calibration performance was assessed based on agreement between predicted and actual survival rates in the calibration curve. Decision curve analysis (DCA) was performed to determine the clinical usefulness of the nomogram by calculating the net benefits at different threshold probabilities in the training and test cohort. Please see the workflow for this study in Supplementary Figure 1.
Results
Clinical Characteristics
The final study population consisted of 200 patients (median age, 58 years; range, 21–83 years): 118 of the 200 patients (59%) (median age, 61 years; range, 21–83 years) were included from institution 1 and constituted a training cohort; 82 of the 200 patients (41%) (median age, 56 years; range, 31–82 years) were included from institution 2 and constituted a test cohort. The main clinical characteristics of patients in the training and test cohorts are shown in Table 1. Demographic and pretreatment clinical characteristics did not show significant differences between the training and test cohorts.
 |
Table 1 Main Baseline Demographic and Clinical Characteristics of Patients in the Training and Validation Cohorts |
Prognostic Model Building
Univariate and multivariate Cox regression analyses were performed to determine preoperative clinical risk factors associated with survival (Table 2). In univariate analysis, there were significant correlation between γ-glutamyl transferase (GGT), tumor number, level of PVTT and OS. The multivariate analysis was then performed to identify factors distinguished in univariate analysis. Result showed that GGT, tumor number, and level of PVTT, were independent risk factors for prognosis of DEB-TACE treated HCC with PVTT, and a prognostic model with these parameters was built for prediction of OS. The cut-off value obtained was 2.00, which dichotomized the cohort into high-risk group with risk score >2 and low-risk group with risk score ≤2:
 |
Table 2 Preoperative Clinical Risk Factors for Overall Survival in Patients with HCC |
(Value assigned: GGT ≤ 50 U/L = 0, GGT > 50 U/L = 1; tumor number ≤3 = 1–3, tumor number >3 = 4; Vp1 = 1, Vp2 = 2, Vp3 = 3, Vp4 = 4).
The Schoenfeld residual test also showed that the preoperative clinical features and the final preoperative clinical model satisfy the PH assumption with P-value >0.05, as shown in Table 3 and Figure 1. The predictive model yielded a C-index of 0.88 (95% CI, 0.79–0.97) in the training set, and 0.87 (95% CI, 0.75–0.99) in the test set.
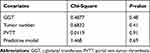 |
Table 3 Test the Proportional Hazards Assumption of Clinical Characteristic Using Schoenfeld Residual Test |
 |
Figure 1 Visualization of Schoenfeld residual test of proportional hazards assumption for clinical characteristics. |
Survival Outcomes
In the training cohort, the 6-, 12- and 18-month survival rates were 88.9%, 68.9% and 51.9%, respectively, for patients in the low-risk group, and 42.8%, 7.1% and 0%, respectively, for patients in the high-risk group (P-value <0.0001; Table 4 and Figure 2A). The same cut-off value of risk score was used in the test cohort with the 6-, 12- and 18-month survival rates of 89.0%, 67.0% and 56.8%, respectively, in the low-risk group, and 35.7%, 21.6% and 11.1%, respectively, in the high-risk group (P-value = 0.00014; Table 4 and Figure 2B).
 |
Table 4 The Median Survival Time and Survival Rate According to Risk Stratification |
Development and Performance of Nomogram
A nomogram was constructed to predict 6-, 12- and 18-month OS, based on the identified prognostic factors (Figure 3). ROC curves for the nomogram at 6-, 12- and 18-month showed satisfied discrimination, with an AUC of 0.765, 0.803 and 0.809 in the training cohort (Figure 4A), respectively, and 0.772, 0.724 and 0.746 in the test cohort, respectively (Figure 4B). Time-dependent ROC (tdROC) curves were also performed to show the trend of AUC values over time in both training and test cohorts (Figure 5). The calibration curve demonstrated good agreement between predicted and actual survival rates in the training (Figure 6A–C) and test (Figure 6D–F) cohorts. The decision curve showed good performance of the nomogram in terms of clinical application, which added more benefit than either a treat-all or treat-none scheme (Figure 7).
 |
Figure 3 A nomogram to predict the 6-, 12- and 18-month survival rates for patients in the training cohort. |
 |
Figure 4 ROC curves for the nomogram at 6-, 12- and 18-month in the training cohort (A) and test cohort (B). |
 |
Figure 5 Time-dependent ROC curves to show the trend of AUC values over time both in the training and test cohorts. |
 |
Figure 6 Calibration curves to evaluate the performance of the nomogram in 6-, 12- and 18-month survival rates in the training (A–C) and test (D–F) cohorts. |
Discussion
To the best of our knowledge, this is the first study focusing on the prognostic model construction and validation of HBV-associated HCC with PVTT following DEB-TACE in a real-life eastern cohort. In this study, we proposed a predictive model with a corresponding nomogram based on GGT, tumor number and level of PVTT, which were found to be significantly associated with the survival of patients in the training cohort. Importantly, this model was validated on a test cohort with satisfactory performance, implying its potential use as a promising tool for the prediction of survival.
Many studies have investigated the prognostic factors for TACE-treated HCC with PVTT. Luo et al21 conducted a prospective study to explore the survival benefit and prognostic factors of TACE in patients with HCC and PVTT, and they found that level of PVTT, tumor number, tumor size, and serum bilirubin level were independent prognostic factors for survival. In a retrospective study that enrolled 3126 consecutive TACE-treated patients who suffered from HCC with PVTT, their multivariate analysis showed that Child-Pugh class and level of PVTT were the independent prognostic factors affecting survival.22 Liang et al23 proposed that the presence of ascites, arteriovenous fistula and TACE response were significant prognostic factors, which needed further validation as only 57 patients were retrospectively enrolled. In addition, tumor burden and extrahepatic metastasis were also demonstrated to be closely associated with OS in selected patients with HCC and segmental PVTT.24 Based on these potential factors, various prognostic models and scoring systems for survival prediction were developed.11,14–16,25 However, for DEB-TACE-treated patients with HCC and PVTT, few studies have been reported.
A most recently published study demonstrated that the higher Child-Pugh classification and liver tumor burden were independent prognostic factors associated with poor survival for HCC patients with PVTT treated by DEB-TACE.26 Interestingly, these significant risk factors revealed by their study were consistent with another retrospective study conducted by Gorodetski et al,9 but not with ours. The reasons for this discrepancy might be that the sample sizes for these 2 studies were relatively small with only 58 and 38 patients enrolled, respectively, and no validations were performed to prove the reliability of their results. In addition, another previous retrospective study conducted in patients with HCC and PVTT following DEB-TACE found that age, the extent of liver involvement and weight were also independent prognostic factors for survival.27 Although lots of potential survival predictors were disclosed, only few prognostic models were developed or validated. Peisen et al17 reported that the established mHAP-II score could be used for the survival prediction of HCC following DEB-TACE. Nevertheless, this model was validated in western cohorts with alcohol abuse contributing most of the aetiology, and patients with HCC staged BCLC C only contributed 19% of the whole cohort, indicating that the performance of mHAP-II score was still controversial if the model was applied in eastern HBV-associated HCC with PVTT cohort. In order to find the most suitable population for DEB-TACE treatment, Prajapati et al28 proposed a BCLC C HCC Prognostic (BCHP) staging system based on Child-Pugh class, Eastern Cooperative Oncology Group (ECOG) performance status (PS), tumor size, site of venous thrombus, metastasis, and serum level of creatinine and AFP. However, 61.3% of the patients with HCC were caused by HCV infection, and no validation was performed. Therefore, the results of our study were more applicable to HBV-associated HCC in the eastern population.
GGT is a glycoprotein mainly located on the plasma membranes of hepatocytes and has the function of extracellular catabolism of glutathione.29 It was widely accepted that GGT had a close relationship with the risk of cancer, and a positive association between GGT and overall cancer outcome was found by a meta-analysis, which contained 10 cohort studies.30 In our study, GGT was proved to be one of the independent prognostic factors affecting survival of patients with HCC treated by DEB-TACE. Notably, a recently published study reported that pretreatment of high levels of serum GGT was associated with poor OS in patients with advanced HCC treated with TACE,31 which was consistent with our results. In fact, Zhang et al32 reported earlier that GGT could be used to predict survival of patients with intermediate HCC who received TACE, and higher serum GGT level was closely correlated with worse prognosis, even in small HCC and normal AFP subgroup. Subsequently, Guiu et al33 proposed that no validation was performed by the above-mentioned study and the applicability of their conclusions in European populations needs to be verified. Therefore, an external validation was conducted in a group of 88 patients, and the authors demonstrated that GGT was an independent predictor of OS in European population as well. However, the underlying correlation between GGT and DEB-TACE therapy effect is still unclear. Previous studies postulated that reactive oxygen species (ROS) produced by GGT-mediated metabolism and GGT-dependent resistance to platinum drugs might contribute to tumor progression,29,34 which needed to be further verified in our cohort.
There were several limitations in our study. First, as this study was retrospectively designed, bias might be inevitable most of the time. Second, although the results of our training cohort were validated in an independent cohort from another center, more validations needed to be conducted by different centers to demonstrate the generalizability of our results. Finally, the duration of our follow-up time was not long enough, thus the long-term effect of DEB-TACE on patients with HCC and PVTT was still unclear. Therefore, follow-up with longer time should be done in the future. Actually, Sorafenib remains a unique TKI for the treatment of advanced HCC able to block signal transduction mediated by the mitogen-activated protein kinase (MAPK) pathway; however, HCC cells often presented an intrinsic and acquired resistance to TKIs, due to several mutations and loss of functions. Recent breakthroughs could propose the strategy of combined targeted therapy in HCC, and the multi-pathways inhibition, together with more frequently customized treatments such as DEB-TACE, could eventually open the door for better results in the treatment of HCC.5
In conclusion, the prognostic model that we developed based on these factors showed satisfactory performance in both the training and the validation cohort, and we then built a nomogram for better visualization and application of the model, which needs further validation in the future. Our study holds the potential that this model can be improved with the increase in sample size and the development of statistical methods, and is expected to become a practical tool for the prediction of survival in clinic with the help of artificial intelligence, which might be the main knowledge gaps for clinicians needing to tackle, in the next few years.
Funding
This work was funded by Major Project of Science and Technology Innovation 2030 - “New Generation of Artificial Intelligence” (2020AAA0109503).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
3. Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol. 2014;10(3):153–161.
4. Lu J, Zhang XP, Zhong B-Y, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4(9):721–730. doi:10.1016/S2468-1253(19)30178-5
5. Gnoni A, Licchetta A, Memeo R, et al. Role of BRAF in hepatocellular carcinoma: a rationale for future targeted cancer therapies. Medicina. 2019;55. doi:10.3390/medicina55120754.
6. Rizzo A, Ricci AD, Brandi G. Immune-based combinations for advanced hepatocellular carcinoma: shaping the direction of first-line therapy. Fut Oncol. 2021;17(7):755–757. doi:10.2217/fon-2020-0986
7. Rizzo A, Ricci AD, Brandi G. Atezolizumab in advanced hepatocellular carcinoma: good things come to those who wait. Immunotherapy. 2021;13(8):637–644. doi:10.2217/imt-2021-0026
8. Dai Y, Jiang H, Jiang H, et al. Optimal timing of combining sorafenib with trans-arterial chemoembolization in patients with hepatocellular carcinoma: a meta-analysis. Transl Oncol. 2021;14(12):101238. doi:10.1016/j.tranon.2021.101238
9. Gorodetski B, Chapiro J, Schernthaner R, et al. Advanced-stage hepatocellular carcinoma with portal vein thrombosis: conventional versus drug-eluting beads transcatheter arterial chemoembolization. Eur Radiol. 2017;27(2):526–535. doi:10.1007/s00330-016-4445-9
10. Park J, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
11. Han G, Berhane S, Toyoda H, et al. Prediction of survival among patients receiving transarterial chemoembolization for hepatocellular carcinoma: a response‐based approach. Hepatology. 2020;72(1):198–212. doi:10.1002/hep.31022
12. Raoul J-L, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi:10.1016/j.ctrv.2018.11.002
13. Han T, Yang X, Zhang Y, et al. The clinical safety and efficacy of conventional transcatheter arterial chemoembolization and drug-eluting beads-transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a meta-analysis. Biosci Trends. 2019;13(5):374–381. doi:10.5582/bst.2019.01153
14. Pinato DJ, Arizumi T, Allara E, et al. Validation of the hepatoma arterial embolization prognostic score in European and Asian populations and proposed modification. Clin Gastroenterol Hepatol. 2015;13:1204–1208.e2. doi:10.1016/j.cgh.2014.11.037
15. Kim BK, Shim JH, Kim SU, et al. Risk prediction for patients with hepatocellular carcinoma undergoing chemoembolization: development of a prediction model. Liver Int. 2016;36(1):92–99. doi:10.1111/liv.12865
16. Cappelli A, Cucchetti A, Cabibbo G, et al. Refining prognosis after trans-arterial chemo-embolization for hepatocellular carcinoma. Liver Int. 2016;36(5):729–736. doi:10.1111/liv.13029
17. Peisen F, Maurer M, Grosse U, et al. Predictive performance of the mHAP-II score in a real-life western cohort with hepatocellular carcinoma following trans-arterial chemoembolisation with drug-eluting beads (DEB-TACE). Eur Radiol. 2020;30(7):3782–3792. doi:10.1007/s00330-020-06734-8
18. Park Y, Kim BK, Park JY, et al. Feasibility of dynamic risk assessment for patients with repeated trans-arterial chemoembolization for hepatocellular carcinoma. BMC Cancer. 2019;19(1):363. doi:10.1186/s12885-019-5495-6
19. Kudo M, Kitano M, Sakurai T, Nishida N. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: the outstanding achievements of the liver cancer study group of Japan. Digest Dis. 2015;33(6):765–770. doi:10.1159/000439101
20. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. doi:10.1093/biomet/81.3.515
21. Luo J, Guo R-P, Lai ECH, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi:10.1245/s10434-010-1321-8
22. Lv W-F, Liu K-C, Lu D, et al. Transarterial chemoembolization for hepatocellular carcinoma combined with portal vein tumor thrombosis. Cancer Manag Res. 2018;10:4719–4726. doi:10.2147/CMAR.S166527
23. Liang H, Cui P, Guo Q, et al. Prognostic factors of hepatocellular carcinoma patients with portal vein tumor thrombosis treated with transcatheter arterial chemoembolization. Asia Pac J Clin Oncol. 2017;13(5):e331–e341. doi:10.1111/ajco.12606
24. Kim JH, Shim JH, Yoon H-K, Ko H-K, Kim JW, Il Gwon D. Chemoembolization related to good survival for selected patients with hepatocellular carcinoma invading segmental portal vein. Liver Int. 2018;38(9):1646–1654. doi:10.1111/liv.13719
25. Zhao S-M, Qiu L-W, Zhao H, et al. Prognostic nomogram for hepatocellular carcinoma patients after transarterial chemoembolization based on des-γ-carboxy prothrombin reactivity and modified Response Evaluation Criteria in Solid Tumors. J Cancer Res Ther. 2021;17(3):707–714. doi:10.4103/jcrt.JCRT_651_20
26. Zhou T-Y, Chen S-Q, Wang H-L, et al. Safety and efficacy of drug-eluting bead transarterial chemoembolization with CalliSpheres® microsphere for hepatocellular carcinoma with portal vein tumor thrombus: a preliminary study. J Cancer. 2021;12(15):4522–4529. doi:10.7150/jca.54650
27. Akinwande O, Kim D, Edwards J, et al. Is radioembolization ((90)Y) better than doxorubicin drug eluting beads (DEBDOX) for hepatocellular carcinoma with portal vein thrombosis? A retrospective analysis. Surg Oncol. 2015;24(3):270–275. doi:10.1016/j.suronc.2015.06.008
28. Prajapati HJ, Kim HS. Treatment algorithm based on the multivariate survival analyses in patients with advanced hepatocellular carcinoma treated with trans-arterial chemoembolization. PLoS One. 2017;12(2):e0170750. doi:10.1371/journal.pone.0170750
29. Kunutsor SK. Gamma-glutamyltransferase—friend or foe within? Liver Int. 1723–1734;36(2016). doi:10.1111/liv.13221
30. Long Y, Zeng F, Shi J, Tian H, Chen T. Gamma-glutamyltransferase predicts increased risk of mortality: a systematic review and meta-analysis of prospective observational studies. Free Radic Res. 2014;48(6):716–728. doi:10.3109/10715762.2014.902055
31. Guo J, Liu S, Gao S, et al. γ-Glutamyltranspeptidase as a prognostic biomarker in advanced hepatocellular carcinoma treated with transarterial chemoembolization. J Vasc Interv Radiol. 2021;32(3):419–428.e2. doi:10.1016/j.jvir.2020.07.020
32. Zhang JB, Chen Y, Zhang B, et al. Prognostic significance of serum gamma-glutamyl transferase in patients with intermediate hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol. 2011;23(9):787–793. doi:10.1097/MEG.0b013e32834902dd
33. Guiu B, Deschamps F, Boulin M, et al. Serum gamma-glutamyl-transferase independently predicts outcome after transarterial chemoembolization of hepatocellular carcinoma: external validation. Cardiovasc Intervent Radiol. 2012;35(5):1102–1108. doi:10.1007/s00270-011-0293-9
34. Daubeuf S, Balin D, Leroy P, Visvikis A. Different mechanisms for gamma-glutamyltransferase-dependent resistance to carboplatin and cisplatin. Biochem Pharmacol. 2003;66(4):595–604. doi:10.1016/s0006-2952(03)00343-5
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.