Back to Journals » International Journal of General Medicine » Volume 16
Development and Validation of a Clinical-Based Severity Scale for Patients with Cerebral Venous Thrombosis
Authors Li M, Wan S, Wang N, Chen J, Duan J, Chen J, Zhang X, Meng R, Ji X
Received 17 September 2023
Accepted for publication 16 October 2023
Published 25 October 2023 Volume 2023:16 Pages 4783—4794
DOI https://doi.org/10.2147/IJGM.S437457
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Min Li,1,2,* Shuling Wan,1,* Nanbu Wang,3 Jiahao Chen,4 Jiangang Duan,5 Jian Chen,6 Xuxiang Zhang,7 Ran Meng,1,2 Xunming Ji2,6
1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Beijing Institute for Brain Disorders, Capital Medical University, Beijing, People’s Republic of China; 3Department of Neurology, The First Affiliated Hospital, Guangzhou University of Chinese Medicine, Guangzhou, People’s Republic of China; 4Department of Neurobiology, School of Basic Medical Sciences, Capital Medical University, Beijing, People’s Republic of China; 5Department of Emergency, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 6Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 7Department of Ophthalmology, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ran Meng, Department of Neurology, Xuanwu Hospital, Capital Medical University, 45 Changchun Road, Xicheng District, Beijing, 100053, People’s Republic of China, Tel +86-13693080599, Email [email protected] Xunming Ji, Beijing Institute for Brain Disorders, Capital Medical University, 10 You an Men Wai Road, Fengtai District, Beijing, 100069, People’s Republic of China, Tel +86-13911077166, Email [email protected]
Introduction: Cerebral venous thrombosis (CVT) is a rare subtype of stroke. However, existing scales were insufficient to evaluate the overall severity of CVT. The aim of this study is to develop and validate a CVT severity scale.
Methods: Items 1– 11 were directly derived from NIHSS. New items were generated from a literature review and focus group discussion. A total of 170 CVT patients were prospectively recruited from 26 top tertiary hospitals in China Mainland from January 2021 to May 2022 to validate the CVT severity scale. The CVT severity scale, NIHSS, mRS and GCS were rated at admission. The lumbar puncture opening pressure was also recorded. Twenty randomly selected CVT patients were rated with the CVT severity scale again 24 hours later. The clinical outcome of CVT was evaluated by mRS at 6 months after baseline.
Results: We successfully established a CVT severity scale with 18 items. Exploratory factor analysis showed that 18 items were attributed to factor 1 (focal neurological deficits), factor 2 (diffuse encephalopathy), factor 3 (intracranial hypertension) and factor 4 (cavernous sinus syndrome). CVT severity scale was positively correlated with ICP, NIHSS and mRS, and negatively correlated with GCS at baseline. CVT severity scale > 3 or factor 3 > 2 indicated intracranial hypertension. CVT severity scale > 10 indicated poor clinical outcome at 6 months of follow-up. Meanwhile, CVT severity scale showed high internal consistency and test–retest reliability.
Conclusion: The CVT severity scale included 18 items encompassing 4 domains of focal neurological deficits, diffuse encephalopathy, IH and cavernous sinus syndrome. CVT severity scale correlated well with ICP, NIHSS, mRS and GCS. Patients with CVT severity scale > 10 can be defined as severe CVT. The CVT severity scale may serve as a valid and reliable tool for measuring the overall severity of CVT.
Keywords: cerebral venous thrombosis, clinical manifestations, severity scale, reliability, validity
Introduction
Cerebral venous thrombosis (CVT) is a rare subtype of stroke which account for 0.5–1% of all strokes.1,2 The manifestations of CVT presented as a decreased level of consciousness, headache, tinnitus, visual impairment, papilledema, focal neurological deficits, seizures, psychiatric symptoms, cranial nerve palsies and neck discomfort.1,3,4 These clinical manifestations can be divided into four domains: focal neurological deficits, diffuse encephalopathy, intracranial hypertension (IH) and cavernous sinus syndrome.5,6
Most of previous studies on CVT used intracranial pressure (ICP), National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale (mRS) and Glasgow Coma Scale (GCS) to evaluate the severity of CVT.4,7–9 However, ICP was only capable of evaluating the severity of IH.8,10,11 NIHSS were designed for acute arterial stroke to assess the severity of focal neurological deficits.12,13 mRS was used only to measure the global disability.13,14 A series of studies also used GCS to discriminate the severity of CVT.9,15 However, GCS only represents the level of consciousness.
Unfortunately, none of ICP, NIHSS, mRS or GCS were sufficient to represent an overall severity of CVT. Therefore, a new scale which represents an overall severity of CVT will be essential. The aim of this study is to develop and validate a proper CVT severity scale. In this study, the CVT severity scale was designed based on NIHSS. Original items of NIHSS were preserved, and new items were added to make it more suitable for CVT.
Scale Development
Methods
Literature Review
A literature review was conducted to gather the clinical manifestations of CVT. Two independent investigators (Min Li and Nanbu Wang) carefully searched MEDLINE, EMBASE, Web of Science, Cochrane Controlled Trials Register Database, Scopus and Google Scholar from January 1966 to December 2021 by using the following keywords “symptom” or “sign” or “presentation” or “manifestation” in combination with “cerebral venous thrombosis” or “venous sinus thrombosis” or “cortical venous thrombosis” or “cavernous sinus thrombosis”. Clinical manifestations which were not included in NIHSS were recorded.
Focus Group Discussion
New items were generated and delivered to a focus group with 5 experts (2 neurologists, 1 neurosurgeon, 1 epidemiologist, 1 ophthalmologist) to discuss whether the items were relevant, understandable and unambiguous. Items with an endorsement rate higher than 0.6 were added. The weighting score of each enrolled item was related to its clinical importance.
Results
Except for the clinical manifestations included in NIHSS, ophthalmoplegia, epilepsy, headache, nausea, vomiting, decreased visual acuity, papilledema, tinnitus, vertigo, hearing loss, mental disorder and neck discomfort were the most frequent clinical manifestations of CVT after a literature review. In the CVT severity scale, items 1–11 were directly derived from NIHSS. Seven new items including epilepsy, headache, tinnitus, papilledema, ophthalmoplegia, mental disorder and neck discomfort were added and graded (items 12–18) whereas 5 new items (nausea, vomiting, decreased visual acuity, vertigo and hearing loss) were discarded in focus group discussion. Items 12–14 (epilepsy, headache and tinnitus) was scored on a 4-point scale (0–3) whereas item 15 (papilledema) was scored on a 5-point scale (0–4). Items 16–18 (ophthalmoplegia, mental disorder and neck discomfort) are offered with three options (0–2). The final CVT severity scale is shown in Table 1.
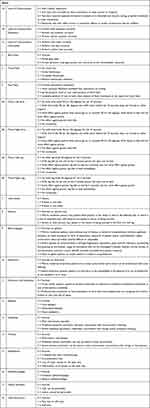 |
Table 1 Severity Scale for Cerebral Venous Thrombosis |
Discussions
We successfully established a CVT severity scale with 18 items. Items 1–11 were directly inherited from NIHSS. Items 12–18 were new items.
The acute symptomatic seizures after arterial stroke account for 4% of all patients.16 In comparison to arterial stroke, acute symptomatic seizures occur in 35–50% of CVT patients.17 These seizures more commonly occur in CVT patients with structural lesions.17 Therefore, epilepsy was added as item 12.
In patients with CVT, IH is a very common presentation.18 Headache, tinnitus and papilledema are a triad of IH.19 They were added as item 13–15. Heckmann JG et al20 and Visvanathan V et al21 reported that cavernous sinus thrombosis may also result in enlarged ophthalmic veins and thereby lead to papilledema. Either IH or cavernous sinus thrombosis may give rise to papilledema. Frisen grade was widely used for rating papilledema as previously described.22 Grades in item 15 (papilledema) was designed based on Frisen grade.
The third, fourth and sixth cranial nerve palsy may occur in the presence of cavernous sinus thrombosis.23 Cavernous sinus thrombosis may involve unilateral or bilateral cavernous sinus.23 In addition, IH may lead to unilateral or bilateral sixth cranial nerve palsy24,25 Thus, ophthalmoplegia was added as item 16.
Nzwalo H et al26 reported that mental disorder was the predominant initial manifestations of CVT. A series of studies also revealed that mental disorders predicted unfavorable clinical outcome in CVT patients.27,28 Therefore, mental disorder was added as item 17. Neck pain and stiffness were reported in CVT-induced hemorrhagic venous infarction and acute subarachnoid hemorrhage.29–31 In the CVT severity scale, neck discomfort was added as item 18.
As known, nausea and vomiting are secondary symptoms of headache in CVT patients.6 Decreased visual acuity is the secondary symptom of papilledema.19 For this reason, nausea, vomiting and decreased visual acuity were discarded in focus group discussion. Pons et al32 conducted a systemic review and found that hearing loss and vertigo occurred in association with tinnitus in CVT patients. Therefore, hearing loss and vertigo were discarded in focus group discussion.
Scale Validation
Methods
Subject Recruitment
CVT patients were prospectively recruited from 26 top tertiary hospitals in China Mainland (CCC cohort, NCT 03919305) from January 2021 to May 2022. The inclusion criterion was neuroimaging (magnetic resonance venography, computed tomography venography, magnetic resonance black-blood imaging and digital subtraction angiography) confirmed CVT as previously described.10 This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee of Xuanwu Hospital (2019[006]). All participants signed an informed consent form.
Data Collection
The CVT severity scale, NIHSS, mRS and GCS were rated by the attending doctor at admission. The lumbar puncture opening pressure was recorded as ICP. An ICP > 250 mmH2O was defined as IH.33 Twenty randomly selected CVT patients were rated with the CVT severity scale again 24 hours later. The clinical outcome of CVT was evaluated by mRS at 6 months after baseline. mRS of 0–2 was classified as good outcome, and mRS of 3–6 was classified as poor outcome.8
Statistical Analysis
The factorability of the correlation matrix was assessed by using Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy and Bartlett’s test of sphericity. Exploratory factor analysis was performed with 4 factors to determine the structural validity of the scale. Factors were interpreted when loadings were greater than 0.3. Intercorrelation between continuous variables was assessed by Pearson correlation coefficient. Comparison between two groups of continuous data was assessed by independent t-test. The sensitivity, specificity, cutoff value and area under curve were calculated using receiver operating characteristic (ROC) curves. Internal consistency was analyzed by Cronbach’s α coefficient. Test–retest reliability was analyzed by repeated measurement. Continuous data were expressed as mean ± SEM. Dichotomous data were expressed as number (percentage). A p value <0.05 was considered statistically significant. SPSS (Version 19.0, Chicago, IL, USA) and GraphPad Prism (Version 5.0, La Jolla, CA, USA) were used for all statistical analyses.
Results
A total of 170 CVT patients were recruited to validate the CVT severity scale. The demographic features and clinical manifestations of CVT patients were shown in Table 2. The KMO measurement of sampling adequacy was 0.86, and the Bartlett’s test of sphericity was significant (χ2 = 1930.99, P < 0.001). Exploratory factor analysis (Table 3) showed factor 1 (focal neurological deficits) shared items 1–12. Factor 2 (diffuse encephalopathy) shared item 17 and 18. Factor 3 (IH) shared items 13–15. Factor 4 (cavernous sinus syndrome) shared item 15 and 16.
 |
Table 2 Baseline Characteristics and Clinical Manifestations of Patients with Cerebral Venous Thrombosis |
 |
Table 3 Rotated Factor Loadings for the Severity Scale |
Pearson correlation analysis was performed to compare the CVT severity scale with ICP, NIHSS, mRS and GCS at baseline. Remarkably, the CVT severity scale is positively correlated with ICP (Figure 1A, R2 = 0.046, p = 0.013), NIHSS (Figure 1B, R2 = 0.952, p < 0.001) and mRS (Figure 1C, R2 = 0.491, p < 0.001), respectively. In addition, the CVT severity scale is negatively correlated with GCS (Figure 1D, R2 = 0.690, p < 0.001).
CVT severity scale of 3.5 was calculated as a cutoff value to predict IH with a sensitivity of 81.11% and specificity of 54.76% (Figure 2A, AUC = 0.6964, p < 0.001). It is suggested that the CVT severity scale >3 indicated the presence of IH. Further analysis revealed that patients with CVT severity scale >3 suffered from higher ICP compared with those with CVT severity scale ≤3 (Figure 2B, p < 0.001). Factor 3 (IH) of 2.5 was also identified as a cutoff value to predict IH with a sensitivity of 73.33% and specificity of 88.1% (Figure 2C, AUC = 0.8460, p < 0.001). It is suggested that factor 3 >2 indicated the presence of IH. Further analysis revealed that patients with factor >2 suffered from higher ICP compared with those with factor ≤2 (Figure 2D, p < 0.001).
Results from ROC curve also showed that the CVT severity scale >10.5 indicated mRS >2 at 6 months of follow-up (Figure 3A, AUC = 0.6818, p = 0.0368). It is suggested that the CVT severity scale >10 independently predicts poor clinical outcome. mRS in patients with CVT severity scale >10 is significantly lower than those with CVT severity scale ≤10 (Figure 3B, p = 0.001).
Cronbach’s α coefficient for total score was 0.829, showing that CVT severity scale exhibited a high internal consistency. No significant difference was found between the first and the second test, showing a high test–retest reliability (F = 0.063, p = 0.805).
Discussions
It is challenging to clinically diagnose CVT since it may manifest in a wide range of nonspecific clinical signs. Neuroimaging is therefore essential for the diagnosis of CVT.34 CVT patients were enrolled in this investigation after neuroimaging confirmation using either magnetic resonance venography, computed tomography venography, magnetic resonance black-blood imaging, or digital subtraction angiography.
Results from KMO measurement of sampling adequacy and the Bartlett’s test of sphericity showed that the CVT severity scale is suitable for exploratory factor analysis. Results from exploratory factor analysis revealed that 4 factors (focal neurological deficits, diffuse encephalopathy, IH and cavernous sinus syndrome) shared items 1–18. Each factor shared at least 2 items. It is suggested that the CVT severity scale covered four domains of the clinical manifestations of CVT.
ICP, NIHSS, mRS and GCS were widely used to evaluate the severity of a certain aspect of CVT in substantial researches.4,7–9 It is noteworthy that CVT severity scale was positively correlated with ICP, NIHSS and mRS, and negatively correlated with GCS at baseline. It is demonstrated that the CVT severity scale correlates well with all the existing scales.
In this study, we found that the CVT severity scale >3 or factor 3 >2 indicated IH. However, factor 3 showed higher sensitivity and specificity than the whole CVT severity scale. By evaluating factor 3, clinicians will be able to predict whether CVT patients suffered from IH prior to lumbar puncture.
Our results also showed that the CVT severity scale >10 indicated poor clinical outcome at 6 months of follow-up. Patients with CVT severity scale >10 had significantly lower mRS than those with CVT severity scale ≤10 at 6 months of follow-up. Therefore, severe CVT can be defined as CVT severity scale >10.
Cronbach’s α coefficient and repeated measurement showed a high internal consistency and test–retest reliability. However, the limitation to this study is the relatively small sample size.
Conclusions
We established a CVT severity scale with 18 items encompassing 4 domains of focal neurological deficits, diffuse encephalopathy, IH and cavernous sinus syndrome. The CVT severity scale was positively correlated with ICP, NIHSS and mRS, and negatively correlated with GCS at baseline. The CVT severity scale >3 or factor 3 >2 indicated IH. The CVT severity scale >10 indicated poor clinical outcome at 6 months of follow-up and can be defined as severe CVT. Therefore, the CVT severity scale may serve as a valid and reliable tool for measuring the overall severity of CVT.
Data Sharing Statement
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the ethics committee of Xuanwu Hospital (2019[006]).
Consent to Participate
All participants signed a letter of consent for participation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by National Natural Science Foundation of China (81371289, 82101390) and Natural Science Foundation of Beijing Municipality (7212047). The funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Disclosure
Min Li and Shuling Wan are co-first authors for this study. All authors declare that they have no competing interests in this work.
References
1. Bai C, Wang Z, Stone C., et al. Pathogenesis and management in cerebrovenous outflow disorders. Aging Dis. 2021;12(1):203–222. doi:10.14336/AD.2020.0404
2. Idiculla PS, Gurala D, Palanisamy M, Vijayakumar R, Dhandapani S, Nagarajan E. Cerebral venous thrombosis: a comprehensive review. Eur Neurol. 2020;83(4):369–379. doi:10.1159/000509802
3. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162–170. doi:10.1016/S1474-4422(07)70029-7
4. Duman T, Uluduz D, Midi I, et al. A multicenter study of 1144 patients with cerebral venous thrombosis: the VENOST study. J Stroke Cerebrovasc Dis. 2017;26(8):1848–1857. doi:10.1016/j.jstrokecerebrovasdis.2017.04.020
5. Coutinho JM. Cerebral venous thrombosis. J Thromb Haemost. 2015;13(Suppl 1):S238–44. doi:10.1111/jth.12945
6. Silvis SM, de Sousa DA, Ferro JM, Coutinho JM. Cerebral venous thrombosis. Nat Rev Neurol. 2017;13(9):555–565. doi:10.1038/nrneurol.2017.104
7. Wu Y, Zhou L, Yao M, et al. Elevated fasting blood glucose is predictive of the severity and poor outcome in nondiabetic patients with cerebral venous thrombosis. J Neurol Sci. 2020;417(117017):117017. doi:10.1016/j.jns.2020.117017
8. Hu Y, Meng R, Zhang X, et al. Serum neuron specific enolase may be a marker to predict the severity and outcome of cerebral venous thrombosis. J Neurol. 2018;265(1):46–51. doi:10.1007/s00415-017-8659-9
9. Kowoll CM, Kaminski J, Weiss V, et al. Severe cerebral venous and sinus thrombosis: clinical course, imaging correlates, and prognosis. Neurocrit Care. 2016;25(3):392–399. doi:10.1007/s12028-016-0256-8
10. Li M, Pan L, Gao X, Hou J, Meng R, Ji X. Low diastolic blood pressure predicts good clinical outcome in patients with cerebral venous thrombosis. Front Neurol. 2021;12:649573.
11. Wang L, Duan J, Bian T, et al. Inflammation is correlated with severity and outcome of cerebral venous thrombosis. J Neuroinflammation. 2018;15(1):329. doi:10.1186/s12974-018-1369-0
12. Fischer U, Arnold M, Nedeltchev K, et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36(10):2121–2125. doi:10.1161/01.STR.0000182099.04994.fc
13. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603–612. doi:10.1016/S1474-4422(06)70495-1
14. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–1096. doi:10.1161/01.STR.0000258355.23810.c6
15. Coutinho JM, Zuurbier SM, Bousser MG, et al. Effect of endovascular treatment with medical management vs standard care on severe cerebral venous thrombosis: the TO-ACT randomized clinical trial. JAMA Neurol. 2020;77(8):966–973. doi:10.1001/jamaneurol.2020.1022
16. Feyissa AM, Hasan TF, Meschia JF. Stroke-related epilepsy. Eur J Neurol. 2019;26(1):18–e3. doi:10.1111/ene.13813
17. Mehvari Habibabadi J, Saadatnia M, Tabrizi N. Seizure in cerebral venous and sinus thrombosis. Epilepsia open. 2018;3(3):316–322. doi:10.1002/epi4.12229
18. Ferro JM, Canhao P, Aguiar de Sousa D. Cerebral venous thrombosis. Presse Med. 2016;45(12 Pt 2):e429–e50. doi:10.1016/j.lpm.2016.10.007
19. Wall M. Update on idiopathic intracranial hypertension. Neurol Clin. 2017;35(1):45–57. doi:10.1016/j.ncl.2016.08.004
20. Heckmann JG, Tomandl B. Cavernous sinus thrombosis. Lancet. 2003;362(9400):1958. doi:10.1016/S0140-6736(03)15013-1
21. Visvanathan V, Uppal S, Prowse S. Ocular manifestations of cavernous sinus thrombosis. BMJ Case Rep. 2010;2010(1):bcr0820092225. doi:10.1136/bcr.08.2009.2225
22. Sinclair AJ, Burdon MA, Nightingale PG, et al. Rating papilloedema: an evaluation of the Frisen classification in idiopathic intracranial hypertension. J Neurol. 2012;259(7):1406–1412. doi:10.1007/s00415-011-6365-6
23. Plewa MC, Tadi P, Gupta M. Cavernous Sinus Thrombosis. In: StatPearls. Treasure Island (FL): StatPearls; 2021.
24. Cho DY, Evans KN, Weed MC, Lee A, Susarla SM. Bilateral squamosal suture craniosynostosis presenting with abducens nerve palsy and severe papilledema. World Neurosurg. 2020;138:344–348. doi:10.1016/j.wneu.2020.03.079
25. Ding D, Chen CJ, Starke RM, Liu KC, Crowley RW. Rapid recovery of bilateral abducens nerve palsies after venous sinus stenting for idiopathic intracranial hypertension. J Neurol Sci. 2015;357(1–2):335–337. doi:10.1016/j.jns.2015.07.047
26. Nzwalo H, Rodrigues F, Carneiro P, Macedo A, Ferreira F, Basilio C. Clinicoepidemiological profile of cerebral venous thrombosis in Algarve, Portugal: a retrospective observational study. J Neurosci Rural Pract. 2015;6(4):613–616. doi:10.4103/0976-3147.165417
27. Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664–670. doi:10.1161/01.STR.0000117571.76197.26
28. Wasay M, Kaul S, Menon B, et al. Asian study of cerebral venous thrombosis. J Stroke Cerebrovasc Dis. 2019;28(10):104247. doi:10.1016/j.jstrokecerebrovasdis.2019.06.005
29. Hassan A, Ahmad B, Ahmed Z, Al-Quliti KW. Acute subarachnoid hemorrhage. An unusual clinical presentation of cerebral venous sinus thrombosis. Neurosciences. 2015;20(1):61–64.
30. Hoang TPT, Perazzini C, Ngo DHA, Saby C, Bendjelid SM, Boyer L. Cerebral venous thrombosis: report of 2 cases of hemorrhagic venous infarction. Radiol Case Rep. 2020;15(8):1295–1300. doi:10.1016/j.radcr.2020.05.009
31. Wasay M, Kojan S, Dai AI, Bobustuc G, Sheikh Z. Headache in cerebral venous thrombosis: incidence, pattern and location in 200 consecutive patients. J Headache Pain. 2010;11(2):137–139. doi:10.1007/s10194-010-0186-3
32. Pons Y, Verillaud B, Ukkola-Pons E, Sauvaget E, Kania R, Herman P. Pulsatile tinnitus and venous cerebral thrombosis: report of a case and literature review. Rev Laryngol Otol Rhinol (Bord). 2012;133(3):163–164.
33. Favoni V, Pierangeli G, Toni F, et al. Idiopathic Intracranial Hypertension Without Papilledema (IIHWOP) in chronic refractory headache. Front Neurol. 2018;9:503. doi:10.3389/fneur.2018.00503
34. Oliveira IM, Duarte JÁ, Dalaqua M, Jarry VM, Pereira FV, Reis F. Cerebral venous thrombosis: imaging patterns. Radiol Bras. 2022;55(1):54–61. doi:10.1590/0100-3984.2021.0019
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



