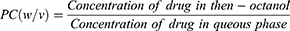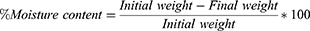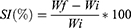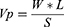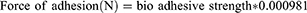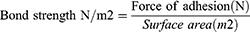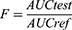Back to Journals » Drug Design, Development and Therapy » Volume 16
Development, in vitro Evaluation, and in vivo Study of Adhesive Buccal Films for the Treatment of Diabetic Pediatrics via Trans Mucosal Delivery of Gliclazide
Authors Gaber DA, Alburaykan AI, Alruthea LM, Aldohan NS, Alharbi RF, Aljohani AR, Albilaihi HM, Adogim SS
Received 31 October 2022
Accepted for publication 2 December 2022
Published 13 December 2022 Volume 2022:16 Pages 4235—4250
DOI https://doi.org/10.2147/DDDT.S394523
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Dalia A Gaber,1,2 Abeer I Alburaykan,3 Lama M Alruthea,3 Njoud S Aldohan,3 Raneem F Alharbi,3 Alhanoof R Aljohani,3 Helah M Albilaihi,3 Somaiah S Adogim3
1Department of Quality Control & Quality Assurance, Holding Company for Biological Products and Vaccines, Cairo, Egypt; 2Department of Pharmaceutics, College of Pharmacy, AL-Qassim University, Al-Qassim, Kingdom of Saudi Arabia; 3College of Pharmacy, Al- Qassim University, Al-Qassim, Kingdom of Saudi Arabia
Correspondence: Dalia A Gaber, Tel +20 1013379892, Fax +2 224154781, Email [email protected]
Objective: Development and evaluation of bucco-adhesive films of Gliclazide for pediatric use.
Methods: Sixteen films were formulated using a different combination of Gelatin, Hydroxy propyl methyl cellulose (HPMC), polyvinyl alcohol, Hydroxy propyl cellulose (HPC), chitosan, polyethylene glycol, sodium alginate, and carbopol. Compatibility study for drug and polymers was conducted using differential scanning calorimetry method and Fourier transform infrared spectroscopy. All films were examined for drug content, weight variation, thickness, swelling index, muco-adhesion and folding endurance. In vitro drug release has been completed for two hours. Stability studies were conducted at 4°C, 25°C, and 40°C for selected films. The optimized formulation based on in vitro data was selected for a bioavailability study in rabbits.
Results: The selected film formula (carbopol 2%, HPMC 2%) did not demonstrate interactions between the drug and polymers, while it showed accepted content, muco-adhesion, and mechanical properties. The in vitro release study showed rapid and complete release of drug from films. Stability studies confirmed accepted stability of the selected film at 4°C and 25°C, but the film get hard with few particles at 40°C. The bioavailability studies conducted showed that there was 2.1 fold increase in the AUC0-24 of selected film compared with oral tablets.
Conclusion: Bucco adhesive films of Gliclazide is a promising dosage form for the treatment of diabetes in children.
Keywords: diabetic, chitosan, pediatric, stability, bioavailability
Introduction
Diabetes mellitus (DM) is a chronic disease in which blood sugar increases above normal levels. Insulin, a hormone released by pancreas cells, is responsible for regulating blood glucose levels.1 Type I diabetes occurs when the pancreas does not produce any insulin, and type 2 diabetes (T2D) occurs when the pancreas does not produce enough insulin or the body’s cells do not react to insulin.2 Sulphonylureas (SUs) is one of the vital groups used for the treatment of T2D. They are one of the second-line agents that proved their efficacy and safety for long-term use, in addition to its reasonable cost.3 SUs work by stimulating the release of insulin from pancreatic B-cells. Gliclazide (GZ) is one of the second-generation sulfonylurea drugs (Figure 1) that are on the World Health Organization’s (WHO) essential medicines list.4 GZ is the drug of choice for the treatment of type II diabetes in the children and elderly since it is less possible to create hypoglycemia.5 Researchers reported that Tmax of GZ is between 2 to 8 hours after a single oral dosage due to its limited solubility and pH-dependency.6 GZ has a small volume of distribution and a high plasma protein binding rate of 94.2%, and it undergoes significant hepatic metabolic biotransformation to create many inactive metabolites.7 The diversity in GZ absorption observed among individuals which might be due to its early breakdown in the stomach, which leads to higher variability in intestinal absorption.8,9 The traditional oral dosage formulations have limited bioavailability as a result of this process.10 Moreover, tablets and capsules are not suitable for infants and children and solution dosage forms showed low patient compliance due to the unaccepted taste and inaccurate dose.11,12 A Buccal region has arisen as an attractive alternative to traditional oral dosage forms, that it provides a highly vascularized area with no enzymatic activity, no drug degradation, rapid onset of action, and self-use by the patient, In the market, there are several commercial buccal drug delivery dosage forms, including buccal tablets, sprays, mucoadhesive, sublingual lozenges, chewing gum, films, and oral mucosal solutions.13,14 Buccal films are thin, accurate-dose, soft form easily applied by adults and children.15 To assure the retention of drug on the buccal mucosa, the buccal film should has an accepted mucoadhesive properties. Ideal mucoadhesive properties and suitable drug release pattern are depending on the polymers used for film formulation.16,17 Mucoadhesive polymers have received a lot of attention as buccal film carriers due to their capacity to create, intimate and persistent contact with mucosa and enhance medication absorption.18 However films formed by more than one polymer usually exhibit superior properties compared to those formed by an individual polymer.19 Researchers reported a large number of successful polymers in the area of bucco-adhesive dosage forms.16,18 Gelatin is a natural, non-immunogenic, non-antigenic, biocompatible, and biodegradable bioadhesive polymer, with molecular weights 20–200 kDa on average. Gelatin showed a good ability to form gels and enlargement depending on its concentration-that help the forming of a mechanical bond with mucin covering the buccal cavity.19 Polyvinyl alcohol (PVA) is an uncharged hydrophilic biodegradable polymer. It has been widely employed in medication delivery systems as a carrier polymer.20 Chitosan (CHN) is a polysaccharide made by the deacetylation of chitin, the world’s most prevalent polysaccharide after cellulose. Both -OH and -NH2 groups of chitosan help in forming hydrogen bonds with mucin.21 Carbopol (CP) is a synthetic white, dry powder.22 It is a water soluble hydrophilic polymer having water sorption property, it swells in water for more than one hundred times its original volume. It has been demonstrated to form a strong adhesion to the mucus membrane.22 The cellulose derivatives such as hydroxypropyl methylcellulose, and hydroxypropyl cellulose are most commonly used in the design of mucoadhesive preparations.23 HPMC is a non-ionic neutral cellulose derivative that has mild mucoadhesive characteristics. On the other hand, HPC is a completely odorless, tasteless, water soluble polymer that has been commonly used as a thickening agent and a film coating material for control drug release.24 Sodium alginate (SA) is a natural hydrophilic polymer derived from marine brown algae.25 It is an ionic polymer with thinking, gel forming, and stabilizing properties.26 Because of its biocompatibility and biodegradability, it has been intensively studied in the pharmaceutical field.27 The study aimed to design a bucco adhesive film of GZ for pediatric use. Gelatin with different polymers in different ratios were used for preparing sixteen buco adhesive film of GZ. Drug/polymers compatibility was checked using differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FT-IR). Films also were characterized for muco-adhesive properties and in-vitro drug release. Finally, in vivo pharmacokinetic parameters were tested for the selected optimized film in comparison to commercial tablets as a reference.
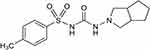 |
Figure 1 Chemical structure of gliclazide. |
Materials and Methods
Material
Gliclazide (GZ) and Gelatin were purchased from Sigma-Aldrich (Germany). Hydroxypropyl Methylcellulose (HPMC), hydroxypropyl cellulose (HPC) and polyvinyl alcohol (PVA) were a kind gift from El Kahera Pharmaceuticals (Cairo, Egypt). Chitosan (M.Wt 1526.5), Carbopol 971 P – CP- (M.Wt 94.05), and Polyethylene glycol 4000 (PEG) were purchased from Fisher Chemical (Leicestershire UK). Sodium alginate powder –SA-(M.Wt 418.23), Glycerol, Potassium dihydrogen phosphate, sodium hydroxide, potassium chloride and calcium chloride were purchased from May & Baker (Dagenham, England). Glipizide (Internal standard) and Gliclazide® commercial tablets were supplied by SIGMA pharmaceutical Industries, Egypt.
Methods
The formulation of gliclazide bucco- adhesive films were completed based on the solvent casting technique. Sixteen GZ bucco- adhesive films were prepared. Different concentrations of gelatin, PVA, CHN, HPMC, CP, HPC, PEG, and SA dispersions or solutions were prepared and mixed for 2 hours at 75 rpm resulting in different combinations for the film formulations according to Table 1. Gelatin (1%) was prepared by the addition of gelatin to distilled water under constant stirring. Then, PVA solution (21% w/w) was prepared by dissolving PVA in distilled water under constant stirring and heating at 80°C until complete dissolution. CHN (2%) was prepared by adding the required amount of CHN to acetic acid (1% V/V). HPMC (2%), HPC (2%), SA (1%), and CP (1.5%) were prepared by the addition of HPMC, HPC, SA, and CP to distilled water under constant stirring. Glycerol as a plasticizer was added to some formulations and then poured into suitable molds. The films were left to dry for 24–48 hours in a well-aerated space at room temperature. The films were then detached, wrapped in aluminum foil, and kept in well-closed containers for further investigations.28
 |
Table 1 Drug/ Polymer Composition and Concentration in the Different Formulations of GZ Mucoadhesive Films |
Determination of the Partition Coefficient of the Drug Between the Batch and Buccal Mucosa
The partition of GZ between the formula and buccal mucosa was determined using n-octanol: water system. Water and n-Octanol were pre-saturated with each other for at least 12 hours then one gram of GZ was dissolved in 5 mL of the n-octanol phase and shaken for 24 h against 5 mL aqueous phase in a sealed container at room temperature. n-octanol phase was then separated and the amount of GZ was assayed by UV spectrophotometer at 238 nm.29 The partition coefficient of GZ between the aqueous and organic phases was expressed as:
Characterization of Gliclazide Bucco-Adhesive Films (GZ-BF)
Differential Scanning Calorimetry Analysis
Thermal behavior of GZ and selected GZ-BF formulations components were considered using the differential scanning calorimetric method (DSC) (Mettler Toledo, Switzerland). In aluminum pans, five milligrams samples were heated at a rate of 10°C/minute between 50–200°C under a nitrogen flow of fifty mL/min.
Fourier Transform Infrared (FTIR) Spectroscopy
The possible interactions between GZ and polymers in films were showed. FTIR studies were presented to verify the possible interaction using FTIR spectrophotometer (Shimadzu 1800, Japan). Samples powder was blended with Potassium bromide powder and pellets were made by press.
Surface Study Using Scanning Electron Microscope (SEM)
The Surface morphology study was done for the optimal film (F10) using SEM (Mettler Toledo, Tokyo, Japan). One millimeter of the film was attached to SEM sampling stumps by double side tape and coated uniformly with a thin sheet of gold and the soft image was taken.
Weight Variation Test of Gliclazide Bucco-Adhesive Films
Ten films of each formula were weighed and the average weight of 10 films was calculated.30
Thickness of Gliclazide Bucco-Adhesive Films
The thickness of three films from each formula was measured using micrometre screw gauge at three different sites (middle, upper, and lower part), and the average thickness was calculated.30
Moisture Content Percentage
Three patches from each formula were weighed accurately and heated at 100–120 °C in a hot air oven for one hour. The dried films were reweighed.19 The percentage of moisture content was estimated through the following equation:
Surface pH
Three films from each formula were stirred individually in 20 mL of distilled water for half an hour; pH was then measured using a microprocessor pH meter.14
Flexibility of GZ-BF Preparations
Three films from each formula were selected randomly. Each film was folded at the same position until cracking occurred. The value of the film’s folding endurance was represented by the number of film folds before cracking. The mean folding endurance ± SD was considered.31
Percentage Swelling of GZ-BF Preparations
Three films from each prepared formulation were weighed and immersed individually in 30 mL of phosphate buffer solution (pH =6.8) for 60 minutes. Every 15 minutes the film was removed from the buffer, and excess liquid was removed by filter paper, weighted, and returned in the buffer. The swelling index (SI) will be estimated by adopting the following equation, where Wf is the weight after each immersing, Wi is the initial weight of the film:32
Drug Content Uniformity of the Films
Three films from each prepared formulation were dispensed separately in 100 mL phosphate buffer solution (pH 6.8), and stirred for one hour, GZ content in each film was analyzed by UV spectrophotometer at 238 nm. The results were calculated as a percentage and listed as the average of three trials ±SD.8
Measurement of Water Vapor Through Films
Blank films from all formulations were tested for water vapor permeation. Two grams of dried anhydrous calcium chloride were taken in an empty vial, and at the top of the vial blank films were fixed. The vials were weighed and they were kept in desiccators containing a saturated solution of potassium chloride, to maintain RH at 75±5%. The desiccator was tightly closed. The vials were weighed every day for seven days. Table 2 shows the rate of permeation for each film after seven days. The water vapor transmission rate (Vp) was expressed by the following equation:33
 |
Table 2 Different Physicochemical Parameters of the GZ Bucco-Adhesive Films |
Where, W: weight of water vapor transmitted in mg, L: thickness of the film in mm, S: surface area of the film in mm2.
Measurement of Mucoadhesive Strength
The tensile experiment was used to assess the strength of the bonds formed between the film and mucosa membrane. The test was done on a selected formulation based on the method published by Guptha et al.34 The sheep cheek pouch was used as a model membrane and phosphate buffer (pH 6.6) was used as a isotonic fluid. In a petri dish, a sheep cheek pouch was stacked onto the inner surface. Then the isotonic phosphate buffer was added. Two sides balance was used, it was made equal before the study, and five gram weight was used on the left side. The Petri dish was kept under the right hand of the balance. The films were stuck on to a lower side of the hanging assembly. Twenty microliters of phosphate buffer (pH 6.8) was added to the mucosal surface. The weight from the left pan was removed and kept untouched for 3 minutes. Then the weights on the left-hand side were added gradually and slowly until the film just detached from the membrane surface. The excess weight on the left pan was considered as adhesive strength, the following equations were used to calculate the force of adhesion (N) and bond strength:
Measurement of Mucoadhesive Time
The mucoadhesive duration of the buccal films was assessed using sheep buccal tissue. The time required until the film detached from the sheep buccal tissue in a stirred beaker was used to measure the mucoadhesive performance. A fresh sheep buccal tissue was glued on the side of the beaker. The films were attached to sheep tissue by applying light fingertip force for 30 seconds. The beaker was then filled with 500 mL phosphate buffer (pH 6.8), stirred at 50 rpm, and kept at 37°. The time taken by the film to detach from the porcine tissue was observed and recorded as muco-adhesion time.35
In vitro Drug Release Studies
The in vitro drug release studies for GZ bucco-adhesive batches were done in phosphate buffer solution (pH 6.8, 250 mL) at 37°C using a modified dissolution apparatus. The modified dissolution apparatus consisted of a 500 mL beaker with a magnetic stirrer (adjusted at 75 rpm) and a thermo-regulated hot plate was adopted for adjusting the temperature. The film was placed in a basket chamber and immersed in a dissolution medium. One-milliliter samples were withdrawn at predetermined time intervals (0, 5, 10, 20, 30, 40, 60, 90, and 120 minutes) for all formulation batches. For each sample withdrawn, an equivalent milliliter of phosphate buffer was added to the medium to keep the constant volume and sink condition. A five-fold dilution of each of the withdrawn sample was made and the diluted drug solutions were subsequently analyzed spectrophotometrically at 238 nm.30
Accelerated in vitro Stability Study
A stability study was conducted to study the physical changes, drug loss, and muco-adhesion characteristics of batches upon storage. The stability of the selected GZ films was assessed by wrapping the batches in an aluminum foil and storing it for 3 months at 4°C, 25°C, and 40°C and constant humidity. At constant time intervals (15, 30, 45, 60, and 90 days) the physical appearance, flexibility, moisture content, drug content, and muco-adhesion strength were evaluated by the method described in the previous sections.20
Bioavailability Study of GZ from the Selected Mucoadhesive Film
Based on the previous mucoadhesin and release results; F10 was selected for further studies in vivo. The study was in agreement with the stated principles of animal care published by the European center for the validation of alternative methods (NIH publication No. 8023, revised 1987), and was approved by the committee of research ethics, Al-Qassim University. Twelve healthy rabbits weighing 2.5 to 3 kg were selected and checked for the absence of any diseases just before the beginning of the study. Rabbits were divided into three groups equally and randomly with free access to water before and during the experiment. The dose of GZ was adjusted based on the rabbit weight. The film was located in the buccal membrane with the help of a clip for the first group. Tablets of the commercial product were crushed and the weight of the tablet with an equivalent amount of GZ was suspended in distilled water and given to rabbits in the second group via oral gavage. One mL of normal saline was given to rabbits in the third group as a control. Blood samples (0.5 mL) were withdrawn from the ear vein at 0.5, 1, 2, 3, 4, 6, 8, and 12 h after dosing in heparinized tubes. The collected blood samples were centrifuged (Hettich Zentrifugen, Germany) at 3500 RPM for 15 min and were stored in the freezer at −20°C until further work.36 The GZ concentration in plasma was assayed according to Binhashim et al, method after validation for selectivity, linearity, precision, accuracy, and stability just before the start of the study.37
Calculation of GZ Concentration in Plasma and Statistical Analysis of Data
All the presented pharmacokinetic parameters were deliberate from the plasma concentrations versus time curve. GZ plasma concentrations are showed as the mean ± SD. Maximum concentration, time to reach maximum concentration, and the area under the curve was reported as measured. The relative bioavailability (F) with commercial tablets was calculated as the following:
Statistical Estimation of the Results
All statistical analysis of the data were calculated by SPSS Statistics 17 (Armonk, NY, USA) using one-way ANOVA and extended LSD post hoc test for the pharmacokinetic parameters, and P value <0.01 was considered significant.
Result and Discussion
In the study, GZ mucoadhesive films were prepared through the solvent casting method which could be termed a simple and cost effective technique. The study involved the use of the following polymers gelatin, PVA, CHN, HPMC, CP, HPC, PEG, and SA. Different polymer content and concentration in addition to drug polymer ratio were evaluated. Physicochemical evaluation of the films, drug loading, in vitro releases, and in vivo performance was studied. All other processing factors were retained unvaried during the investigation.
Partition Coefficient Study
The partition coefficient of GZ in n-octanol water system was found to be 2.27 which predicts an accepted diffusion of the drug through the mucoadhesive buccal batches.8,10 Partition coefficient octanol-water of gliclazide, suggesting that GZ is a highly permeable drug. Furthermore, it shows that the transcellular passive pathway is the main permeation route of the drug.8 Results support the findings that GZ is a good candidate to deliver via the buccal route.
Surface Morphology
Figure 2 displays the scanning electron microscope captured image for the surface morphology of the selected formula (F10) and its corresponding blank (drug-free) formulation. The blank film (Figure 2A) shows extended, nearly network structures with an even and smooth loops and a lot of pores. F10 (Figure 2B) displayed also a network structure with smooth, straight lines with no evidence of drug particles aggregation which shows a uniform distribution of the drug without precipitation or aggregation.35
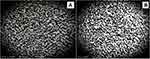 |
Figure 2 SEM image of plain buccal film (A) and drug loaded film (F10) (B). |
DSC Thermal Analysis
The DSC curve of pure GZ showed a single endothermic response corresponding to the melting point of the drug. The onset of melting was detected at 170.8 °C and the corresponding heat of fusion was 171.8 J/g (Figure 3A). The DSC thermograms of pure gelatin, chitosan, and carbopol (Figure 3B–D) showed a broad peak from 60°C to 100°C reflecting the typical thermal performance of the loss of surface water. However, the physical mixtures showed the characteristic melting peak of the drug with some broadening. The presence of the characteristic melting endotherm of GZ in the DSC thermograms of the physical mixtures designates the absence of interaction between the drug and the used polymers.38,39
 |
Figure 3 DSC thermogram for (A) gliclazide, (B) gelatin, (C) chitosan, and (D) carbopol. |
Fourier-Transform Infrared (FT-IR) Spectroscopy
FTIR test was used to describe the possible interactions between the drug and the other polymers in the solid state. The IR spectra of the selected formula (F-10) and polymers are compared with the spectrum of gliclazide in Figure 4A. The IR spectrum of GZ was characterized by the absorption of carbonyl (C=O) sulphonyl urea group and NH group at 1701 cm−1 and 3270 cm−1, respectively. In the spectra of F-10, the carbonyl (C=O) sulphonyl urea group was moved towards slightly higher frequencies at 1713 cm−1 and 1719 cm−1, respectively but the NH group band peak broaden in the F-10. The sulphonyl group peak were located at 1340 cm−1 and 1172 cm−1 in pure GZ. In the buccal batch, the symmetric vibration peak of S=0 band was shifted from 1340 cm−1 to 1349 cm−1 with a decrease in the frequency. The symmetric stretching band of S=0 was shifted from 1152 cm−1 to 1120 cm−1 with decreased frequencies also. Significant vibrations noticed in the spectrum of PEG was the C-H stretching at 2875 cm−1 and the C-O stretching at 1115cm−1. The appearance of the characteristic peaks of the drug and polymers in the FTIR spectra of the physical mixtures of GZ with HPC, PVA, and carbopol (Figure 4B) exposed the absence of physicochemical interactions between the drug and polymers.38,40
 |
Figure 4 FTIR spectrum of GZ (A), and mixture blend of F10 components (B). |
Characterization of GZ Bucco-Adhesive Films
Weight Variation
Table 2 shows the average weight of the films, as well as the standard deviation of the weight measurements. Results reveal that the average weight of all formulated films ranged between 0.047±0.002 and 0.081±0.003. The recorded average weight shows the appropriateness of prepared film for buccal use.41
Thickness, Moisture Content and Surface pH
The thickness of prepared formulations was ranged between 0.16±0.002 and 0.22±0.005 for F9 and F2 respectively (Table 2), which could be explained based on the polymer content in each formula. The thickness of the formulations was suitable for pediatric use in the buccal cavity without irritation or bulkiness feel for the patients.42 The moisture contents percentage of the prepared formulations ranged between 2.15±0.051 to 4.94±0.021 for F4 and F14 respectively. The difference in the moisture content between films might be due to the differences in the polymer content and concentration. The optimum moisture content in the film is needed to assure the optimal flexibility, stability, and non-susceptibility for microbial growth.43 The results revealed the significant effect of CP on the increase in film moisture content. While the films composed of chitosan showed a decrease in the moisture content. A negligible difference in the percentage of moisture in the films composed of CHN was reported. The high content of carboxylic acid groups in carbopol helps in enhancing its swelling ability. This could interpret the high moisture content in formulation (F9-F16). The low moisture content in formulations F1-F8 could be due to the presence of chitosan in its content and the compactness of the film network. The alkaline and acidic pH may cause inflammation or irritation to buccal mucosa and may also affect the hydration of polymers and drug release. Hence the surface pH of films was determined to adjust both mucoadhesion and drug release. All the films showed a pH ranging ranged between 6.2 and 7.06 for F15 and F1 respectively, films showed pH close to that of the buccal mucosa which enhances its activity and compatibility in buccal cavity. Results revealed accepted drug loading higher than 97.2% for all films formulations as shown in Table 2. This indicates the efficiency of the solvent casting technique in formulating films with a high loading.44 Non-significant differences in loading efficiency were detected between different films at p level <0.5. Minimum differences in drug loading between films could be due to the variation in content and concentration of polymers.
Mechanical Strength and Flexibility of Films
Figure 5 showed the value of the film’s folding endurance which is represented by the number of film folds before cracking. F1 and F14 showed the minimum and maximum folding endurance, respectively (100 ± 11 and 195 ± 10). Significant increase in folding endurance for F5, F6, F13 and F14, compared to the other formulations which could be explained based on the presence of PEG in these formula increases its endurance. Graphs also showed that CP increases the flexibility of formula while lower flexibility was observed for CHN films. Folding endurance gives a good idea about the behavior of the batch upon use in the buccal cavity and during storage. Firm films may fragment during storage and may lose part of the drug. In addition, firm film has a low ability to adjust in the buccal cavity after use. Also, it may induce discomfort, irritation, distress and loss of part of the drug after application. Folding endurance results showed a suitable elasticity of the films.14,17
 |
Figure 5 Film’s folding endurance for formulations F1-F16. |
Swelling Behavior of GZ-BF Formulations
The swelling behaviors of all formulations are presented in Figure 6. Figure 6A showed the swelling behavior of F1, F2, F3, and F4 which shows an accelerated increase in size began after 15 minutes and sustain up to 40 minutes then a decrease in size was observed. The swelling behavior of films is influenced by their structure and polymeric content. Polymeric hydrogels (HPMC, HPC) are three dimensional cross linked networks that have the ability to absorb water and swell without losing their shape. Their swelling behavior is affected mainly by external conditions (ie pH, temperature). Under the experiment condition the HPMC, HPC did not swell to its optimal value due to the limited swelling of these polymers at test pH.45 Swelling behavior of F5, F6, F7 and F8 showed in Figure 6B. Rapid swelling was observed in F5 and F6 which could be expressed based on the PEG content in the formula which helps in the rapid absorption of water and extraordinary increase in size. F7 and F8 showed no change in size during 1 hour, which could be due to the presence of SA in both formulations which reported that it, does not absorb water at pH 5–7. Swelling results showed a slow steady rate in F9 and F10, and a rapid continuous swelling rate for F11 and F12 (Figure 6C). F11, F12 showed a higher swelling ratio than F9 and F10 due to the more hydrophilic properties of HPC, in addition to the presence of hydroxyl group in the molecules which play an important role in water uptake and matrix integrity of swollen polymer.46 Rapid absorption of water and swelling by PEG with some degradation of CP are shown in F13, F14, while non-observable swelling was noticed for F15, and F16 (Figure 6D).
 |
Figure 6 (A–D) Swelling behavior of GZ buccal batch films in phosphate buffer. |
Water Vapor Permeation Rate
Water vapor permeation studies showed that all studied films were having good permeability to water vapor. Vp ranged between 6.50±0.14 and 10.11±0.15 for F16 and F5 respectively. Results revealed that the vapor permeation is depending on the polymer content of each formulation. A higher permeation rate was observed with chitosan formulations which is attributed to the nature of chitosan which is amine polysaccharides that form a thin hydrated gel that allows a higher rate of vapour permeation. The combination of chitosan with other polymers (HPMC, HPC, SA) showed a decrease in permeation due to the increase in gel strength. So lower permeation of carbopol formulations may be due to the highly cross-linked characters which produce highly viscous coherent matrix that hinders vapor permeation. Relevant results were reported by Semalty et al for enalapril maleate mucoadhesive buccal film.33
Mucoadhesion Strength and Time
Based on swelling, folding, and vapor transition results. Formulations were selected for further study. Figure 7 shows the mucoadhesion strength of the selected formulations. The films showed adhesion forces ranging between 0.11 ± 0.07 and 0.35 ± 0.04 N and bond strength ranged from 0.08 ± 0.02 to 0.26 ± 0.12 N/m2. All films under investigation showed adhesion time of more than 3.12±0.19 hours which is sufficient time to guarantee a complete release of the drug prior to film detachment. The buccal mucus layer is composed of glycoproteins, lipids, electrolytes, and water that covered the oral cavity. Both chitosan and carbopol have several advantages as a drug carrying polymers in buccal dosage forms, they have good compatibility with other hydrophilic polymers, accepted biocompatibility with mucosa, and an outstanding reputation in incorporating drugs.47 Many studies reported the use of chitosan/carbopol alone or in a combination with other polymers for the design of mucoadhesive buccal films. Chitosan films have been reported to have good mucoadhesive features as it has a positive amino group that is able to form attractions with the buccal mucus layer in the cavity.48 F4, F8 showed a stronger mucoadhesion force compared with F2, that can be interpretated based on the presence of HPC, and SA that both are hydrophilic polymers forms a viscous sticky gel which increase the mucoadhesive force of the film. Significant increase in mucodhesin was observed with carbopol films p˂0.5. The carbopol polymer hydrated in an aqueous medium and forms a gel. The high hydration ability of carbopol improves its adhesive force and facilitates long residence. The higher mucoadhesive force of F10 compared to other buccal films might be due to the HPMC content. HPMC is a hydrophilic matrix, which contains a large number of hydroxyl groups which capable to form a strong viscous gel in contact with the aqueous media, therefore leading to the development of a robust gel through hydrogen bonding that penetrates strongly into the mucous layer. The results are in a good coordination with the results reported by Gilhotra et al and El Sharawy et al.17,42
 |
Figure 7 Mucoadhesion force for the selected GZ buccal batch films. |
Drug Release Study
Figure 8 shows the in vitro release profiles of gliclazide from the selected formulations. Chitosan-based films; F2, F4, and F8 revealed 50–60% amount of GZ release within 30 minutes and 65–80% after one hour with non-significant increase in the amount of drug released up to two hours. The slower drug release from CN-based films (F2, F4, F8) might be ascribed to the slow swelling and erosion rate of the CN polymer. In addition the limited swelling ability decrease the drug diffusion from compact film matrix, that around 75–85% of the drug is only released after two hours. While carbopol based films showed 75–95% of GZ released after one hour. F10 displayed the fastest and complete drug release within two hours relative to all other formulations. The differences in the drug release from chitosan films (F2, F4, and F8) and carbopol films (F10, F12, F14, F16) were significant (P < 0.5). The high drug release rate from the CP-based films (F10, F12, F14, and F16) can be related to the rapid water absorption by the highly hydrophilic polymer that improved both the wetting and swelling behavior that help in enhancing penetration of water into the film, and thus increased drug release. Though, F10 (carbopol /HPMC) showed a significant increase in drug release (P < 0.5) compared to the other carbopol films. The increase in the of drug release from F10 compared to the other carbopol films (F12, F14, F16) might be related to the presence of HPMC polymer in the formula with CP that both are hydrophilic polymers with a high ability to adsorb water and swell.48 Related results were mentioned by Nair et al for rizatriptan and by Mady et al for miconazole-urea.43,44
 |
Figure 8 Release profile of GZ from selected buccal films (F2, F4, F8, F10, F12, F14 and F16) in 0.1N HCl buffer for 2 hours. |
Stability Study
The stability of GZ buccal films at different temperatures is an essential concern, to study the probability of drug degradation and/or film shrinkage or dryness upon storage. Based on release results F10, F12 and F14 were selected to be studied at three different temperatures ie 4°C, 25°C, and 40°C. The three formulations were assayed for its physical appearance, flexibility, moisture content, drug content, mucoadhesion time and mucoadhesion strength at the following intervals 15, 30, 45, 60 and 90 days. No significant physical changes in the texture, color and appearance of three films were observed all over the 90 days at 4°C and 25°C. At 40°C hardness and darking in the color were observed for the three tested films. Table 3 shows the results of the stability study; it reported that neither significant drug loss nor crystal growth was observed for the three films at 4°C and 25°C over 90 days. A non-significant decrease in drug content was observed at 25°C; that 98.9±1.5, 95.8±2.8 and 97.0±2.1 was recorded for F10, F12 and F14 respectively. However, an observable decrease in drug and moisture content occurred at 40°C at the end of 3 months. These results may be due to the high temperature that causes degradation of the drug and loss of water from films.49 In addition a remarkable decrease in muco-adhesion strength was observed for all films at 40°C which can be explained based on the dryness of the film and a significant reduction in moisture content that decrease the ability of film to adhere with mucosa.50
 |
Table 3 Stability of Selected GZ Films (F10, F12, F14) in Term of Folding Endurance, Moisture Content, Drug Content and Muco-Adhesion Strength at 4°C, 25°C, and 40°C After 90 Days |
In vivo Bioavailability Study Results
GZ-Buccal film (F10) showed optimal results in folding endurance, moisture content, muco-adhesion strength, release behavior and stability studies; so it was selected for further examination for in vivo activity. Binhashim et al method was followed for detecting concentration of GZ in plasma. The plasma concentrations curve of GZ after buccal administration of F10 and oral use of equivalent amount of commercial tablets showed that F10 enhanced the absorption of GZ compared with market tablets. Maximum plasma concentrations (Cmax) of GZ were 3.30 ±0.81 and 2.16± 0.28 μg/mL for F10 and market tablets, respectively (Table 4 and Figure 9). There was about 1.53 fold enhancement of Cmax of GZ from F10 as compared to oral reference tablets. The area under the plasma concentration–time curve (AUC) of GZ after 12 hours was 16.51±2.86 μg/mL h for oral tablets. The AUC of GZ after buccal administration of F10 was 23.06 ± 4.2 μg/mL h. Tmax (the time to reach the maximum plasma concentration) was significantly different at p level 0.5, it was 1.5±0.12 h for F10 and 3.2±0.16 for commercial tablets. The significant enhancement in the bioavailability of GZ from F10 over oral tablets could be interpreted on the base of faster absorption from highly vascularized buccal area and the bypass of liver metabolism pathway in addition to the accepted parameters of the designed buccal dosage form (ie adhesion force, adhesion time, release characteristics).50–52 Buccal films of rizatriptan, propranolol, and duloxetine hydrochloride showed higher bioavailability compared with its commercial tablets.30,42,43
 |
Table 4 Mean Pharmacokinetic Parameters of GZ After Buccal Use of F10, and Oral Administration of Commercial Tablets to Rabbits |
 |
Figure 9 GZ plasma concentration (mean ± S.E) time profiles in rabbits after administration of buccal film (F10) and commercial tablets. |
Conclusion
Improving the bioavailability of GZ was achieved by loading the drug in a buco-adhesive film. Different polymers in different ratios were tested for producing optimized formula. F10 composed of 1% gelatin, 2%HPMC, 3%PVC and 2% CP was found to be the optimal formula based on mucoadhesion, flexibility and in vitro release parameters. Optimum GZ buccoadhesive film showed a marked improvement (2.1 fold) in the bioavailability of GZ compared to convention tablets. The ease of administration, the absence of irritation and pain in addition to the high bioavailability make GZ-buccoadhesive film an ideal and promising dosage form for pediatrics.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wahyuni Y. Improving the quality of life of patients with diabetes mellitus type 2 with treatment adherence. Media Keperawatan Indonesia. 2021;4(3):244. doi:10.26714/mki.4.3.2021.244-256
2. Verma S, Gupta M, Popli H, Aggarwal G. Diabetes mellitus treatment using herbal drugs. Int J Phytomed. 2018;10(1):1. doi:10.5138/09750185.2181
3. Gaber DA, Alhuwaymili AS, Alhawas HS. Synthesized nano particles of glimepiride via spray freezing into cryogenic liquid: characterization, antidiabetic activity, and bioavailability. Drug Deliv. 2022;29:364–373. doi:10.1080/10717544.2021.2018524
4. Singh A, Singh R. Is gliclazide a sulfonylurea with difference? A review in 2016. Expert Rev Clin Pharmacol. 2016;9(6):839–851. doi:10.1586/17512433.2016.1159512
5. Singh R, Ahmed A, Bhattacharya K. Pharmacoscintigraphic evaluation and antidiabetic efficacy of gliclazide-loaded 99mTc-labelled mucoadhesive microspheres. Future J Pharma Sci. 2021;7(1). doi:10.1186/s43094-021-00322-3
6. Sarkar A, Tiwari A, Bhasin PS, Mitra M. Pharmacological and pharmaceutical profile of gliclazide: a review. J Appl Pharm Sci. 2011;1(9):11–19.
7. Alkhamis KA, Allaboun H, Al-Momani WY. Study of the solubilization of gliclazide by aqueous micellar solutions. J Pharm Sci. 2003;92:839–846. doi:10.1002/jps.10350
8. Varshosaz J, Talari R, Mostafavi SA, Nokhodchi A. Dissolution enhancement of gliclazide using in situ micronization by solvent change method. Powder Technol. 2008;187:222–230. doi:10.1016/j.powtec.2008.02.018
9. Saharan VA, Choudhury PK. Dissolution rate enhancement of gliclazide by ordered mixing. Acta Pharm. 2011;61:323–334. doi:10.2478/v10007-011-0021-7
10. Hosmani AH, Thorat YS. Optimization and pharmacodynamic evaluation of solid dispersion of gliclazide for dissolution rate enhancement. Lat Am J Pharm. 2011;30:1590–1595.
11. Ahmady A, Samah NH. A review: gelatine as a bioadhesive material for medical and pharmaceutical applications. Int J Pharm. 2021;608:121037. doi:10.1016/j.ijpharm.2021.121037
12. Padhi S, Nayak A, Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother. 2020;131:110708. doi:10.1016/j.biopha.2020.110708
13. Jacob S, Nair AB, Boddu SH, Gorain B, Sreeharsha N, Shah J. An updated overview of the emerging role of patch and film-based buccal delivery systems. Pharmaceutics. 2021;13(8):1206. doi:10.3390/pharmaceutics13081206
14. Singh R, Sharma D, Garg R. Review on mucoadhesive drug delivery system with special emphasis on buccal route: an important tool in designing of novel controlled drug delivery system for the effective delivery of pharmaceuticals. J Dev Drugs. 2017;6(01):1–2.
15. Aframian D, Davidowitz T, Benoliel R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis. 2006;12(4):420–423. doi:10.1111/j.1601-0825.2005.01217.x
16. Bala R, Khanna S, Pawar P, Arora S. Orally dissolving strips: a new approach to oral drug delivery system. Int J Pharm Investig. 2013;3(2):67. doi:10.4103/2230-973X.114897
17. Gilhotra RM, Ikram M, Srivastava S, Gilhotra N. A clinical perspective on mucoadhesive buccal drug delivery systems. J Biomed Res. 2014;28(2):81–97. doi:10.7555/JBR.27.20120136
18. Boddupalli B, Mohammed Z, Nath R, Banji D. Mucoadhesive drug delivery system: an overview. J Adv Pharm Technol Res. 2010;1(4):381. doi:10.4103/0110-5558.76436
19. Shaikh R, Singh TR, Garland MJ, Woolfson AD, Donnelly RF. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci. 2011;3(1):89. doi:10.4103/0975-7406.76478
20. Jovanović M, Tomić N, Cvijić S, Stojanović D, Ibrić S, Uskoković P. Mucoadhesive gelatin buccal films with propranolol hydrochloride: evaluation of mechanical, mucoadhesive, and biopharmaceutical properties. Pharmaceutics. 2021;13(2):273. doi:10.3390/pharmaceutics13020273
21. Falath W, Sabir A, Jacob KI. Novel reverse osmosis membranes composed of modified PVA/Gum Arabic conjugates: biofouling mitigation and chlorine resistance enhancement. Carbohydr Polym. 2017;155:28–39. doi:10.1016/j.carbpol.2016.08.058
22. Irikura K, Ekapakul N, Choochottiros C, Chanthaset N, Yoshida H, Ajiro H. Fabrication of flexible blend films using a chitosan derivative and poly (trimethylene carbonate). Polymer J. 2021;53(7):823–833. doi:10.1038/s41428-021-00470-6
23. Priyanka R, Prabhu R. Carbopol 71G-NF polymer –the next pillar of oral solid dosage form. Magna Scientia Adv Res Rev. 2020;1:010–017. doi:10.30574/msarr.2020.1.1.0018
24. Kamel S, Ali N, Jahangir K, Shah S, El-Gendy A. Pharmaceutical significance of cellulose: a review. J Express Polym Lett. 2008;2:758–778. doi:10.3144/expresspolymlett.2008.90
25. Martin Pastor M, Stoyanov E. Mechanism of interaction between hydroxypropyl cellulose and water in aqueous solutions: importance of polymer chain length. J Polymer Sci. 2020;58(12):1632–1641. doi:10.1002/pol.20200185
26. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–126. doi:10.1016/j.progpolymsci.2011.06.003
27. Krishnan V, Sasikumar S, Dass F, Vijayaraghavan R. Effect of Pore forming agents on the physical characteristics and release kinetics of levofloxacin hemihydrate from floating alginate drug delivery system - an in vitro study. J Trends Biomater Artif Organs. 2010;24:139–145.
28. Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57:1666–1691. doi:10.1016/j.addr.2005.07.003
29. Marin EL, Modamio P. Transdermal absorption of celiprolol and bisoprolol in human skin in vitro. Int J Pharma. 1998;173:141–148.
30. Mohamad SA, Salem H, Yassin HA, Mansour HF. Bucco-adhesive film as a pediatric proper dosage form for systemic delivery of propranolol hydrochloride: in-vitro and in-vivo evaluation. Drug Des Devel Ther. 2020;14:4277. doi:10.2147/DDDT.S267317
31. Pedacchia A, Adrover A. Study of release kinetics and diffusion coefficients in swellable cellulosic thin films by means of a simple spectrophotometric technique. Chem Eng Res Des. 2014;92(11):2550–2556. doi:10.1016/j.cherd.2014.03.017
32. Ganji F, Vasheghani FS, Vasheghani FE. Theoretical description of hydrogel swelling: a review. Iran Polym J. 2010;19(5):375–398.45.
33. Semalty A, Semalty M, Nautiyal U. Formulation and evaluation of mucoadhesive buccal films of enalapril maleate. Indian J Pharm Sci. 2010;72(5):571–575. doi:10.4103/0250-474X.78522
34. Gupta A, Garg S, Khar RK. Measurement of bioadhesive strength of muco-adhesive buccal tablets: design of an in-vitro assembly. Indian Drugs. 1992;30:152–155.
35. Kumria R, Nair AB, Goomber G, Gupta S. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016;23(2):471–478. doi:10.3109/10717544.2014.920058
36. Gaber D, Abdoun S, Alfuraihy A, Altasan B, Alsubaiyel A. Superhydrophobic surface for enhancing the bioavailability of salbutamol sulfate from cross-linked microspheres: formulation, characterization, and in vivo evaluation. Drug Des Devel Ther. 2021;15:2869–2884. doi:10.2147/DDDT.S309078
37. Binhashim NH, Alvi SN, Hammami MM. A validated reversed phase HPLC assay for the determination of gliclazide in human plasma. Saudi J Med Pharm Sci. 2022;10B:1128–1132.
38. Sapkal NP, Kilor VA, Bhusari KP, Daud AS. Evaluation of some methods for preparation of gliclazide- β-cyclodextrin inclusion complexes. Trop J Pharm Res. 2007;6(4):833–840. doi:10.4314/tjpr.v6i4.14667
39. Stetinova V, Polaskova A, Smetanova L, Kholova D, Herout V, Kvetina J. Toxicological studies, membrane transport and pharmacodynamic effect of gliclazide in rats. Toxicol Lett. 2008;180:58–59. doi:10.1016/j.toxlet.2008.06.639
40. Mapa B, Araújo L, Silva-Barcellos N, Caldeira T, Souza J. Gliclazide: biopharmaceutics characteristics to discuss the biowaiver of immediate and extended release tablets. Appl Sci. 2020;10(20):7131. doi:10.3390/app10207131
41. Li L, Li J, Si S. Effect of formulation variables on in vitrorelease of a water-soluble drug from chitosan-sodium alginate matrix tablets. Asian J Pharma Sci. 2015;10(4):314–321. doi:10.1016/j.ajps.2014.09.002
42. El Sharawy AM, Shukr MH, Elshafeey AH. Formulation and optimization of duloxetine hydrochloride buccal films: in vitro and in vivo evaluation. Drug Deliv. 2017;24(1):1762–1769. doi:10.1080/10717544.2017.1402216
43. Nair AB, Shah J, Jacob S, et al. Development of mucoadhesive buccal film for rizatriptan: in vitro and in vivo evaluation. Pharmaceutics. 2021;13(5):728. doi:10.3390/pharmaceutics13050728
44. Mady OY, Donia AM, Al-Madboly LA. Miconazole-urea in a buccal film as a new trend for treatment of resistant mouth fungal white patches. Front Microbiol. 2018;9:837. doi:10.3389/fmicb.2018.00837
45. Yildiz Pekoz A, Sedef Erdal M, Okyar A, et al. Preparation and in- vivo evaluation of dimenhydrinate buccal mucoadhesive films with enhanced bioavailability. Drug Dev Ind Pharm. 2016;42:916–925. doi:10.3109/03639045.2015.109147061
46. Koland M, Charyulu RN, Vijayanarayana K, Prabhu P. In vitro and in vivo evaluation of chitosan buccal films of ondansetron hydrochloride. Int J Pharm Investig. 2011;1:64–71. doi:10.4103/2230-973X.85967
47. Tejada G, Barrera M, Piccirilli G, et al. Development and evaluation of buccal films based on chitosan for the potential treatment of oral candidiasis. AAPS PharmSciTech. 2017;18(4):936–946. doi:10.1208/s12249-017-0720-652
48. Bahri-Najafi R, Tavakoli N, Senemar M, Peikanpour M. Preparation and pharmaceutical evaluation of glibenclamide slow release mucoadhesive buccal film. Res Pharm Sci. 2014;9(3):213–223.
49. Mohamad SA, Sarhan HA, Abdelkader H, Mansour HF. Vitamin B12-loaded buccoadhesive films as a noninvasive supplement in vitamin B12 deficiency: in vitro evaluation and in vivo comparative study with intramuscular injection. J Pharm Sci. 2017;106:1849–1858. doi:10.1016/j.xphs.2017.03.04025
50. Svensson O, Arnebrant T. Mucin layers and multilayers— physicochemical properties and applications. Curr Opin Colloid Interface Sci. 2010;15:395–405. doi:10.1016/j.cocis.2010.05.015
51. Dixit RP, Puthli SP. Oral strip technology: overview and future potential. J Control Release. 2009;139:94–107. doi:10.1016/j.jconrel.2009.06.01411
52. Trastullo R, Abruzzo A, Saladini B, et al. Design and evaluation of buccal films as pediatric dosage form for trans mucosal delivery of ondansetron. Eur J Pharm Biopharm. 2016;105:115–121. doi:10.1016/j.ejpb.2016.05.026
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.