Back to Journals » Journal of Inflammation Research » Volume 16
Development and Validation of a Rapid and Efficient Prognostic Scoring System for Sepsis Based on Oxygenation Index, Lactate and Glasgow Coma Scale
Authors Lai Q, Xia Y, Yang W, Zhou Y
Received 13 May 2023
Accepted for publication 10 July 2023
Published 18 July 2023 Volume 2023:16 Pages 2955—2966
DOI https://doi.org/10.2147/JIR.S418531
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Qiang Lai,1– 3 Yiqin Xia,1– 3 Wentao Yang,1– 3 Yiwu Zhou1– 3
1Emergency Department, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 2Laboratory of Emergency Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 3Disaster Medical Center, Sichuan University, Chengdu, Sichuan, People’s Republic of China
Correspondence: Yiwu Zhou, Emergency Department, West China Hospital of Sichuan University, Chengdu, Sichuan, People’s Republic of China, Email [email protected]
Objective: To develop a concise scoring system for efficient and rapid assessment of sepsis prognosis applicable to emergency departments.
Methods: This was a single-center retrospective cohort study of patients with sepsis. In this study, a new scoring system (oxygenation index, lactate, and Glasgow coma scale: GOL) was developed through a derivation group, and then the GOL was validated using a validation group. Multivariate logistic regression analysis was performed to investigate the relationship between GOL and 28-day adverse outcomes. The GOL was compared with the previous scoring system using receiver operating characteristic curves (ROC) and decision analysis curves. The endpoints of this study were mortality, mechanical ventilation (MV), and admission to the intensive care unit (AICU).
Results: 608 patients were included in the derivation group and 213 patients in the validation group, with 131 and 42 deaths, respectively. In the validation group, lactate (Lac), oxygenation index (PaO2/FiO2), and Glasgow coma scale score (GCS), the three best performers in predicting 28-day mortality from receiver operating characteristic curves, were used to construct the GOL. The higher the GOL score, the higher the incidence of death, MV and AICU within 28 days. Multifactorial logistic regression analysis showed that when the GOL was greater than 1, it was an independent risk factor for 28-day mortality, MV, and AICU. In predicting 28-day mortality, GOL was superior to the quick Sequential Organ Failure Assessment (qSOFA), Mortality in Emergency Department Sepsis Score (MEDS), Systemic Inflammatory Response Syndrome Score (SIRS), and Modified Early Warning Score (MEWS), and was comparable to the Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA).
Conclusion: The GOL is a simple, rapid, and accurate method for early identification of patients at increased risk of in-hospital death from sepsis.
Keywords: sepsis, GCS, lactic acid, oxygenation index, PaO2/FiO2, prognosis
Introduction
Sepsis is a life-threatening systemic inflammatory response syndrome caused by infection.1 It is estimated that tens of millions of severe sepsis cases occur worldwide each year and at least more than 5 million people die from sepsis.2,3 As the world ages, the morbidity and mortality of sepsis are likely to increase further.3–5 Early recognition and appropriate management of sepsis can improve the prognosis.6 Therefore early assessment and stratified management of sepsis are important.
Since sepsis is a time-related disease and the first medical contact for most of these patients is in the emergency department (ED).7,8 Therefore, identifying and stratifying the management of septic patients with poor outcomes and high risk of death at an early stage is what emergency departments need to face.
Systems commonly used to assess the severity of sepsis include the Acute Physiologic and Chronic Health Evaluation (APACHE) II,9 Sequential Organ Failure Assessment (SOFA),10 quick Sequential Organ Failure Assessment (qSOFA),2 Mortality in Emergency Department Sepsis Score (MEDS),11 Systemic Inflammatory Response Syndrome (SIRS) score, and Modified Early Warning Score (MEWS).12 APACHE II and SOFA are often used to assess the risk level of patients in the ICU but are rarely used for early assessment in the emergency department outside the ICU. It is also not easy to perform rapid and reproducible measurements and assessments due to the number of items involved. MEWS involves more items and it does not perform better than qSOFA.13 Previous studies have shown that qSOFA is more specific than SIRS but less sensitive and not suitable as a screening tool at the emergency bedside.14–16 MEDS has a good prognostic value for sepsis, but the overall assessment ability is average and involves laboratory tests and imaging, which require more time to complete.17–19 Therefore, there is no efficient, concise, and rapid assessment method to meet the need for rapid and accurate assessment of sepsis in the emergency department.
Previous studies have shown a strong correlation between lactate(Lac),20–22 oxygenation index23 and Glasgow coma scale score (GCS) score24,25 and sepsis prognosis. Of these, Lac and oxygenation index can be obtained within minutes by bedside arterial blood gas analysis, while Glasgow coma scale score GCS scores can be obtained quickly when the physician examines the patient. Combined with our previous experience in combining indicators from bedside arterial blood gas analysis to rapidly assess disease prognosis in our study.26 Therefore, we attempted to combine Lac, oxygenation index and, GCS scores to assess the prognosis of sepsis.
Materials and Methods
Study Design
This is a single-center retrospective cohort study. The study complied with the Declaration of Helsinki and the study protocol was approved by the Human Ethics Committee of West China Hospital, Sichuan University. We followed up all included patients by telephone.
Study Population
The study used data from the West China Hospital retrospective sepsis database. This single-center database retrospectively included data from all adult (≥18 years old) patients who developed sepsis in the ED of West China Hospital of Sichuan University between July 2015 and June 2016. In this study, only patients with a first diagnosis of sepsis according to sepsis-3 were eligible for inclusion. Patients were excluded if they were pregnant, in cardiac or respiratory arrest, or taking vasoactive drugs before admission.
Data Collection
Demographic data, in addition to data on vital signs at admission, medical history, laboratory data and, final diagnosis, were obtained from the patient’s electronic medical records in the ED. These data have been reviewed by a trained study coordinator. Hematological analytes including PH, pCO2, pO2, lactate (Lac) white blood cell count (WBC), PLT, hemoglobin and hematocrit (HCT) have been analyzed using the Cobas-b-123 system (Roche) (ABG analyzer) and have been analyzed using the automated hematology analysis system Beckman Coulter LH750 (Beckman Coulter Corporation, Brea, CA, USA) for analysis. Albumin, urea nitrogen, creatinine, cystatin C (Cys-C), total bilirubin, alanine aminotransferase (AST) and aspartate aminotransferase (ALT) have been analyzed using an Architect c16000 analyzer (Abbott Diagnostics Inc.).D-dimer has been analyzed using a Sysmex CA-7000 analyzer (Siemens Medical Diagnostics Inc.) The D-dimer values shown in this study are in D-dimer units, not fibrinogen equivalent units. PCT had been measured with a Cobas S6000 Hitachi (Roche Diagnostics Inc.). Oxygenation index has been calculated from arterial partial pressure of oxygen/inhaled oxygen concentration. Physicians stratified risk for sepsis according to APACHE II and SOFA based on the patient’s baseline clinical characteristics.
Study Follow-Up and Primary Endpoints
All patients with sepsis received a 28-day follow-up. The primary endpoint was all-cause mortality during follow-up. Secondary endpoints were adverse outcomes, including mechanical ventilation (MV) and admission to the intensive care unit (AICU). All sepsis patients underwent a structured telephone interview with an emergency physician to determine all-cause mortality and other adverse outcomes at 28 days. All in-hospital data were matched to hospital records (Figure 1).
Statistical Analysis
Categorical variables were presented as frequencies and percentages, whereas continuous variables were presented as means ± standard deviation (SD) for normally distributed data or medians and interquartile range (IQR) for abnormally distributed data. Construct time-dependent receiver operating characteristic (ROC) curves to compare prognostic performance and determine optimal cut points for different metrics. The area under the curve (AUC) was compared using the non-parametric method proposed by Hanley and McNeil.27 Construction of a new scoring system using the two highest AUC values from arterial blood gas analysis together with GCS. Univariate analysis was performed with the new score as a variable along with other variables between the survival and death groups. The indicators with significant differences in the univariate analysis were then subjected to multivariate logistic regression analysis to determine whether the new score was an independent predictor of mortality. Discriminative power analysis with AUROC; calibration capability analysis with Hosmer-Lemeshow. The newly constructed scoring system was then used to compare patient characteristics. Parametric patient characteristics were compared using one-way ANOVA tests, whereas nonparametric characteristics were compared using Kruskal–Wallis H-tests; categorical data were compared using χ2-tests. The relationship between the new scoring system and the various endpoints was examined using multivariate logistic regression models. Decision curve analysis was used to quantify the clinical utility of the new scoring model.28 p-values <0.05 were considered statistically significant. Data analyses were performed using SPSS version 25.0 (IBMCorp, Armonk, NY, USA) and Stata version MP17.0 (StataCorp LP, College Station, TX, USA). Graphing with GraphPad Prism version 9.4.1.681.
Results
Clinical Characteristics of the Derivation Group
A total of 841 patients with sepsis were enrolled in the study; however, 20 patients were lost to follow-up and were therefore excluded from the study population. Thus, the final study population consisted of 821 patients; the mean age at admission was 57 ± 18 years, and 64.3% of the patients were male. The first 608 patients were then included in the derivation group according to the order of admission, and the remaining 213 patients were included in the validation group.
In the derivation group, the mean age was 58 years, of which 64.1% were men. The median SOFA and APACHE II scores were 5 (IQR 3–7) and 15 (IRQ 12–20), respectively. During the 28-day follow-up period, there were 131 deaths (Table 1).
 |
Table 1 Comparison of Base Information of Derivation and Validation Groups |
Establishment of the New Scoring System
The area under the ROC curve (AUC) for predicting 28-day mortality using Lac (0.645, P<0.001) or oxygenation index (0.678, P<0.001) was greater compared with other indices from arterial blood gas analysis (<0.6). In addition, the GCS score, one of the components of SOFA and APACHE II, is an important predictor of disease prognosis, and the AUC for a GCS score less than 15 to predict 28-day mortality in this study was (0.604, P<0.001). Since the GCS score is also simple and easy to obtain, a new scoring system was formed using the oxygenation index, Lac, and GCS scores, and named GOL based on the initials of these three indicators. The calculation of GOL is shown in Table 2.
 |
Table 2 Weight and Score of Scoring Items in the New Scoring System (GOL) |
Clinical characteristics among sepsis patients according to GOL are shown in Table 3. Heart and breathing rates, Lac, urea nitrogen, AST, and PCT were significantly increased with increasing GOL. Conversely, systolic blood pressure (SBP), diastolic blood pressure (DBP), SPO2, PH, Oxygenation index, Albumin, and Fibrinogen significantly decreased with decreasing GOL. The other clinical characteristics did not vary significantly based on GOL (p > 0.05).
 |
Table 3 Relationship Between Clinical Characteristics and GOL Scores of Patients with Sepsis in the Derivation Group |
Then, we performed univariate analysis of GOL, SOFA, APACHE II, age, gender, vital signs, GCS, arterial blood gas analysis indexes and laboratory indexes, and then those with significant differences (p < 0.05) were then subjected to multivariate logistic regression analysis. The results showed that GOL, SOFA, APACHE II, GCS, Lac, and albumin were independent predictors of 28-day mortality in sepsis (p < 0.05) (Table 4).
 |
Table 4 Univariate Analysis and Multivariate Logistic Regression Analysis Between Survival and Death Groups in the Derivation Group |
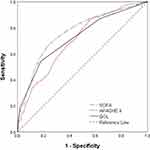
The AUROC value for GOL predicting 28-day mortality was 0.723 (95% CI: 0.672–0.775, OR=2.916, P<0.001) (Figure 2), and its Jorden index reached a maximum of 0.364 when GOL ≥2(Sensitivity, 54.2%; Specificity, 82.18%). The logistic regression analysis Hosmer-Lemeshow goodness-of-fit test shows that GOL has good calibration ability (p=0.327).
GOL and the Severity of Sepsis
Sepsis patients with higher GOL had higher SOFA and APACHE II scores when compared to patients with lower GOL. For those with GOL of 0, 1, 2, and 3, the respective median SOFA scores were 3 (IQR 1–5), 5 (IQR 3–7), 7(IQR 5–10), and 14 (IQR 10–17) (p < 0.001), and the respective mean APACHE II scores were 12 (IQR 9–16), 16 (IQR 12–20), 19 (IQR 14–24) and 25 (IQR 23–34) (p < 0.001) (Figure 3).
GOL and in-Hospital Adverse Outcomes
The 28-day mortality, MV, and AICU rates for patients gradually increased as GOL increased. The 28-day mortality rate of patients with scores of 3 or 2 or 1 was 8.2 or 4.3 or 2.1 times higher than that of patients with scores of 0, respectively (Figure 4A). The MV rate of patients with scores of 3 or 2 or 1 was 6.0 or 4.9 or 3.0 times higher than that of patients with scores of 0, respectively (Figure 4B). The AICU rate of patients with scores of 3 or 2 or 1 was 5.2 or 4.3 or 2.6 times higher than that of patients with scores of 0, respectively (Figure 4C).
Univariate logistic regression models indicated that the GOL was positively associated with the risks of mortality, MV, and AICU. After adjusting for these potential confounders in the multivariate logistic regression analysis, the GOL was independently associated with MV and AICU. However when adjusted for risk factors, GOL=1 was not an independent predictor of 28-day mortality, but only when GOL≥2 was its independent predictor. (Table 5).
 |
Table 5 Logistic Regression Analysis Regarding Correlations Between Clinical Outcomes and GOL |
Comparison Between GOL and Other Prognostic Scores
We assessed the performances of qSOFA, MEWS, MEDS, APACHE II and SOFA in the prediction of 28-day mortality. The C-index for the GOL was significantly better than that of qSOFA (0.675, 95% CI =0.636–0.712), MEWS (0.621, 95% CI = 0.581–0.659), and MEDS (0.634, 95% CI =0.594–0.672). In addition, there was no significant difference between GOL and APACHE II scores (0.701, 95% CI =0.663–0.737) and SOFA scores (0.764, 95% CI=0.728–0.797). Decision curve analysis showed that for threshold probabilities above 0.18, GOL had a higher net benefit compared to APACHE II, and for threshold probabilities above 0.43, GOL had a higher net benefit than SOFA (Figure 5).
Validation of the Ability of GOL to Predict the Prognosis of Sepsis
In the validation group, the mean age was 56 ±19 years, of which 64.8% were male. The median SOFA and APACHE II scores were 5 (IQR 2–8) and 15 (IRQ 10–20), respectively. There were 42 deaths during the 28-day follow-up period (Table 1). OLG score has good discrimination (AUROC=0.785, p<0.001) and calibration (Hosmer-Lemeshow goodness-of-fit test, p=0.774) in the validation group. We compared the performances of GOL, APACHE II, and SOFA in the prediction of 28-day death, AICU, and MV. We found that GOL was superior in predicting AICU (p= 0.030) and MV (p= 0.008) compared with APACHE II, while both were comparable in predicting 28-day death (p= 0.902). The GOL and the SOFA were comparable in their ability to predict 28-day death (p= 0.735) and AICU (p= 0.498), while the SOFA outperformed the GOL in predicting MV (p= 0.011) (Table 6).
 |
Table 6 AUC Comparison of Adverse Outcome Prediction Based on GOL, APACHE II, and SOFA in Validation Cohort |
Discussion
In the present study, we derived and validated a new scoring system, GOL, to assess sepsis prognosis. We found that higher GOL scores were associated with more severe sepsis and more poor prognosis. After adjusting for confounders, when the GOL score was greater than 1 still independently predicted mortality, AICU, and MV within 28 days of admission. And in subgroup analysis, GOL was also an independent risk factor for poor prognosis. In addition, we compared GOL with other scores and found that GOL had better predictive power than qSOFA, MEWS, and MEDS, and was comparable to APACHE II and SOFA. The performance of GOL was also validated in the validation group.
In the validation group, GOL predicted 28-day death and AICU as well as APACHE II and SOFA, and GOL demonstrated better performance than APACHE II in predicting MV.
In addition, the GOL score consists of only three indicators: oxygenation index, Lac, and simplified GCS score, and its greatest advantage is its simplicity and speed. The presence of each variable contributes one point to a total 3-point score. Among them, the oxygenation index is the ratio of arterial partial pressure of oxygen to the concentration of inhaled gaseous oxygen (PaO2/FiO2), which is an important indicator for assessing acute lung injury (ALI) or acute respiratory distress syndrome (ARDS),29 a common clinical complication of sepsis.30 Previous studies have shown that cytokines in the course of sepsis can mediate the aggregation of large numbers of immune cells, activating intracellular signaling pathways and further releasing large numbers of cytokines. Inflammatory cells are continuously activated, creating a vicious cycle that eventually leads to a cytokine storm. The lung epithelial cell injury and endothelial cell injury caused by these cytokine storms may ultimately lead to ALI/ARDS.31–34 ARDS is classified into different levels of severity depending on the oxygenation index: mild (200 mm<PaO2/FiO2≤300mmHg), moderate (100mmHg<PaO2/FiO2≤200mmHg), and severe (PaO2/FiO2<100 mmHg).29 In this study, we found that the oxygenation index showed a significant inverse relationship with poor prognosis and a cut-off value of 271, with a significantly higher incidence of poor prognosis when the oxygenation index was less than 271. This is similar to the SOFA score, where the lower the oxygenation index, the higher the SOFA score and the higher the incidence of poor prognosis.
Previous studies have shown that early lactate levels are associated with organ dysfunction and mortality in the ICU and emergency department,35–37 with elevated lactate levels positively correlating with increased mortality.38 Serum lactate levels >2 mmol/L are recommended in sepsis 3.0 as the main criteria for clinical differentiation of septic shock.1 The higher the lactate level, the worse the outcome.39 Earlier studies showed a specificity of 96% for lactate concentrations >4 mmol/L in predicting in-hospital mortality in non-hypotensive patients.2,40 Our study showed that lactate was an independent risk factor for 28-day mortality in sepsis, and mortality was significantly higher when serum lactate was greater than 2.3 mmol/L.
As two objective indicators in the new scoring system. Both arterial partial pressure of oxygen and Lac in the oxygenation index can be quickly obtained within minutes by a bedside arterial blood gas analyzer.
The level of consciousness is often used as an indicator to assess the prognosis of a disease. The GCS score, as a common assessment tool for the level of consciousness, has been used directly or indirectly to assess the risk level and prognosis of a variety of diseases.2,9–12,41,42 As the only subjective indicator in the new scoring system, we found a significant difference between a GCS score equal to 15 and less than 15 in predicting 28-day death in patients with sepsis. Therefore, we simplified the assessment process of the GCS score by contributing 1 point to the new scoring system once the GCS score was less than 15.
Limitations
The present study has several limitations. One, the study design was single-center and retrospective, and thus vulnerable to potential selection bias. Second, because of the increasing attention and motivation for the treatment of sepsis, we collected relatively old data on sepsis cases, which may be biased for current use. Third, in this study, the new scoring system was no worse than the APACHE II system, but slightly inferior to SOFA in predicting MV. Fourth, the GCS score is a subjective score, which has implications for the rigor of the new scoring system. Therefore, further multicenter prospective studies are needed to further validate the results of this study.
Conclusions
The new scoring system GOL, created in this study by combining Lac, oxygenation index, and GCS scores, can effectively predict 28-day mortality in patients with sepsis with significantly better performance than SIRS, qSOFA, MEWS, and MEDS, and comparable performance to APACHE II and SOFA. Its advantage is that it is rapid, concise, and can be used as a reference tool for prognostic assessment of sepsis in the emergency department.
Acknowledgment
We would like to thank all the volunteers who took part in this study and all the participants for their contribution to data collection and analysis.
Funding
This study was supported by the Funds of Technology Department of Sichuan Provincial (No. 2023YFS0242, and No.23ZDYF0887).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):762–774. doi:10.1001/jama.2016.0288
3. Fleischmann-Struzek C, Mellhammar L, Rose N, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1552–1562. doi:10.1007/s00134-020-06151-x
4. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi:10.1097/01.CCM.0000194535.82812.BA
5. Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561(7721):45–56. doi:10.1038/s41586-018-0457-8
6. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247.
7. Sivayoham N, Blake LA, Tharimoopantavida SE, Chughtai S, Hussain AN, Rhodes A. Treatment variables associated with outcome in emergency department patients with suspected sepsis. Ann Intensive Care. 2020;10(1):136.
8. Loritz M, Busch HJ, Helbing T, Fink K. Prospective evaluation of the quickSOFA score as a screening for sepsis in the emergency department. Intern Emerg Med. 2020;15(4):685–693.
9. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829.
10. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi:10.1007/BF01709751
11. Shapiro NI, Wolfe RE, Moore RB, Smith E, Burdick E, Bates DW. Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med. 2003;31(3):670–675. doi:10.1097/01.CCM.0000054867.01688.D1
12. Subbe CP, Kruger M, Rutherford P, Gemmel L. Validation of a modified Early Warning Score in medical admissions. QJM. 2001;94(10):521–526. doi:10.1093/qjmed/94.10.521
13. Brunetti E, Isaia G, Rinaldi G, et al. Comparison of diagnostic accuracies of qSOFA, NEWS, and MEWS to identify sepsis in older inpatients with suspected infection. J Am Med Dir Assoc. 2022;23(5):865–871 e862. doi:10.1016/j.jamda.2021.09.005
14. Usman OA, Usman AA, Ward MA. Comparison of SIRS, qSOFA, and NEWS for the early identification of sepsis in the Emergency Department. Am J Emerg Med. 2019;37(8):1490–1497. doi:10.1016/j.ajem.2018.10.058
15. van der Woude SW, van Doormaal FF, Hutten BA, J Nellen F, Holleman F. Classifying sepsis patients in the emergency department using SIRS, qSOFA or MEWS. Neth J Med. 2018;76(4):158–166.
16. Oduncu AF, Kiyan GS, Yalcinli S. Comparison of qSOFA, SIRS, and NEWS scoring systems for diagnosis, mortality, and morbidity of sepsis in emergency department. Am J Emerg Med. 2021;48:54–59. doi:10.1016/j.ajem.2021.04.006
17. Williams JM, Greenslade JH, Chu K, Brown AF, Lipman J. Severity scores in emergency department patients with presumed infection: a prospective validation study. Crit Care Med. 2016;44(3):539–547. doi:10.1097/CCM.0000000000001427
18. Hsieh CC, Yang CY, Lee CH, Chi CH, Lee CC. Validation of MEDS score in predicting short-term mortality of adults with community-onset bacteremia. Am J Emerg Med. 2020;38(2):282–287. doi:10.1016/j.ajem.2019.05.002
19. Zhang G, Zhang K, Zheng X, Cui W, Hong Y, Zhang Z. Performance of the MEDS score in predicting mortality among emergency department patients with a suspected infection: a meta-analysis. Emerg Med J. 2020;37(4):232–239. doi:10.1136/emermed-2019-208901
20. Suetrong B, Walley KR. Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest. 2016;149(1):252–261. doi:10.1378/chest.15-1703
21. Yu B, Chen M, Zhang Y, et al. Diagnostic and prognostic value of interleukin-6 in emergency department sepsis patients. Infect Drug Resist. 2022;15:5557–5566. doi:10.2147/IDR.S384351
22. Xie Y, Zhuang D, Chen H, Zou S, Chen W, Chen Y. 28-day sepsis mortality prediction model from combined serial interleukin-6, lactate, and procalcitonin measurements: a retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2023;42(1):77–85. doi:10.1007/s10096-022-04517-1
23. Cai C, Qiu G, Hong W, Shen Y, Gong X. Clinical effect and safety of continuous renal replacement therapy in the treatment of neonatal sepsis-related acute kidney injury. BMC Nephrol. 2020;21(1):286. doi:10.1186/s12882-020-01945-z
24. Lu HX, Du J, Wen DL, et al. Development and validation of a novel predictive score for sepsis risk among trauma patients. World J Emerg Surg. 2019;14:11. doi:10.1186/s13017-019-0231-8
25. Jiang Z, Bo L, Xu Z, et al. An explainable machine learning algorithm for risk factor analysis of in-hospital mortality in sepsis survivors with ICU readmission. Comput Methods Programs Biomed. 2021;204:106040. doi:10.1016/j.cmpb.2021.106040
26. Lai Q, Wei W, He Y, Cheng T, Han T, Cao Y. A rapid prognostic score based on bedside Arterial Blood Gas Analysis (ABG) established for predicting 60-day adverse outcomes in patients with acute pancreatitis in the emergency department. J Inflamm Res. 2022;15:5337–5346. doi:10.2147/JIR.S381438
27. Bewick V, Cheek L, Ball J. Statistics review 13: receiver operating characteristic curves. Crit Care. 2004;8(6):508–512. doi:10.1186/cc3000
28. Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6. doi:10.1136/bmj.i6
29. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi:10.1001/jama.2012.5669
30. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi:10.1007/s00134-017-4683-6
31. Englert JA, Bobba C, Baron RM. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome. JCI Insight. 2019;4(2). doi:10.1172/jci.insight.124061
32. Huppert LA, Matthay MA, Ware LB. Pathogenesis of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2019;40(1):31–39. doi:10.1055/s-0039-1683996
33. Lee C, Choi WJ. Overview of COVID-19 inflammatory pathogenesis from the therapeutic perspective. Arch Pharm Res. 2021;44(1):99–116. doi:10.1007/s12272-020-01301-7
34. Kim JS, Lee JY, Yang JW, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–329. doi:10.7150/thno.49713
35. Scott HF, Donoghue AJ, Gaieski DF, Marchese RF, Mistry RD. The utility of early lactate testing in undifferentiated pediatric systemic inflammatory response syndrome. Acad Emerg Med. 2012;19(11):1276–1280. doi:10.1111/acem.12014
36. Jansen TC, van Bommel J, Schoonderbeek FJ, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–761. doi:10.1164/rccm.200912-1918OC
37. Scott HF, Brou L, Deakyne SJ, Kempe A, Fairclough DL, Bajaj L. Association between early lactate levels and 30-day mortality in clinically suspected sepsis in children. JAMA Pediatr. 2017;171(3):249–255. doi:10.1001/jamapediatrics.2016.3681
38. Singer AJ, Taylor M, Domingo A, et al. Diagnostic characteristics of a clinical screening tool in combination with measuring bedside lactate level in emergency department patients with suspected sepsis. Acad Emerg Med. 2014;21(8):853–857. doi:10.1111/acem.12444
39. Nichol AD, Egi M, Pettila V, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. 2010;14(1):R25. doi:10.1186/cc8888
40. Aduen J, Bernstein WK, Khastgir T, et al. The use and clinical importance of a substrate-specific electrode for rapid determination of blood lactate concentrations. JAMA. 1994;272(21):1678–1685. doi:10.1001/jama.1994.03520210062033
41. Prytherch DR, Smith GB, Schmidt PE, Featherstone PI. ViEWS--Towards a national early warning score for detecting adult inpatient deterioration. Resuscitation. 2010;81(8):932–937. doi:10.1016/j.resuscitation.2010.04.014
42. Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57(12):1698–1703. doi:10.1136/gut.2008.152702
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.