Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Development and Validation of a Prognostic Model for Transarterial Chemoembolization in Unresectable Hepatocellular Carcinoma Based on Preoperative Serum Prealbumin
Authors Xu L, Zhao D, Tian P, Ding J, Jiang Z, Ni G, Hou Z, Ni C
Received 1 September 2023
Accepted for publication 2 December 2023
Published 13 December 2023 Volume 2023:10 Pages 2239—2250
DOI https://doi.org/10.2147/JHC.S433245
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Lin Xu,* Dongxu Zhao,* Pengcheng Tian,* Jiaan Ding,* Zhengyu Jiang, Guanyin Ni, Zhongheng Hou, Caifang Ni
Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Caifang Ni, Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China, Email [email protected] Zhongheng Hou, Department of Interventional Radiology, Huzhou Central Hospital, Huzhou, People’s Republic of China, Email [email protected]
Purpose: We aimed to develop a prognostic nomogram utilizing preoperative serum prealbumin levels to predict the overall survival (OS) in patients undergoing transarterial chemoembolization (TACE) for unresectable hepatocellular carcinoma (HCC).
Patients and Methods: A total of 768 individuals with unresectable HCC who underwent TACE at three medical facilities in Suzhou between January 2007 December 2018 were included. The patient cohort was assigned to a training set (n = 461) and a validation set (n = 307). Cox regression analysis identified independent prognostic factors, which were then used to construct a prognostic nomogram. Internal validation was performed in the testing group, and its effectiveness and capability were evaluated with reference to the concordance index (C-index), area under the curve (AUC), calibration curve, and decision curve analysis (DCA).
Results: Independent risk factors identified through Cox regression analyses included the BCLC stage, cirrhosis, invasion, tumor number, preoperative serum PALB, performance status (PS), and tumor size. The nomogram demonstrated a C-index of 0.734 (95% confidence interval (CI): 0.710– 0.758) in the training set and 0.717 (95% CI: 0.678– 0.756) in the validation set, indicating strong discriminatory ability. The nomogram also demonstrated favorable discriminatory performance with AUC values of 0.873, 0.820, and 0.833 for 1-, 2-, and 3-year OS, respectively, in the training set, and 0.854, 0.765, and 0.724 in the validation set. The AUC value of the nomogram (0.843) was significantly higher than that of the four conventional staging systems. Moreover, calibration graphs confirmed a strong concordance between the predicted and observed results. Furthermore, DCA underscored the significant clinical utility of the nomogram. Additionally, the low-risk group exhibited considerably superior rates of survival compared to the high-risk group.
Conclusion: The developed nomogram demonstrated excellent prognostic capability, which served as a valuable tool for personalized clinical decision-making for patients with HCC.
Keywords: hepatocellular carcinoma, nomogram, prealbumin, overall survival, prognosis
Introduction
The prevalence and mortality rates of hepatocellular carcinoma (HCC) have witnessed a significant rise over the years. While surgery remains the primary approach for patients with early stage HCC, most individuals are diagnosed at an advanced stage, which makes them ineligible for surgical treatment. In such cases, transarterial chemoembolization (TACE) is the preferred therapy across the world, as it offers localized management and improved patient survival.1,2 Nevertheless, the prognosis for patients with advanced-stage HCC differs significantly due to variables such as tumor load, initial liver condition, and treatment choice.3 Unfortunately, the existing clinical approach lacks highly precise and responsive indicators to predict the prognosis in patients with HCC.4 Therefore, there is a crucial need to develop a more precise predictive model to forecast the clinical outcomes and guide the treatment of patients diagnosed with advanced-stage HCC.
Recent times have witnessed an increasing focus on utilizing serum prealbumin as a predictive biomarker across various cancer types. Transthyretin, also called serum prealbumin, is a small protein produced in the hepatocytes. It serves as a reliable and responsive indicator of nutritional and inflammatory status as well as liver function. Its short half-life makes it advantageous for assessing short-term nutritional changes within the body.5 Moreover, serum prealbumin remains largely unaffected by liver diseases, blood transfusions, or the administration of human albumin.6 These characteristics make serum prealbumin a promising candidate for a prognostic biomarker. Several trials have documented a strong correlation between prealbumin levels in the blood and the prognosis of various cancer types.7 Nevertheless, the correlation between preoperative serum prealbumin concentrations and the extended outcomes in individuals with unresectable HCC undergoing TACE is not fully elucidated. Hence, this retrospective analysis aims to clarify the prognostic significance of preoperative serum prealbumin levels in patients with unresectable HCC undergoing TACE.
Materials and Methods
Patients
This retrospective study focused on the clinical data from patients diagnosed with unresectable HCC undergoing TACE at three different medical facilities. The study spanned from January 1, 2007, to December 31, 2018. The medical ethics committees of all three institutions approved this study, and informed consent was obtained from all patients and their families. The qualified patients were randomly assigned to two groups: a training set (n = 461) and a validation set (n = 307). The inclusion criteria for the patients are as follows: ① a confirmed pathological diagnosis of HCC, ② ineligibility for surgical resection, ③ no prior tumor treatment before TACE, and ④ absence of medical conditions that could impact serum prealbumin levels, such as cardiovascular diseases, respiratory tract infections, or autoimmune diseases. The exclusion criteria included the following: ① concurrent other malignant tumors, ② non-HCC confirmed by pathology, ③ prior tumor-related treatment pre-TACE, ④ conditions impacting serum prealbumin or severe underlying diseases, and ⑤ inconsistent follow-up, loss to follow-up, or incomplete clinical data.
Data Collection
Clinical data encompassed gender, age, Child–Pugh classification, tumor characteristics (size, number, and location), liver cirrhosis, ascites, hepatitis B virus (HBV), alanine aminotransferase (ALT), aspartate aminotransferase (AST), prealbumin (PALB), albumin (ALB), bilirubin, alpha-fetoprotein (AFP), Barcelona clinic liver cancer (BCLC) stage, performance status (PS), liver vascular invasion, and prothrombin time (PT).
Measurement of Prealbumin
Pre-TACE assessments included prealbumin level measurements, complete blood count analysis, biochemical tests, and tumor marker evaluations. Prealbumin levels <170 mg/L were classified as low, while levels >170 mg/L were in the normal group. The normal reference range for prealbumin was 170–400 mg/L, and none of the individuals in the normal group displayed levels exceeding the maximum threshold.
TACE Procedure
Patients received conventional transarterial chemoembolization (cTACE) treatment using the modified Seldinger method, wherein the femoral artery located on the right side was punctured. With the assistance of digital subtraction angiography (DSA), a 2.7 F microcatheter (Terumo, Japan) was guided as close to the tumor-feeding arteries as possible. After thoroughly mixing with iodized oil (2–20 mL), a combination of 200 mg of oxaliplatin and 20 mg of pirarubicin was gradually administered into these arteries. To accomplish embolization, gelatin sponge particles with a size range of 300–500 μm were inserted into the tumor-supplying arteries until the particles completely stopped moving at the tip of the catheter. Furthermore, a concentrated accumulation of iodized oil was detected within the tumor region, accompanied by the emergence of minor portal vein branches surrounding the tumor. To achieve tumor devascularization, every available tumor-supplying artery was embolized. Post-operatively, liver cancer efficacy was evaluated, and additional TACE treatment was administered if needed.
Follow-Up and Reexamination
Overall survival (OS) was considered the principal endpoint, that is, the time between TACE and death or the latest follow-up extended to December 31, 2020). Enhanced CT or MRI reexaminations were performed one month after TACE, followed by subsequent reexaminations every 2–3 months. Further TACE was based on the radiographic assessment, overall health of the individual, and functioning of the liver and kidneys. In cases where further TACE treatment was considered inappropriate, optimal supportive care was provided.
Statistical Analysis
Data analysis utilized R version 4.2.2. P < 0.05 was deemed statistically significant. Chi-squared test was performed to compare groups, with counts presented as percentages (%). Both univariate and multivariate Cox regression models were used to analyze the patients’ survival and prognosis factors. Multivariate analysis incorporated factors that had P < 0.05 in the univariate analysis. A nomogram, incorporating independent predictors of OS, was also constructed. The discriminative ability of the nomogram was assessed using the C-index. Additionally, its sensitivity and specificity were determined through ROC curves utilizing the TimeROC package. Calibration curves compared predicted OS probabilities with Kaplan–Meier estimations of survival at 1-, 2-, and 3-years post-surgery to gauge the accuracy of the predicted probabilities of OS. Decision curve analysis (DCA) assessed the clinical significance of the nomogram. On the basis of median risk scores, the nomogram also classified individuals into high- and low-risk groups.
Results
Patient Characteristics
The study involved 768 individuals diagnosed with HCC. The median OS was 18.5 months, with a 95% confidence interval (CI) of 17.6–19.4. Notably, a substantial discrepancy was noted in the albumin levels between the training and validation groups (P < 0.05). However, no statistically significant discrepancies were observed in the other clinical characteristics between the two groups, (P > 0.05). For a thorough understanding of individual clinical traits, refer to Table 1.
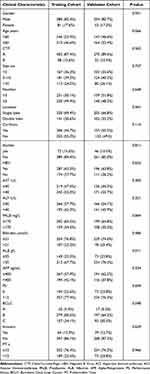 |
Table 1 Baseline Differences Between Training and Validation Cohort |
Determination of Prognostic Factors for OS
Univariate Cox regression analysis detected significant correlations (P < 0.05) between OS and factors such as AFP, ALB, ascites, AST, BCLC, cirrhosis, HBV, invasion, location, number, PALB, PS, PT, and size. Multivariate Cox regression analysis incorporating these factors identified BCLC stage, cirrhosis, invasion, number, PALB, PS, and size as significant independent risk factors impacting patients with HCC’s OS (P < 0.05). For a comprehensive overview of the outcomes from the univariate and multivariate Cox regression analyses, refer to Table 2.
 |
Table 2 Univariate and Multivariate Cox Regression Analyses of OS in Training Cohort |
Prognostic Nomogram for OS
The nomogram (Figure 1) integrated autonomous risk factors for predicting the 1-, 2-, and 3-year OS in individuals with unresectable HCC treated with TACE. Its performance, assessed in the training and validation sets, yielded a C-index of 0.734 (95% Cl 0.710–0.758) and 0.717 (95%Cl 0.678–0.756), respectively. The ROC analysis (Figure 2) demonstrated AUC values of 0.873, 0.820, and 0.833 for 1-, 2-, and 3-year OS predictions in the training cohort, and 0.854, 0.765, and 0.724 in the validation cohort, confirming its robust discriminatory ability (AUC > 0.7, high discriminatory capabilities). The accuracy of the model was confirmed through a remarkable consistency secured between the predicted and observed 1-, 2-, and 3-year OS values, as evidenced by the calibration curves (Figure 3).
 |
Figure 1 Nomogram for predicting the OS of unresectable hepatocellular carcinoma patients in the training cohort at 1-, 2-, and 3 years. |
 |
Figure 2 ROC curve and AUC of the nomogram to predict 1-, 2-, and 3-year OS in training cohort (A) and validation cohort (B). |
Prognostic Assessment and Risk Stratification
These curves were constructed by performing 1000 bootstrap resamples for both cohorts. DCA (Figure 4) underscored the nomogram’s superior net advantage within a reasonable probability range, emphasizing its significant clinical usefulness.
The nomogram’s risk score effectively classified individuals into high- and low-risk categories, with the low-risk group exhibiting remarkably better survival rates than the high-risk group in both the training and validation cohorts (Figure 5).
 |
Figure 5 Kaplan-Meier curves of unresectable hepatocellular carcinoma patients in the low-risk and high-risk groups in training cohort (A) and validation cohort (B). |
Performance Comparison with Conventional Prognostic Staging Systems
The nomogram outperformed four conventional prognostic staging systems, with an AUC for OS of 0.843 (Figure 6). This value significantly surpassed BCLC (AUC: 0.773), JIS (AUC: 0.608), CUPI (AUC: 0.626), and ITA.LI.CA (AUC: 0.649) systems, confirming its superior prognostic prediction capabilities.
 |
Figure 6 AUC and 95% CI of the Nomogram and Conventional Prognostic Staging Systems. |
Discussion
Early identification of HCC remains challenging, leading to a predominance of patients being diagnosed in the intermediate to advanced stages, associated with increased risk of recurrence and metastasis, resulting in suboptimal outcomes8 Moreover, HCC is an extremely heterogeneous and cancerous neoplasm,9 displaying notable disparities in the way it responds to treatment and prognosis of different individuals. Although several studies10–13 have developed models for forecasting the OS of patients with HCC after surgical resection, there is a scarcity of uncomplicated and efficient models for forecasting OS in patients with unresectable HCC undergoing TACE. In this study, we constructed a novel model comprising seven predictive factors (BCLC, cirrhosis, invasion, number, PALB, PS, and size) to accurately predict the prognostic value of patients with unresectable HCC undergoing TACE.
During acute-phase responses, the liver produces prealbumin or transthyretin, a protein with a short lifespan of 2–3 days. Acting as a precursor to albumin, prealbumin reflects recent nutritional status, albumin reserves, and the systemic inflammatory response.7,14 Low prealbumin levels have been correlated with malnutrition, inflammation, and weakened immune function, all of which negatively impact the patient’s prognosis.15 Notably, the prognosis of patients with malignant tumors has been strongly influenced by their nutritional status after surgery.16 In clinical practice, albumin is frequently utilized to evaluate nutritional status and liver function, as opposed to prealbumin. Nevertheless, certain studies indicate that prealbumin exhibits greater specificity and sensitivity,17 emerging as a valuable marker for assessing nutritional status and prognosis. Moreover, inflammation contributes to the increase of vascular endothelial growth factor and platelet-derived growth factor, which induce the formation of neovascularization associated with tumors. Additionally, it can trigger extracellular matrix development via the transformation of growth factor-beta, resulting in unfavorable outcomes for individuals diagnosed with HCC.18,19 Prealbumin has been reported to indicate the body’s acute inflammatory state and has the potential to be a prognostic biomarker for tumors.20 Although the exact effect of prealbumin on the prognosis of individuals with HCC is not completely known, it is widely accepted that the tumor microenvironment promotes tumor development, progression, invasion, and metastasis by causing immune suppression, ultimately affecting patient prognosis.21 Moreover, changes in lymphocyte subgroups and functions are critical in the treatment of cancerous growths, with lymphocyte levels partially indicating the immune function of the individual.22,23 Conversely, prognosis is enhanced by serum prealbumin, which increases lymphocyte maturation and boosts the immune response. As a result, immune suppression in the tumor microenvironment is alleviated.24 Hence, the dependable prognostic value of serum prealbumin levels in patients with HCC is evident.
Following the initial diagnosis of HCC, medical professionals evaluate patients utilizing clinical staging methods to categorize them according to the level of tumor progression, which helps in determining subsequent treatment plans and evaluating treatment efficacy. The BCLC staging system, widely utilized in clinical practice, is considered the leading method for forecasting the outcome of patients with HCC.25,26 The BCLC staging system primarily assesses the prognosis of patients with HCC based on factors such as tumor burden, liver function status, physical condition, liver functional reserve, and tumor-related symptoms.27,28 Despite its superiority in prognostic prediction compared to other staging systems,29 the BCLC staging system possesses certain limitations such as the subjective factors involved in assessing the patient’s physical condition and the extensive heterogeneity in prognosis within a given category.30 For example, assessment of the severity of ascites and hepatic encephalopathy may vary from one assessor to another.
The PS score is employed to assess the daily functional ability of individuals with tumors, acting as a measure of their general well-being. Additionally, it is utilized to evaluate the outcome of patients with cancer.31,32 Similar to the BCLC staging system, the PS score also depends on subjective elements and lacks objective benchmarks for assessing the daily functional ability of patients with cancer.33 Liver function and physical fitness primarily affect the prognosis of individuals with HCC.34 From a pathological standpoint, tumor size, number, and intrahepatic vascular invasion are indicative of tumor malignancy and disease progression.35
Integrating preoperative serum prealbumin levels into a prognostic model provides valuable insights into its potential as a predictive biomarker. The established model utilizes preoperative prealbumin as an unbiased serum biochemical indicator, addressing the limitations of subjective elements, as seen in conventional BCLC staging systems and PS scoring. By effectively utilizing both subjective and objective indicators, this model accurately forecasts the prognosis of patients with unresectable HCC undergoing TACE treatment. Furthermore, it provides additional information beyond traditional clinical parameters, allowing for a more accurate assessment of patient prognosis. Thus, the inclusion of prealbumin offers insights into the patient’s nutritional and inflammatory status, enhancing the understanding of treatment response and overall survival.
To investigate the prognostic significance of preoperative serum prealbumin concentrations in unresectable HCC undergoing TACE, we used multivariable Cox regression analysis. The analyses revealed various autonomous variables, such as BCLC stage, cirrhosis, invasion, tumor number, PALB score, PS, and tumor size, that could predict the outcomes of patients undergoing TACE for unresectable HCC. To incorporate these variables and offer precise forecasts of survival percentages at 1, 2, and 3 years, a nomogram was developed. The results demonstrated the significant predictive ability of the nomogram, utilizing preoperative serum prealbumin levels, in predicting the prognosis of patients receiving TACE for inoperable HCC, highlighting its potential to assist in individualized clinical decision-making.
The prognostic model utilizing preoperative serum prealbumin levels holds significant clinical implications. It facilitates the stratification of patients with unresectable HCC undergoing TACE based on their risk level, allowing clinicians to tailor treatment strategies accordingly. Identification of patients with a higher likelihood of unfavorable outcomes (low levels of prealbumin and other negative prognostic factors) enables the consideration of more aggressive treatment modalities, such as a combination of therapies or liver transplantation. Conversely, patients with a more positive prognosis may benefit from less invasive treatments. Moreover, the model serves as a foundation for personalized treatment plans, optimizing decisions and potentially enhancing patient outcomes. Notably, patients with low prealbumin levels can receive nutritional support and interventions to improve their, prealbumin levels, thereby enhancing their overall well-being and treatment response.
The prognosis of patients with HCC is closely correlated to the tumor load, liver function, the patient’s overall health, and treatment modalities.33 Improving the prognosis of HCC involves several aspects, including early diagnosis, treatment choice, patient’s lifestyle, and rehabilitation. Early diagnosis is a vital key to improving the prognosis of HCC. Regular screening also play an important role in early diagnosis. Surgical resection is the recommended and therapeutically effective treatment for patients with very early or early HCC without vascular invasion.36 In addition, liver transplantation is a viable option that can offer a chance of long-term treatment of critical cases in terms of improving the prognosis of patients with HCC. Conversely, for patients who cannot undergo surgical resection, interventional therapies such as TACE, radiofrequency ablation, and microwave therapy, can be used to reduce the size of the tumor and control its growth, thereby prolonging patient survival. On the other hand, targeted drugs such as sorafenib have become the standard treatment for patients with intermediate and advanced unresectable HCC.37 Patients with HCC often present with comorbidities such as liver failure, ascites, esophageal varices, and so on. Thus, the timely and effective management of these comorbidities can improve the prognosis of patients. Lifestyle management, including diet, physical activity, and smoking cessation can help reduce symptoms, improve immune system function, reduce recurrence risk, and improve prognosis. Furthermore, immunotherapy, especially checkpoint inhibitors, is emerging as the most promising approach to improving the prognosis of patients with HCC. It activates the body’s immune system to attack the tumor cells and has demonstrated its efficacy in some patients. Clinical prognostic models, such as the one presented here, lay the foundation for personalized treatment plans for patients with HCC, optimizing therapeutic decisions for improved prognostic outcomes.
While our study offers valuable insights, certain limitations warrant acknowledgment. The retrospective nature of this study introduces the possibility of selection biases, emphasizing the need for prospective cohort studies for validation. The lack of external verification limits generalizability to diverse patient groups. Moreover, further assessment of the model’s relationship with pathological and clinical parameters in a prospective cohort is essential for conclusive evidence. The exclusion of individuals undergoing drug-eluting bead transarterial chemoembolization requires additional investigation for predictive prognosis within this particular subset. Addressing these limitations will contribute to a more comprehensive understanding of the subject and improve the clinical significance of our findings.
Conclusion
The establishment of the TACE prognostic model for inoperable liver cancer, using preoperative serum prealbumin levels, represents a valuable tool for risk stratification and informed treatment decisions. The integration of prealbumin significantly enhances the model’s precision, providing crucial insights into patients’ nutritional and inflammatory states. This model enables the implementation of personalized treatment approaches, thereby optimizing patient care and positively impacting the prognosis of individuals with HCC undergoing TACE. Nevertheless, it is imperative to undertake further prospective cohort studies and external validation to comprehensively explore the clinical significance and wider applicability of this prognostic model.
Abbreviations
HCC, Hepatocellular carcinoma; TACE, Transarterial chemoembolization; OS, Overall survival; C-index, Concordance index; AUC, Area Under the Curve; DCA, Decision curve analysis; CTP, Child-Turcotte-Pugh; HBV, Hepatitis B Virus; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; PALB, Prealbumin; ALB, Albumin; AFP, Alpha-fetoprotein; PS, Performance status; BCLC, Barcelona Clinic Liver Cancer; PT, Prothrombin Time; CUPI, Chinese University Prognostic; JIS, Japanese Integrated Staging; ITA.LI.CA, Italian Liver Cancer; cTACE, Conventional transarterial chemoembolization; DSA, Digital subtraction angiography; DEB-TACE, Dead transarterial chemoembolization.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University and was conducted in strict accordance with the principles of the Declaration of Helsinki. As this study was retrospective, the committee waived the requirement of informed consent. All patient-related data used in this study complied with privacy protection regulations.
Acknowledgments
The patients participating in this study are sincerely acknowledged.
Funding
This work was supported by the National Natural Science Foundation of China Project [grant number 82272094].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi:10.1038/s41575-020-00395-0
2. Zhong BY, Jin ZC, Chen JJ, Zhu HD, Zhu XL. Role of transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Clin Transl Hepatol. 2023;11(2):480–489. doi:10.14218/JCTH.2022.00293
3. Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. 2020;21(21):8165. doi:10.3390/ijms21218165
4. Pinero F, Dirchwolf M, Pessoa MG. Biomarkers in hepatocellular carcinoma: diagnosis, prognosis and treatment response assessment. Cells. 2020;9(6):1370. doi:10.3390/cells9061370
5. Loftus TJ, Brown MP, Slish JH, Rosenthal MD. Serum levels of prealbumin and albumin for preoperative risk stratification. Nutr Clin Pract. 2019;34(3):340–348. doi:10.1002/ncp.10271
6. Huo RR, Liu HT, Deng ZJ, et al. Dose-response between serum prealbumin and all-cause mortality after hepatectomy in patients with hepatocellular carcinoma. Front Oncol. 2020;10:596691. doi:10.3389/fonc.2020.596691
7. Ranasinghe RN, Biswas M, Vincent RP. Prealbumin: the clinical utility and analytical methodologies. Ann Clin Biochem. 2022;59(1):7–14. doi:10.1177/0004563220931885
8. Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20(4):203–222. doi:10.1038/s41575-022-00704-9
9. Zhu GQ, Tang Z, Huang R, et al. CD36(+) cancer-associated fibroblasts provide immunosuppressive microenvironment for hepatocellular carcinoma via secretion of macrophage migration inhibitory factor. Cell Discov. 2023;9(1):25. doi:10.1038/s41421-023-00529-z
10. Wang YY, Xiang BD, Ma L, et al. Development and validation of a nomogram to preoperatively estimate post-hepatectomy liver dysfunction risk and long-term survival in patients with hepatocellular carcinoma. Ann Surg. 2021;274(6):e1209–e1217. doi:10.1097/SLA.0000000000003803
11. Wang JC, Hou JY, Chen JC, et al. Development and validation of prognostic nomograms for single large and huge hepatocellular carcinoma after curative resection. Eur J Cancer. 2021;155:85–96. doi:10.1016/j.ejca.2021.07.009
12. Ruiz E, Pineau P, Flores C, et al. A preoperative nomogram for predicting long-term survival after resection of large hepatocellular carcinoma (>10 cm). HPB. 2022;24(2):192–201. doi:10.1016/j.hpb.2021.06.006
13. Kim JM, Kwon CHD, Joh JW, et al. Nomograms in hepatectomy patients with hepatitis B virus-related hepatocellular carcinoma. J Gastrointest Surg. 2019;23(8):1559–1567. doi:10.1007/s11605-018-04074-z
14. Smith SH. Using albumin and prealbumin to assess nutritional status. Nursing. 2017;47(4):65–66. doi:10.1097/01.NURSE.0000511805.83334.df
15. Watanabe T, Shibata M, Nishiyama H, et al. Serum levels of rapid turnover proteins are decreased and related to systemic inflammation in patients with ovarian cancer. Oncol Lett. 2014;7(2):373–377. doi:10.3892/ol.2013.1735
16. Arends J. Struggling with nutrition in patients with advanced cancer: nutrition and nourishment—focusing on metabolism and supportive care. Anna Oncol. 2018;29:ii27–ii34.
17. Li J, Du M, Li H, et al. Low prealbumin levels were associated with increased frequency of hepatic encephalopathy in hepatitis B virus (HBV)-related decompensated cirrhosis. Med Sci Monit. 2023;29:e937772–937771. doi:10.12659/MSM.937772
18. Chen S, Shen B, Wu Y, et al. The relationship between the efficacy of thermal ablation and inflammatory response and immune status in early hepatocellular carcinoma and the progress of postoperative adjuvant therapy. Int Immunopharmacol. 2023;119:110228. doi:10.1016/j.intimp.2023.110228
19. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12(12):681–700. doi:10.1038/nrgastro.2015.173
20. Myron Johnson A, Merlini G, Sheldon J, Ichihara K. Scientific division committee on plasma proteins IFoCC, Laboratory M. Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419–426. doi:10.1515/CCLM.2007.051
21. Yang L, Li A, Wang Y, Zhang Y. Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy. Signal Transduct Target Ther. 2023;8(1):35. doi:10.1038/s41392-022-01304-4
22. Ferrero A, Vassallo D, Geuna M, et al. Immunonutrition in ovarian cancer: clinical and immunological impact? J Gynecol Oncol. 2022;33(6):e77. doi:10.3802/jgo.2022.33.e77
23. Luo Y, Xue Y, Mao L, et al. Prealbumin as a predictor of prognosis in patients with coronavirus disease 2019. Front Med Lausanne. 2020;7:374. doi:10.3389/fmed.2020.00374
24. Wang WK, Tu CY, Shao CX, et al. Impact of enhanced recovery after surgery on postoperative rehabilitation, inflammation, and immunity in gastric carcinoma patients: a randomized clinical trial. Braz J Med Biol Res. 2019;52(5):e8265. doi:10.1590/1414-431x20198265
25. Goyal P, Salem R, Mouli SK. Role of interventional oncology in hepatocellular carcinoma: future best practice beyond current guidelines. Br J Radiol. 2022;95(1138):20220379. doi:10.1259/bjr.20220379
26. Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21(36):10327–10335. doi:10.3748/wjg.v21.i36.10327
27. Sidali S, Trepo E, Sutter O, Nault JC. New concepts in the treatment of hepatocellular carcinoma. United European Gastroenterol J. 2022;10(7):765–774. doi:10.1002/ueg2.12286
28. Jihye C, Jinsil S. Application of radiotherapeutic strategies in the BCLC-defined stages of hepatocellular carcinoma. Liver Cancer. 2012;1(3–4):216–225. doi:10.1159/000343836
29. He Z, She X, Liu Z, et al. Advances in post-operative prognostic models for hepatocellular carcinoma. J Zhejiang Univ Sci B. 2023;24(3):191–206. doi:10.1631/jzus.B2200067
30. Yang JD, Kim WR, Park KW, et al. Model to estimate survival in ambulatory patients with hepatocellular carcinoma. Hepatology. 2012;56(2):614–621. doi:10.1002/hep.25680
31. Rocha BMM, Dolan RD, Paiva CE, et al. Inflammation and performance status: the cornerstones of prognosis in advanced cancer. J Pain Symptom Manage. 2023;65(4):348–357. doi:10.1016/j.jpainsymman.2022.11.021
32. Dolan RD, Daly L, Sim WMJ, et al. Comparison of the prognostic value of ECOG-PS, mGPS and BMI/WL: implications for a clinically important framework in the assessment and treatment of advanced cancer. Clin Nutr. 2020;39(9):2889–2895. doi:10.1016/j.clnu.2019.12.024
33. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
34. Tumen D, Heumann P, Gulow K, et al. Pathogenesis and current treatment strategies of hepatocellular carcinoma. Biomedicines. 2022;10(12):3202. doi:10.3390/biomedicines10123202
35. de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41(3):374–403. doi:10.1016/j.ccell.2023.02.016
36. Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855. doi:10.1136/gutjnl-2013-306627
37. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi:10.1136/gutjnl-2019-318934
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


