Back to Journals » International Journal of General Medicine » Volume 15
Development and Validation of a Nomogram for Adverse Outcomes of Geriatric Trauma Patients Based on Frailty Syndrome
Authors Zhuang Y , Hao Tu, Feng Q, Tang H, Fu L, Wang Y, Bai X
Received 9 March 2022
Accepted for publication 29 April 2022
Published 7 June 2022 Volume 2022:15 Pages 5499—5512
DOI https://doi.org/10.2147/IJGM.S365635
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yangfan Zhuang,1 Hao Tu,1 Quanrui Feng,2 Huiming Tang,3 Li Fu,1 Yuchang Wang,1 Xiangjun Bai1
1Trauma Center/Department of Emergency and Traumatic Surgery, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China; 2Department of Intensive Care Unit, First Hospital of Wuhan, Wuhan, Hubei Province, People’s Republic of China; 3Department of Intensive Care Unit, Guangzhou First People’s Hospital, School of Medicine, South China University of Technology, Guangzhou, Guangdong, People’s Republic of China
Correspondence: Xiangjun Bai, Trauma Center/Department of Emergency and Traumatic Surgery, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China, Email [email protected]
Purpose: Currently, assessing trauma severity alone in geriatric trauma patients (GTPs) cannot accurately predict the risk of serious adverse outcomes during hospitalization. As an emerging concept in recent years, frailty syndrome is closely related to the poor prognosis of many diseases in elderly patients, including trauma. A logistic model for predicting adverse outcomes in elderly trauma patients during hospitalization was constructed in elderly patients, and the predictive efficacy of the model was verified.
Patients and Methods: Trauma patients aged ≥ 65 years between June 2020 and September 2021 were selected and randomly divided into a training set and validation set at a ratio of 3:1. Mid arm muscle circumference (MAMC) was measured to determine the degree of frailty. LASSO regression was used to screen appropriate variables for the construction of a prognostic model. The logistic regression model was established and presented in the form of a nomogram. Calibration curves and ROC curves were used to verify the performance of the model.
Results: A total of 209 patients were enrolled, including 143 (68.4%) males and 66 (31.6%) females, with an average age of 70.8 ± 4.8 years. Ageless Charlson comorbidity index, BT unit, ISS, GCS, MAMC, prealbumin and lactic acid levels were screened by LASSO regression to construct a prognostic model. The AUC of the ROC analysis prediction model was 0.89 (95% CI 0.80– 0.97) in the validation set. The results of the Hosmer–Lemeshow test for the validation set were χ 2 = 11.23, P = 0.189.
Conclusion: The prognostic model of adverse outcomes in GTPs has good accuracy and differentiation, which can improve the prediction results of risk stratification of GTPs during hospitalization by medical staff and provide a new idea for prognostic prediction.
Keywords: geriatric trauma patients, frailty, MAMC, prognostic model for adverse outcomes
Introduction
Due to the aging population worldwide, the number of older patients suffering trauma has been growing disproportionately. It has been reported that geriatric trauma patients (GTPs) have higher incidence of morbidity and mortality than younger patients.1 Traditional clinical indicators, such as blood pressure and age, are not sufficient to determine who is at high risk for poor prognosis in GTPs.2,3 The current assessment of the risk of serious adverse events in elderly trauma patients relies on various types of trauma scores, of which the more widely used are the trauma severity score (ISS) and trauma revised injury severity score (TRISS).
ISS published in the 1970s, was one of the earliest scoring systems. The severity of trauma is an important factor to be considered in predicting the prognosis of trauma. However, consideration of a single prognostic factor necessarily ignores details, such as the fact that different types of injury mechanisms are assigned the same score.4 The TRISS scoring system, developed in the 1980s, adds physiological indicators, such as a patient’s age, mechanism of injury, respiratory rate and blood pressure, to the prognosis. However, with the improvement of trauma care and statistical theory, many limitations of the TRISS have been discovered. For example, one study showed that the TRISS had a high misclassification rate in elderly patients with severe traumatic brain injury (ISS>20, age >54 years).5 In addition, the TRISS is limited in that it relies on physiological indicators, which are particularly unstable and vary widely based on the quality of out-of-hospital care and time to hospital visits, making it unlikely to be a reliable prognostic predictor in various trauma populations.5
In recent years, the concept of frailties as a driver of poor outcomes in elderly patients with trauma has received increasing attention.6–8 Geriatric experts define frailties as a state of reduced ability to respond to stressors and increased physical vulnerability due to reduced physiological reserves and multisystem disorders that limit the ability to maintain homeostasis and cope with internal and external stresses. Frailty is not uncommon in the elderly and has been proven to be closely related to the poor prognosis of many diseases, including trauma.9 As an indicator of sarcopenia, Mid-arm muscle circumference (MAMC), calculated by measuring mid-upper arm circumference (MUMC) and triceps skinfold (TSF), is a simple and noninvasive anthropometric parameter used to assess geriatric nutritional status and estimate frailty. Noori’s study demonstrated that MAMC, measured as a surrogate of lean body mass, is strongly correlated with the outcomes of maintenance hemodialysis patients.10
Integrating multiple indicators into a single model can improve the predictive value of the prognostic model compared with a single factor. Therefore, we aim to construct a multifactor trauma prognostic model to predict the risk of serious adverse outcomes in elderly trauma patients during hospitalization.
Materials and Methods
Patient Data
We performed a prospective survey between June 2020 and September 2021 to obtain data on patients over the age of 65 treated at the Trauma Center of Tongji Hospital affiliated with the Medical College of Huazhong University of Science and Technology. Patients were excluded if their reason for admission was nontraumatic. Clinical data, including age, sex, mechanism of injury (MOI), ageless Charlson comorbidity index (aCCI), blood transfusion (BT) unit within 24 h of admission, body mass index (BMI), Glasgow Coma Scale (GCS), injury severity score (ISS), shock index (SI), Revised Trauma Score (RTS) and Acute Physiology and Chronic Health Evaluation II (APACHE II), were evaluated and recorded within 24 h of admission. Laboratory tests included hemoglobin (g/L), platelet count (*109/L), albumin (g/L), prealbumin (mg/L), C-reactive protein (mg/L), and lactic acid (mmol/L) at admission.
Anthropometric Measurements
Anthropometric parameters included weight, height, MUMC and TSF. One investigator of our research team was trained to measure these by standardized measurement methods. BMI was calculated as weight divided by the square of height (kg/m2). The MUMC of the dominant arm was measured using an inelastic but flexible measuring tape without compressing the skin. Homolateral TSF thickness was measured with a digital skinfold caliper using standard techniques. The measurement process was repeated 3 times for each patient, and the 2 measurements with the smallest difference were averaged. The MUMC and TSF were reported in centimeters to the first decimal place. MAMC was calculated as follows:
MAMC(cm) = MUAC(cm) – 3.142×TSF(cm)
Frailty
According to a report by the European Working Group on Sarcopenia in Elderly People (EWGSOP) in 2010, there is a lot of overlap between frailty syndrome and sarcopenia, most frail older people exhibit sarcopenia, and some older people with sarcopenia are also frail.11 As an indicator of sarcopenia, Mid-arm muscle circumference (MAMC) was measured to determine the degree of frailty in our study.
Adverse Outcomes
Adverse outcomes were all-cause in-hospital mortality and morbidity. Morbidity included acute or progressive renal injury, cardiovascular complications (cerebrovascular accidents and/or myocardial infarctions), pulmonary complications (acute respiratory distress syndrome and/or pneumonia), urinary tract infection, venous thromboembolic outcomes (pulmonary embolism and/or deep vein thrombosis) and gastrointestinal bleeding. Unplanned admission to the ICU, unplanned intubation and unplanned operation were additional observational indices.
Statistics
Patients were divided into a case group and a control group according to whether adverse outcomes occurred during hospitalization. Normally distributed data are presented as the means and standard deviations (SDs), and nonnormally distributed data are presented as the medians and interquartile ranges (IQRs). Differences between groups were evaluated with the unpaired t test or Mann–Whitney U-test for continuous data. Frequency tables used for the chi-square test or Fisher’s test were generated for categorical variables. Univariate logistic regression was used to explore the risk factors for adverse outcomes during hospitalization. Factors at p<0.2 were entered into a multivariable regression model. Odds ratios (ORs) and 95% CIs were reported as the result in the final model.
Patients were randomly divided into the training set and the validation set at a ratio of 3:1. In the training set, appropriate variables were screened by the least absolute shrinkage and selection operator method (LASSO) regression with a 10-fold cross-validation method, where the λ value was set to 1 standard error (SE). A logistic regression model was developed to predict the occurrence of adverse outcomes during hospitalization, and the predictive efficacy of the prognostic model was tested centrally in the validation set. The final model is represented by the nomogram. The bootstrap method was used for repeated sampling 1000 times for internal validation, and the Hosmer–Lemeshow test was used for goodness of fit. Calibration curves and receiver operating characteristic (ROC) curves were drawn to analyze the differentiation and calibration of the model. Clinical decision curves were used to analyze the clinical value of the model. The statistical analysis was performed using R version 4.0.2 with extension packages.
Results
Descriptive Data
A total of 209 patients were included in the dataset, including 143 (68.4%) males and 66 (31.6%) females, with an average age of 70.8±4.8 years. A total of 131 patients (62.7%) were injured by vehicle collision, followed by falls (22.0%). The mean (SD) BMI was 22.3 (1.9) kg/m2. Hypertension (39.2%), diabetes (28.2%) and coronary heart disease (18.7%) were the most common comorbidities. The most common injured parts of the body were the head/neck (49.8%), thorax (37.8%) and extremities (31.6%). The median (IQR) GCS and ISS were 15 (13–15) points and 14 (9–17) points, respectively. The mean (SD) SI and hemoglobin level at admission were 0.70±0.23 and 103.5±23.8 g/L, respectively, among which 57 patients (27.3%) received a BT 24 h after admission, with an average transfusion volume of 1.00±2.03 U.
Among 209 patients, 68 patients (32.5%) had hospital-acquired pneumonia, 35 patients (16.7%) had traumatic coagulopathy, 47 patients (22.5%) had acute kidney injury, 14 patients (6.7%) had cardiovascular complications, 14 patients (6.7%) had gastrointestinal bleeding, 31 patients (14.8%) had thromboembolism, 8 patients (3.8%) had unplanned transfer to the ICU, 2.9% of patients had an unplanned operation (2.9%), 12.0% of patients had an intubation, and 20 patients died, with a mortality rate of 9.6%. A total of 103 patients with adverse events during hospitalization were included in the case group, and 106 patients were assigned to the control group.
The case group was older (mean (SD): 71.8 (5.1) vs 70.0 (4.4), p=0.013) and had a higher aCCI (median (IQR): 1 (0–2) vs 0 (0–1), p<0.001) than the control group. In addition, the case group had a lower BMI and MAMC than the control patients. Patients in the case group had more severe injuries and a lower GCS than those in the control group. The baseline characteristics of the patients are reported in Table 1.
 |
Table 1 Baseline Characteristics of 209 Participants |
Factors Associated with Adverse Outcomes
According to multivariate logistic analysis, the independent factors associated with adverse outcomes during hospitalization in geriatric trauma patients were sex (OR 0.09; 95% CI 0.02–0.30), aCCI (OR 4.35; 95% CI 2.18–9.56), MAMC (OR 0.55; 95% CI 0.36–0.80), GCS (OR 0.80; 95% CI 0.64–0.97), ISS (OR 1.14; 95% CI 1.01–1.30), erythrocyte infusion volume (OR 1.42; 95% CI 1.06–1.96), albumin (OR 0.85; 95% CI 0.73–0.97) and lactic acid (OR 2.72; 95% CI 1.64–4.88) (Table 2).
 |
Table 2 Logistic Regression for Factors Associated with Adverse Outcomes |
Factors Selected by LASSO Regression with 10-Fold Cross-Validation
The results of LASSO combined with 10-fold cross-validation in 156 patients in the training set are shown in Figures 1 and 2. When λ is taken as the minimum mean square error (λ min, 0.0157854), the AUC of the model is the maximum, and 12 variables are included. When λ is the minimum mean square error plus 1 standard error (λ1 SE, 0.1014697), the model includes 7 variables, and the AUC is also high. In this study, λ is chosen as λ1 SE, in which case the model is simple and the AUC is high.
 |
Figure 1 The relationship between model AUC and log (λ) is shown by LASSO regression with 10-fold cross-validation. |
 |
Figure 2 LASSO regression (dashed line λ=1 SE). |
The relationships between the regression coefficients of the factors and λ are shown in Figure 2. With the increase in λ, the regression coefficient of the variable decreases gradually. When λ was set as 1 SE (0.1014697), the variables included in the final prediction model were aCCI, MAMC, ISS, GCS, BT unit, prealbumin and lactic acid (Table 3).
 |
Table 3 Factors with Regression Coefficients |
Nomogram for Adverse Outcomes
The selected factors (aCCI, MAMC, ISS, GCS, red blood cell infusion, prealbumin and lactic acid) were incorporated into the logistic regression to construct a predictive model for adverse outcomes during hospitalization in elderly trauma patients. The model is shown in the nomogram (Figure 3). Based on the sum of the points assigned to each factor in the nomogram, a higher total point was associated with a higher risk of adverse outcomes. The variance inflation factors (VIFs) of the 7 factors in the model were all less than 5, so it can be considered that there was no collinearity effect among the model factors (Table 4).
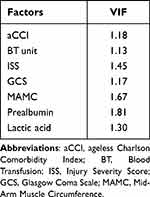 |
Table 4 Multicollinearity Test of the Prognostic Model |
 |
Figure 3 Nomogram prognostic model of serious adverse events in elderly trauma patients during hospitalization. |
Prognostic Model Performance
Calibration
Model accuracy and potential model overfitting were assessed by bootstrap validation of 1000 samples. The bootstrapped calibration plots for the prediction of adverse outcomes in the training set and validation set are shown in Figures 4 and 5, respectively. The Hosmer–Lemeshow goodness of fit test was used to evaluate the predictive bias of the adverse outcomes prognostic model. The detection result of the training set was χ2=6.33, P =0.610. The result of verification of centralized detection was χ2=11.23, P =0.189. The P values of both the training set and validation set were greater than 0.05, indicating that the model fitting degree was acceptable The Brier scores in the training set and validation set were 0.11 (95% CI 0.08–0.14) and 0.15 (95% CI 0.10–0.21), respectively, which were lower than 0.25, suggesting that the prediction accuracy of the model was good. Compared with the ISS, RTS and APACHE II, the prognostic model had a better calibration in both the training and validation sets (Figures 4 and 5).
 |
Figure 4 Calibration diagram of the training set. |
 |
Figure 5 Calibration diagram of the validation set. |
Differentiation
The AUC of the adverse outcomes prognostic model in geriatric trauma patients in the training set was 0.93 (95% CI 0.89–0.97), which was higher than the ISS (AUC 0.67; 95% CI 0.59–0.76), RTS (AUC 0.75; 95% CI 0.68–0.82) and APACHE II (AUC 0.78; 95% CI 0.71–0.85) (Figure 6). Similarly, the AUC of the prognostic model in the validation set was 0.89 (95% CI 0.80–0.97), which was better than the ISS (AUC 0.72; 95% CI 0.58–0.85), RTS (AUC 0.75; 95% CI 0.64–0.86) and APACHE II (AUC 0.79; 95% CI 0.66–0.93) (Figure 7). The prognostic models had good differentiation in both the training and validation sets. For the entire sample, the AUC for the prognostic model was 0.92 (95% CI 0.88–0.95) (Figure 8). The Youden index reached a maximum of 0.68 (0.57–0.77). The specificity was 0.75 (0.65–0.83). The sensitivity was 0.93 (0.87–0.97). The positive predictive value was 0.78 (0.72–0.84). The negative predictive value was 0.92 (0.86–0.97). The positive likelihood ratio was 3.72. The negative likelihood ratio was 0.09. The accuracy of using prognosis model, ISS, RTS and APACHE II to predict adverse outcomes on the entire sample were listed in Table 5.
 |
Table 5 Accuracy of Using Model, ISS, RTS and APACHE II to Predict Adverse Outcomes for the Entire Sample |
 |
Figure 6 ROC curve analysis of the prognostic model and various trauma scores in the training set. |
 |
Figure 7 ROC curve analysis of the prognostic model and various trauma scores in the validation set. |
 |
Figure 8 ROC curve analysis of the prognostic model and various trauma scores in the entire dataset. |
Clinical Decision Curve Analysis
Clinical decision curves using the entire dataset showed that the prognostic model was far from the two extreme reference lines, and when the risk threshold was between 0.1 and 1.0, the net benefit of the prognostic model was higher than that of the ISS, RTS and APACHE II, indicating that the clinical value of the prognostic model was better than that of traditional trauma scores (Figure 9). The risk threshold starts at 0.2, and the number of people identified as having serious adverse events by the prognostic model was close to the real number (Figure 10).
 |
Figure 9 Clinical decision curve for the prognostic model. |
 |
Figure 10 Clinical impact curve for the prognostic model. |
Discussion
In this study, seven risk factors, including aCCI, BT unit, ISS, GCS, MAMC, prealbumin and lactic acid were screened out by LASSO regression combined with 10-fold cross- validation to construct a logistic model to predict the risk of adverse outcomes during hospitalization. The clinical sensitivity of aCCI has been demonstrated in a variety of medical conditions, and progressive increases in CCI have been associated with progressive increases in mortality.12 Massive blood transfusions have been reported to be associated with a variety of complications, and BT units are important in determining the probability of death.13 Prealbumin and lactate have also been reported to be strongly associated with the prognosis of elderly trauma patients.14–17
Many studies have shown that the injury mechanism of elderly patients is different from that of young patients, mainly with low-energy injuries such as falls.18 A multicenter study of 675 elderly patients in Tehran found that falls were the primary mechanism of trauma for the majority (70%) of the elderly.19 This is slightly different from the findings of this study. In this sample, vehicle collisions were the most common injury mechanism for the elderly, with falling ranking second. This is related to China’s large urban population density and high per capita car ownership. Trauma is one of the main causes of death in elderly patients, and the mortality rate of elderly patients is 2–3 times that of young patients (15–30% vs 4–8%).20 However, among the 209 patients in this sample, 20 patients died, with a mortality rate of 9.6%, which is slightly lower than previously reported and may be related to the high rate of prehospital death in China compared with developed countries.
Factors associated with adverse outcomes during hospitalization in elderly patients were sex, aCCI, ISS, GCS, MAMC, red blood cell infusion, albumin, and lactic acid. Although the mean age of patients in the case group was higher than that in the control group, the difference was statistically significant (mean (SD):71.8 (5.1) years vs 70.0 (4.4) years, P=0.013). However, after multivariate analysis, age was not an independent factor influencing the occurrence of serious adverse events in elderly patients during hospitalization. Similarly, the SI was higher in the case group than in the control group (mean (SD): 0.75 (0.25) vs 0.66 (0.19), P =0.006), but after adjustment, the SI was not found to be an independent factor associated with adverse outcomes. Previous studies have also shown that, for elderly trauma patients, indicators such as blood pressure and age are not good enough to identify individuals with poor prognostic risk.2,21 In addition, the incidence of adverse events in elderly men (54.5%) was significantly higher than that in elderly women (37.9%). After adjusting for multivariate analysis, the risk of adverse events in female patients was significantly lower than that in male elderly patients, which was consistent with the results of other studies.22–24
MAMC was shown to be an independent risk factor for serious adverse events in hospitalized elderly trauma patients (OR 0.55; 95% CI 0.36–0.80, P =0.003), and the risk was reduced by 45% for each 1 cm increase in MAMC. Similarly, many studies in recent years have shown an increase in frailty and the risk of morbidity and mortality.7,8,25 Reversal of frailty is possible, especially in the early stages of frailty.26 Therefore, early diagnosis and further identification of patients in the prefrail or frail state and timely intervention are particularly important to reduce and delay the adverse events caused by frailty syndrome.
The prognostic model developed in this study has better differentiation and calibration than the ISS, RTS and APACHE II, with high clinical decision-making value. The included variables were all routine clinical examination items or noninvasive and easy-to-measure indicators, which are easily accepted by patients and have high clinical operability, which could provide a reference value for the treatment of trauma in the elderly in China. However, this study was a single-center observational study with a small number of patients and without external validation set, which limited the clinical representativeness of this prognostic model. Moreover, the time window was limited to the period of hospitalization, and the treatment and recovery after discharge were not further adjusted. Further validation and improvement will be required in the clinical data of large multicenter samples.
Conclusion
The prognostic model developed in this study has good accuracy and differentiation, which can improve the prediction results of risk stratification in elderly trauma patients during hospitalization by medical staff and provide a new idea for prognostic prediction of elderly trauma patients.
Abbreviations
MAMC, Mid-arm muscle circumference; GTPs, Geriatric Trauma Patients; MUMC, Mid-arm circumference; TSF, Triceps skinfold; MOI, Mechanism of injury; aCCI, ageless Charlson Comorbidity Index; BT, Blood transfusion; BMI, Body mass index; GCS, Glasgow coma score; ISS, Injury severity score; SI, Shock index; RTS, Revised Trauma Score; APACHE II, Acute Physiology and Chronic Health Evaluation II; LASSO, the Least Absolute Shrinkage and Selection Operator method; SE, Standard Error; AUC, the Area Under the Curve; 95% CI, 95% Confidence Interval; ROC, Receiver Operating Characteristic; SD, Standard deviation; IQR, Interquartile range; OR, Odds ratio; EWGSOP, The European Working Group on Sarcopenia in Older People; AWGS, the Asia Working Group for Sarcopenia.
Data Sharing Statement
The data are presented within the paper.
Ethics Approval and Consent to Participate
Ethical approval was not required by the Ethics Commission of Tongji Hospital as all data were routinely collected and held as part of usual clinical practice. The need to obtain individual patient’s written consent was waived because it’s an observational study with risk free. All methods were carried out in accordance with relevant guidelines and regulations. Our study was carried out in accordance with the Declaration of Helsinki.
Acknowledgments
The authors would like to thank all staff from the trauma center, Tongji hospital.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 82002101, 81571891, 81772129).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Perdue PW, Watts DD, Kaufmann CR, Trask AL. Differences in mortality between elderly and younger adult trauma patients: geriatric status increases risk of delayed death. J Trauma Injury Infect Critical Care. 1998;45(4):805–810.
2. Brown JB, Gestring ML, Forsythe RM, et al. Systolic blood pressure criteria in the National Trauma Triage Protocol for geriatric trauma: 110 is the new 90. J Trauma Acute Care. 2015;78(2):352–359.
3. Brown CVR, Rix K, Klein AL, et al. A comprehensive investigation of comorbidities, mechanisms, injury patterns, and outcomes in geriatric blunt trauma patients. Am Surg. 2016;82(11):1055–1062.
4. Lefering R. Trauma scoring systems. Curr Opin Crit Care. 2012;18(6):637–640.
5. Demetriades D, Chan LS, Velmahos G, et al. TRISS methodology in trauma: the need for alternatives. Br J Surg. 1998;85(3):379–384.
6. Zhao F, Tang B, Hu C, Zhang L. The impact of frailty on post traumatic outcomes in older trauma patients: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2020;1:87.
7. Tipping CJ, Bilish E, Harrold M, Holland AE, Chan T, Hodgson CL. The impact of frailty in critically ill patients after trauma: a prospective observational study. Aust Crit Care. 2020;33(3):228–235.
8. Pecheva M, Phillips M, Hull P, Carrothers AO, Queally JM. The impact of frailty in major trauma in older patients. Injury. 2020;51(7):1536–1542.
9. Poulton A, Shaw JF, Nguyen F, et al. The Association of frailty with adverse outcomes after multisystem trauma: a systematic review and meta-analysis. Anesth Analg. 2020;130(6):1482–1492.
10. Noori N, Kopple JD, Kovesdy CP, et al. Mid-Arm Muscle Circumference and Quality of Life and Survival in Maintenance Hemodialysis Patients. Clin J Am Soc Nephrol. 2010;5(12):2258–2268.
11. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423.
12. Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson Comorbidity Index: a Critical Review of Clinimetric Properties. Psychother Psychosom. 2022;91(1):8–35.
13. Wu SC, Rau CS, Kuo PJ, Liu HT, Hsu SY, Hsieh CH. Significance of blood transfusion units in determining the probability of mortality among elderly trauma patients based on the geriatric trauma outcome scoring system: a cross-sectional analysis based on trauma registered data. Int J Environ Res Public Health. 2018;15:10.
14. Panigrahi B, LaBoy S, Prosniak R, Mody A. Infection, low prealbumin, low albumin, and renal insufficiency are associated with higher morbidity and mortality in trauma patients with severe head injury. Chest. 2013;144:4.
15. Akella K, Akella S, Chendrasekhar A. Initial prealbumin level and ability to normalize prealbumin are highly predictive of increased mortality and morbidity in elderly trauma patients. Chest. 2017;152(4):346a–346a.
16. Younan DS, Westfall AO, Zaky A, Erdmann N. Neutrophil proportions are comparable to lactic acid in predicting outcomes in trauma patients. J Am Coll Surg. 2018;227(4):E63–E63.
17. Lefering R, Zielske D, Bouillon B, Hauser C, Levy H. Lactic acidosis is associated with multiple organ failure and need for ventilator support in patients with severe hemorrhage from trauma. Eur J Trauma Emerg Surg. 2013;39(5):487–493.
18. Ismail RA, El Sibai RH, Dakessian AV, Bachir RH, El Sayed MJ. Fall related injuries in elderly patients in a tertiary care centre in Beirut, Lebanon. J Emerg Trauma Shock. 2020;13(2):142–145.
19. Ghodsi SM, Roudsari BS, Abdollahi M, Shadman M. Fall-related injuries in the elderly in Tehran. Injury. 2003;34(11):809–814.
20. Kauder D. The geriatric puzzle. Assessment challenges of elderly trauma patients. JEMS. 2000;25(7):
21. Brown CV, Rix K, Klein AL, et al. A comprehensive investigation of comorbidities, mechanisms, injury patterns, and outcomes in geriatric blunt trauma patients. Am Surg. 2016;82(11):1055–1062.
22. Akkose Aydin S, Bulut M, Fedakar R, Ozgurer A, Ozdemir F. Trauma in the elderly patients in Bursa. Ulus Travma Acil Cerrahi Derg. 2006;12(3):230–234.
23. Rogers FB, Osler TM, Shackford SR, et al. A population-based study of geriatric trauma in a rural state. J Trauma. 2001;50(4):604–609.
24. Hukkelhoven CW, Steyerberg EW, Rampen AJ, et al. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg. 2003;99(4):666–673.
25. Rickard F, Ibitoye S, Deakin H, et al. The Clinical Frailty Scale predicts adverse outcome in older people admitted to a UK major trauma centre. Age Ageing. 2021;50(3):891–897.
26. Rodriguez-Manas L, Fried LP. Frailty in the clinical scenario. Lancet. 2015;385(9968):e7–e9.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
