Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Development and Validation of a Prognostic Nomogram for Patients with AFP and DCP Double-Negative Hepatocellular Carcinoma After Local Ablation
Authors Qiao W , Li J, Wang Q, Jin R, Zhang H
Received 2 November 2023
Accepted for publication 26 January 2024
Published 3 February 2024 Volume 2024:11 Pages 271—284
DOI https://doi.org/10.2147/JHC.S442366
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Imam Waked
Wenying Qiao,1– 3,* Jiashuo Li,2,* Qi Wang,2,* Ronghua Jin,2,3 Honghai Zhang1
1Interventional Therapy Center for Oncology, Beijing You’an Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Beijing Di’tan Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Changping Laboratory, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ronghua Jin, Beijing Di’tan Hospital, Capital Medical University, 8 Jingshun East Street, Chaoyang District, Beijing, People’s Republic of China, Tel/Fax +86-13811611118, Email [email protected] Honghai Zhang, Interventional therapy center for oncology, Beijing You’an Hospital, Capital Medical University, 8 Xitoutiao, Youanmenwai Street, Fengtai District, Beijing, People’s Republic of China, Tel/Fax +86-13260398697, Email [email protected]
Purpose: Although alpha-fetoprotein (AFP) and des-gamma-carboxyprothrombin (DCP) have a certain predictive ability for the prognosis of hepatocellular carcinoma (HCC), there are still some cases of aggressive recurrence among patients with AFP and DCP double-negative HCC (DNHC) after local ablation. However, prediction models to forecast the prognosis of DNHC patients are still lacking. Thus, this retrospective study aims to explore the prognostic factors in DNHC patients and develop a nomogram to predict recurrence.
Patients and methods: 493 DNHC patients who underwent the local ablation at Beijing You’an Hospital between January 1, 2014, and December 31, 2022, were enrolled. A part that was admitted from January 1, 2014, to December 31, 2018, was designated to the training cohort (n = 307); others from January 1, 2019, to December 31, 2022, were allocated to the validation cohort (n = 186). Lasso regression and Cox regression were employed with the aim of screening risk factors and developing the nomogram. The nomogram outcome was assessed by discrimination, calibration, and decision curve analysis (DCA).
Results: Independent prognostic factors selected by Lasso-Cox analysis included age, tumor size, tumor number, and gamma-glutamyl transferase. The area under the receiver operating characteristic (ROC) curves (AUCs) of the training and validation groups (0.738, 0.742, 0.836, and 0.758, 0.821) exhibited the excellent predicted outcome of the nomogram. Calibration plots and DCA plots suggest desirable calibration performance and clinical utility. Patients were stratified into three risk groups by means of the nomogram: low-risk, intermediate-risk, and high-risk, respectively. There exists an obvious distinction in recurrence-free survival (RFS) among three groups (p< 0.0001).
Conclusion: In conclusion, we established and validated a nomogram for DNHC patients who received local ablation. The nomogram showed excellent predictive power for the recurrence of HCC and could contribute to guiding clinical decisions.
Keywords: hepatocellular carcinoma, nomogram, prognosis, local ablation, alpha-fetoprotein, des-gamma-carboxyprothrombin
Introduction
Hepatocellular carcinoma (HCC), the sixth most common cancer and the third leading cause of cancer-related death globally, has emerged as a major public health concern.1 China experiences the highest incidence of HCC, accounting for over half of new cases and deaths worldwide.2,3 As a first-line treatment for early-stage HCC, local ablation exhibits the advantages of small trauma, less bleeding, and fewer complications.4 However, despite the presence of some strengths, the high recurrence rate of local ablation remains a therapeutic dilemma for HCC treatment.5,6 Consequently, there is an urgent need to forecast the prognosis of HCC patients after local ablation.
The clinical guidelines recommend imaging examination, liver biopsy, and biomarkers, like alpha-fetoprotein (AFP) and des-gamma-carboxyprothrombin (DCP), to establish the diagnosis of HCC recurrence.7,8 AFP has been identified as a reliable biomarker for the detection of HCC since the 1960s.9,10 Liebman et al claimed a high plasma DCP among 67% of patients with HCC in 1984, suggesting that it could potentially be regarded as a tumor marker.11 Recently, armies of researchers reported the utilization of AFP and DCP in the prognosis of HCC.12,13 Nevertheless, inefficient diagnosis of HCC caused by the low sensitivity of AFP and DCP remains the main reason for the high mortality and poor prognosis.14 Moreover, due to neglect by clinicians and patients, the recurrence rate of patients with negative tumor marker tests is not satisfactory. For these reasons, reliable methods are required to forecast the postoperative outcomes of patients with AFP and DCP double-negative HCC (DNHC).
In the field of HCC with negative tumor markers, most statistical methods are based on univariate and multivariate analyses, which have limitations in overfitting and multicollinearity.15,16 Currently, the application of machine learning (ML) in this field is still lacking. ML is a computer science theory that uses statistical techniques to give artificial intelligence (AI) the capability to progressively enhance the performance on a given task based on a substantial quantity of data.17 It is highly effective in processing voluminous, complex datasets.18 Meanwhile, the performance of ML could be enhanced through continuous training and iteration, which enables an increase in prediction accuracy in clinical studies. Therefore, as a machine learning method that could develop refined models by constructing penalty functions, Lasso regression is employed to identify the independent prognostic factors in our research.
More recently, some studies indicated that locoregional therapy was selected more frequently in DNHC patients, and the 3-year recurrence rate of these patients was approximately 55%.19,20 However, prediction models of recurrence for DNHC patients treated with local ablation are still insufficient at present. Thus, our research serves to develop and verify a dependable nomogram for this group of patients with the aim of guiding the decision of clinicians.
Methods
Patients
A total of 493 HCC patients who underwent ablation at Beijing You’an Hospital from January 2014 to December 2022 were enrolled in this retrospective study. A part that was admitted from January 1, 2014, to December 31, 2018, was designated to the training cohort (n = 307); others from January 1, 2019, to December 31, 2022, were allocated to the validation cohort (n = 186). The diagnosis of HCC was based on the American Association for the Study of Liver Disease (AASLD) guideline.8 Early stage of HCC was defined as Barcelona Clinic Liver Cancer (BCLC) stage 0 or A.21
The inclusion criteria included: (1) age from 18 to 75 years; (2) early-stage HCC treated with ablation; (3) AFP (<20ng/mL) and DCP (<40mAU/mL) double-negative at baseline; (4) Child-Pugh class A or B. The exclusion criteria included: (1) incomplete clinical or follow-up data; (2) received other treatment before ablation; (3) coagulation dysfunction or severe insufficiency of vital organs; (4) distant metastasis or other malignant tumors.
This research received approval from the Ethics Committee of Beijing You’an Hospital, affiliated with Capital Medical University, and implemented according to the Declaration of Helsinki. The requirement of informed consent was waived on account of the retrospective nature of the study.
Clinicopathologic Characteristics
Clinical data were accumulated from electronic patient records, including age, gender, liver cirrhosis, tumor number, tumor size, Child-Pugh class, BCLC stage, prothrombin time (PT), activated partial thromboplastin time (APTT), alanine aminotransferase (ALT), aspartate transaminase (AST), albumin (ALB), total bilirubin (TBIL), direct bilirubin (DBIL), γ-glutamyl transpeptidase (GGT), prealbumin, red blood cell (RBC), neutrophils (Neu), lymphocytes (Lym), and monocytes (Mon).
Ablation Procedure
Under the guidance of triple-phase computed tomography (CT) and magnetic resonance imaging (MRI), local ablation was performed by experienced liver surgeons and interventional radiologists. Before ablation, routine sterilization and toponarcosis were applied near puncture points. In the process of ablation, electrodes were inserted according to the size, number, and location of tumors. In order to achieve complete ablation, the ablation area was estimated to be 0.5–1.0 cm larger than the tumor edge. After ablation, the needle track was heated to block cancer implantation and postoperative bleeding. All patients immediately underwent imaging examinations after ablation, with the objective of assessing technical success and possible complications.
Follow-Up
It was advised that all patients with HCC received regular follow-ups according to clinical guidelines after completing the primary therapy. Commonly, patients were followed up every 3 months in the first year and then every 6 months thereafter. During each follow-up visit, clinicopathologic characteristics and adverse events were recorded. The last day of follow-up was July 1, 2023. Recurrence-free survival (RFS), which was the primary endpoint of this study, was defined as the time from local ablation to the date of first recurrence, last follow-up or death.
Statistical Analysis
Continuous variables were expressed as means and standard deviations, while categorical variables were reported as frequencies and percentages. The differences between groups were compared using Student’s t-test and Chi-square test.
Lasso regression and multivariate Cox regression were performed to identify the independent prognostic factors. Based on these factors, a predictive nomogram model was developed. The receiver operating characteristic curve (ROC) was plotted, and the area under the ROC curve (AUC) was calculated to evaluate the diagnostic value. In addition, calibration and decision curve analysis (DCA) were conducted to validate the calibration performance and clinical utility. In light of nomogram, patients were categorized into low-risk, medium-risk, and high-risk groups. Survival curves were drawn using the Kaplan–Meier method, and Log rank tests were used to compare RFS between three groups.
All analyses in this study were performed using R software (version 4.1.2). A two-tailed P value < 0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 493 DNHC patients who received local ablation between January 1, 2014, and December 31, 2022, were screened and stratified into a training cohort (n=307) and a validation cohort (n=186) by time. Patients’ median follow-up time was 44 months. The last day of follow-up was July 1, 2023.
All patients’ baseline clinicopathologic characteristics are exhibited in Table 1. In both cohorts, most of the patients were male (84.7% vs 78%), the mean age exceeded 50 years (56.61±8.75 vs 57.61±8.51), and the major cause of HCC was HBV infection (92.5% vs 90.3%). Among all patients, 227 (45.9% vs 46.2%) had a history of smoking, 190 (39.1% vs 37.6%) had a history of drinking, and 419 (83.4% vs 87.6%) had cirrhosis. Additionally, 371 (74.3% vs 76.9%) were Child-Pugh class A and 122 (25.7% vs 23.1%) were Child-Pugh class B, while BCLC stage 0 in 174 (33.6% vs 38.2%) cases and BCLC stage A in 319 (66.4% vs 61.8%) cases.
 |
Table 1 Demographics and Clinical Characteristics for Training and Validation Cohorts |
Independent Prognostic Factors of Local Ablation
Lasso regression was adopted with the aim of evaluating the correlation between clinical characteristics and RFS, and the variation features are displayed in Figure 1. The 10-fold cross-validation approach was employed in the iterative analysis. When λ was 0.08 (Log λ = −1.09), a model exhibiting good performance but a minimal quantity of variables was gained.
The variables contained age, gender, cirrhosis, tumor number, tumor size, BCLC stage, RBC, Lym, DBIL, GGT, prealbumin, PT, and APTT. Multivariate Cox regression was further used which revealed age (HR:1.015, 95% CI: 1.002–1.028), tumor number (HR:1.707, 95% CI: 1.346–2.165), tumor size (HR:1.013, 95% CI: 1.002–1.024), and GGT (HR:1.004, 95% CI: 1.002–1.005) as the independent prognostic factors (Table 2).
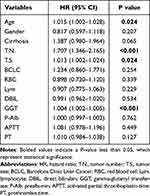 |
Table 2 Multivariate Cox Regression Analysis Based on the Results of Lasso Regression |
Development of the Nomogram
A nomogram was constructed based on the independent prognostic factors (Figure 2). In the training cohort, the ROC curve showed that AUCs of 1-, 3-, and 5-year were 0.738, 0.742, and 0.836, respectively (Figure 3). The outcomes suggested the favorable diagnostic value of the nomogram. The curve of calibration indicated a great agreement between the prediction and practical investigation (Figure 4). DCA curves showed encouraging net benefits, meaning that it had good clinical implementation significance (Figure 5). Patients were classified into low-risk (n=152), intermediate-risk (n=125), and high-risk groups (n=30) in light of nomogram scores (Figure 6). The outcomes indicated that the median RFS was 33.2 months (95% CI: 24.0–41.9) and 13.1 months (95% CI: 4.5–19.5) in the intermediate-risk and high-risk groups, respectively. In the low-risk group, the median RFS was not reached. There existed an obvious distinction in RFS among those three groups (p<0.0001).
Validation of the Nomogram
In order to further verify the reliability of this nomogram, internal validation was performed on it. In the validation cohort, the ROC curve showed that AUCs of 1- and 3-year were 0.758 and 0.821, respectively (Figure 3). The calibration curve (Figure 4) and DCA curves (Figure 5) indicated that the nomogram had good calibration performance and clinical utility. Patients of this cohort were also classified into low-risk (n=113), intermediate-risk (n=57), and high-risk groups (n=16) (Figure 6). Then, KM curves of RFS were drawn, exhibiting that the median RFS was 23.0 months (95% CI: 18.2–27.7) and 10.0 months (95% CI: 1.6–18.4) in intermediate-risk and high-risk groups, while it was not reached in the low-risk group. In agreement with the result from the training cohort, the relapse risk in the high-risk group was significantly higher than low-risk group and intermediate-risk group (p<0.0001).
Discussion
High mortality and recurrence rate of HCC have led to it being one of the major public health concerns worldwide.1,22,23 Over the past decade, the mortality of HCC has been decreased by diverse treatment modalities, but its high recurrence rate still impacts patients’ quality of life. Our study is the first to focus on the prognosis of DNHC patients with local ablation and develop a nomogram, which demonstrates favorable discrimination, calibration, and clinical utility.
AFP and DCP have been widely used as clinical biomarkers for the screening, diagnosis, and prognosis of HCC in recent years.9–14 Though expression in HCC is between 60% and 80%, the genetic regulation of AFP is complicated and has not been fully characterized.24 Some studies have suggested that AFP might regulate the growth of neoplastic and normal cells by several mechanisms, including apoptotic regulation and cytoplasmic signaling modulation.9,25 DCP is an aberrant form of prothrombin, which does not undergo posttranslational modification by means of gamma-carboxylation.12 It is even more accurate than AFP in differentiating HCC patients from those with nonmalignant liver disease or those with other malignant tumors.26 Nevertheless, it was reported in current researches that the specificity of AFP for the diagnosis of HCC is 80–90%, while the sensitivity is only 40–60%.27 The combination of AFP and DCP could increase the sensitivity effectively, but there are also a number of false-negative results. Pan et al found that 12.9% of HCC patients were AFP and DCP double-negative in their study.19
There are several reasons for the negative response of AFP and DCP. Firstly, the levels of AFP and DCP generally rise with disease progression and tumor growth.28,29 Thus, early-stage HCC tumors might not produce enough biomarkers for detection. Additionally, the expression of these biomarkers could vary among patients, with some not exhibiting significant elevations in AFP or DCP naturally.30 Finally, the expression of these biomarkers is related to tumor’s specific biological characteristic. It has been consistently shown that tumor markers are generally undetectable in some types of cancer.31 The occurrence of false-negative results due to a variety of factors and the underlying mechanisms require further exploration.
As effective tumor markers for HCC, the false-negative results of AFP and DCP could impact the patients’ diagnosis and therapy. The prognosis of these patients was then affected accordingly.32,33 According to Ueno et al, the 5-year survival rates of biomarker-negative patients were not as favorable.34 Some studies even indicated that within the group of AFP-negative HCC in baseline, those who turned positive at recurrence had an increased risk of death compared with those who remained AFP-negative at relapse.35,36 At present, most predictive models are developed without considering the prognosis of DNHC patients. Therefore, the nomogram in our study, which could predict the recurrence of DNHC patients precisely, has some implications for clinical practice.
Local ablation is a promising strategy in which electrodes are inserted into the tumor issue in accordance with imaging examinations. Additionally, high-frequency electrodes are applied to destroy HCC cells.37–39 Local ablation, which serves as a first-line therapy for the early HCC stage, is effective in prolonging patients’ overall survival and recurrence-free survival. A study by Lzzo et al claimed that the overall survival and disease-free survival in HCC patients treated with ablation changed between 53.2 ± 3.0 months and 66 months and between 22.0 ± 2.6 months and 39 months, respectively.40 Long-term clinical practice has proven that local ablation offers a valid therapy for patients suffering from early-stage HCC, so we employ this therapy in our study.
Unlike conventional univariate analysis, the Lasso regression that we use can increase prediction accuracy and avoid overfitting risk. The established nomogram based upon Lasso-Cox regression ran greatly in forecasting the recurrence of DNHC patients despite having a minimum number of variables. Based on personalized recurrence risk, the patients were classified into three groups by means of the nomogram. Clinicians could develop more individualized treatment regimens for patients with different prognoses assessed, and this will make the therapy more targeted.
Independent prognostic factors involved in the nomogram include age, GGT, tumor size, and tumor number. Among these factors, there exists one recognized risk factor, age, for recurrence in HCC patients. Elderly patients with HCC present with low immunity, rapid tumor progression, and poor prognosis postoperatively.41 It is shown in a few researches that elderly patients have decreased liver weight and portal blood flow rate, which resulted in weak liver repairability.42,43 Namieno et al indicated that the degree of liver damage and the incidence of liver cirrhosis were outstanding in elderly patients with HCC, while the frequency of high serum AFP values was low.44 Tumor burden that includes tumor size and tumor number is generally considered to be a key feature of cancer. It has been confirmed that patients with high tumor burden are more vulnerable to microvascular infiltration, which may influence patients’ recurrence rate.45,46 In the study of Nagahara et al, there were significantly more cases under TNM Stage III in DNHC patients.18 Therefore, the influence of tumor burden in DNHC patients requires further attention. GGT, a metabolite of glutathione, provided cells with access to an additional source of cysteine, which helped maintain intracellular glutathione and increased resistance to drug toxicity.47 A study by Yang et al showed that GGT levels between groups of HCC patients which classified by AFP were not statistically significant.48 When liver function reports reveal increased GGT, this research prompts us to be alert for the presence of HCC, even if tumor markers are negative.
Although the nomogram demonstrated good predictive performance, there exist some restrictions in this research. First of all, this study was a retrospective study, which inevitably leads to bias. However, the excellent calibration results suggest that this is probably a minor concern. Another limitation is that this research was a single-center study, and the sample size obtained was insufficient. Thus, more cases will be collected in our future research. Moreover, patients receiving local ablation were included in this research, but the validity of this nomogram for patients who underwent surgery is uncertain. Thereby, it is indispensable for more researches to further validate our outcomes.
Conclusion
In summary, a nomogram that could predict the recurrence of DNHC patients after local ablation was developed and validated in our research. With age, tumor size, tumor number and GGT, the nomogram exhibited excellent predictive capability, which was able to help physicians with personalized treatment decision-making.
Data Sharing Statement
All relevant data are available within the manuscript. Further enquiries can be directed to the corresponding author (Honghai Zhang, [email protected]).
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Beijing Youan Hospital, affiliated with Capital Medical University. As a retrospective study, the identities of the individuals included in this study were anonymized using computer-generated ID numbers in order to ensure patient confidentiality. Thus, the requirement of informed consent was waived.
Acknowledgments
The authors highly appreciate all patients who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no external funding.
Disclosure
The authors declare no conflict of interest in this study.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Zou H, Li M, Lei Q, et al. Economic burden and quality of life of hepatocellular carcinoma in Greater China: a systematic review. Front Public Health. 2022;10:801981. doi:10.3389/fpubh.2022.801981
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Chen Z, Xie H, Hu M, et al. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res. 2020;10(9):2993–3036.
5. Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver resection versus local ablation therapies for hepatocellular carcinoma within the Milan criteria: a systematic review and meta-analysis. Ann Surg. 2021;273(4):656–666. doi:10.1097/SLA.0000000000004350
6. Zheng J, Cai J, Tao L, et al. Comparison on the efficacy and prognosis of different strategies for intrahepatic recurrent hepatocellular carcinoma: a systematic review and Bayesian network meta-analysis. Int J Surg. 2020;83:196–204. doi:10.1016/j.ijsu.2020.09.031
7. de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(Suppl 1):S75–87. doi:10.1016/S0168-8278(12)60009-9
8. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
9. Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39(12):2214–2229. doi:10.1111/liv.14223
10. Terentiev AA, Moldogazieva NT. Alpha-fetoprotein: a renaissance. Tumour Biol. 2013;34(4):2075–2091. doi:10.1007/s13277-013-0904-y
11. Yang Y, Li G, Lu Z, Liu Y, Kong J, Liu J. Progression of prothrombin induced by vitamin K absence-II in hepatocellular carcinoma. Front Oncol. 2021;11:726213. doi:10.3389/fonc.2021.726213
12. Hu X, Chen R, Wei Q, Xu X. The landscape of alpha fetoprotein in hepatocellular carcinoma: where are we? Int J Biol Sci. 2022;18(2):536–551. doi:10.7150/ijbs.64537
13. Chen VL, Sharma P. Role of biomarkers and biopsy in hepatocellular carcinoma. Clin Liver Dis. 2020;24(4):577–590. doi:10.1016/j.cld.2020.07.001
14. Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The performance of AFP, AFP-3, DCP as biomarkers for detection of Hepatocellular Carcinoma (HCC): a Phase 3 biomarker study in the United States. Clin Gastroenterol Hepatol. 2023;21(2):415–423.e414. doi:10.1016/j.cgh.2022.01.047
15. Wang X, Mao M, He Z, et al. Development and Validation of a Prognostic Nomogram in AFP-negative hepatocellular carcinoma. Int J Biol Sci. 2019;15(1):221–228. doi:10.7150/ijbs.28720
16. Li W, Han L, Xiao B, Li X, Ye Z. A predictive nomogram of early recurrence for patients with AFP-negative hepatocellular carcinoma underwent curative resection. Diagnostics. 2022;12:5.
17. Deo RC. Machine Learning in Medicine. Circulation. 2015;132(20):1920–1930. doi:10.1161/CIRCULATIONAHA.115.001593
18. Lee S, Mohr NM, Street WN, Nadkarni P. Machine learning in relation to emergency medicine clinical and operational scenarios: an overview. West J Emerg Med. 2019;20(2):219–227. doi:10.5811/westjem.2019.1.41244
19. Nagahara T, Sugihara T, Kihara T, et al. The characteristics and prognosis of alpha-fetoprotein and des-gamma-carboxy prothrombin double-Negative hepatocellular carcinoma at baseline in higher BCLC stages. Cancers. 2023;15(2). doi:10.3390/cancers15020390
20. Pan YX, Sun XQ, Hu ZL, et al. Prognostic values of alpha-fetoprotein and des-gamma-carboxyprothrombin in hepatocellular carcinoma in China: an analysis of 4792 patients. J Hepatocell Carcinoma. 2021;8:657–670. doi:10.2147/JHC.S316223
21. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
22. Ganesan P, Kulik LM. Hepatocellular carcinoma: new developments. Clin Liver Dis. 2023;27(1):85–102. doi:10.1016/j.cld.2022.08.004
23. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
24. Zhao YJ, Ju Q, Li GC. Tumor markers for hepatocellular carcinoma. Mol Clin Oncol. 2013;1(4):593–598. doi:10.3892/mco.2013.119
25. Lin B, Zhu M, Wang W, et al. Structural basis for alpha fetoprotein-mediated inhibition of caspase-3 activity in hepatocellular carcinoma cells. Int J Cancer. 2017;141(7):1413–1421. doi:10.1002/ijc.30850
26. Marrero JA, Su GL, Wei W, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology. 2003;37(5):1114–1121. doi:10.1053/jhep.2003.50195
27. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y
28. Zheng Y, Zhu M, Li M. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(10):2439–2446. doi:10.1007/s00432-020-03331-6
29. Cui SX, Yu XF, Qu XJ. Roles and signaling pathways of Des-γ-carboxyprothrombin in the progression of hepatocellular carcinoma. Cancer Invest. 2016;34(9):459–464. doi:10.1080/07357907.2016.1227445
30. Scheetz L, Park KS, Li Q, et al. Engineering patient-specific cancer immunotherapies. Nat Biomed Eng. 2019;3(10):768–782. doi:10.1038/s41551-019-0436-x
31. Kim MJ, Choi NY, Lee EK, Kang MS. Identification of novel markers that outperform EpCAM in quantifying circulating tumor cells. Cell Oncol. 2014;37(4):235–243. doi:10.1007/s13402-014-0178-4
32. Giannini EG, Marenco S, Borgonovo G, et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. 2012;56(4):1371–1379. doi:10.1002/hep.25814
33. Chen ZZ, Huang L, Wu YH, Zhai WJ, Zhu PP, Gao YF. LncSox4 promotes the self-renewal of liver tumour-initiating cells through Stat3-mediated Sox4 expression. Nat Commun. 2016;7(1):12598. doi:10.1038/ncomms12598
34. Ueno M, Hayami S, Shigekawa Y, et al. Prognostic impact of surgery and radiofrequency ablation on single nodular HCC ⩽5 cm: cohort study based on serum HCC markers. J Hepatol. 2015;63(6):1352–1359. doi:10.1016/j.jhep.2015.07.013
35. Sun Y, Xiong Y, Wang Q, Qiao W, Zhang H, Zhang Y. Development and validation of a nomogram to predict the recurrence of hepatocellular carcinoma patients with dynamic changes in AFP undergoing locoregional treatments. Front Oncol. 2023;13:1206345. doi:10.3389/fonc.2023.1206345
36. Wang Q, Qiao W, Zhang H, et al. Nomogram established on account of Lasso-Cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma. Front Immunol. 2022;13:1019638. doi:10.3389/fimmu.2022.1019638
37. Breen DJ, Lencioni R. Image-guided ablation of primary liver and renal tumours. Nat Rev Clin Oncol. 2015;12(3):175–186. doi:10.1038/nrclinonc.2014.237
38. Chen S, Zeng X, Su T, et al. Combinatory local ablation and immunotherapies for hepatocellular carcinoma: rationale, efficacy, and perspective. Front Immunol. 2022;13:1033000. doi:10.3389/fimmu.2022.1033000
39. Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res. 2005;127(2):208–223. doi:10.1016/j.jss.2005.02.009
40. Izzo F, Granata V, Grassi R, et al. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 2019;24(10):e990–e1005. doi:10.1634/theoncologist.2018-0337
41. Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203(6):865–877. doi:10.1016/j.jamcollsurg.2006.08.026
42. Cannistrà M, Grande R, Ruggiero M, et al. Resection of hepatocellular carcinoma in elderly patients and the role of energy balance. Int J Surg. 2016;33(Suppl 1):S119–125. doi:10.1016/j.ijsu.2016.06.020
43. Wang HW, Hsieh TH, Huang SY, et al. Forfeited hepatogenesis program and increased embryonic stem cell traits in young hepatocellular carcinoma (HCC) comparing to elderly HCC. BMC Genomics. 2013;14(1):736. doi:10.1186/1471-2164-14-736
44. Namieno T, Kawata A, Sato N, Kondo Y, Uchino J. Age-related, different clinicopathologic features of hepatocellular carcinoma patients. Ann Surg. 1995;221(3):308–314. doi:10.1097/00000658-199503000-00014
45. Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11(9):1086–1092. doi:10.1002/lt.20472
46. Lee S, Kang TW, Song KD, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. 2021;273(3):564–571. doi:10.1097/SLA.0000000000003268
47. Hanigan MH. Gamma-glutamyl transpeptidase: redox regulation and drug resistance. Adv Cancer Res. 2014;122:103–141.
48. Yang JG, He XF, Huang B, Zhang HA, He YK. Rule of changes in serum GGT levels and GGT/ALT and AST/ALT ratios in primary hepatic carcinoma patients with different AFP levels. Cancer Biomark. 2018;21(4):743–746. doi:10.3233/CBM-170088
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






