Back to Journals » Journal of Pain Research » Volume 17
Development and Evaluation of a Virtual Reality Simulator for Spinal Cord Stimulation: A Randomized Controlled Trial
Authors Kim JY , Jang Y , Yoon EJ, Lee W, Kim J, Koh JC
Received 10 October 2023
Accepted for publication 30 January 2024
Published 7 February 2024 Volume 2024:17 Pages 543—552
DOI https://doi.org/10.2147/JPR.S443909
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Dawood Sayed
Supplementary video of “Virtual reality simulator for spinal cord stimulator” [443909].
Views: 78
Ji Yeong Kim,1,* Yookyung Jang,2,* Eun Jang Yoon,1 Wootaek Lee,1 Jaewoo Kim,2 Jae Chul Koh2
1Department of Anesthesiology and Pain Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea; 2Department of Anesthesiology and Pain Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Republic of Korea
*These authors contributed equally to this work
Correspondence: Jae Chul Koh, Department of Anesthesiology and Pain Medicine, Korea University Anam Hospital, Korea University College of Medicine, 73, Goryeodae-ro, Seongbuk-gu, Seoul, 02841, Republic of Korea, Tel +82-2-920-5632, Fax +82-2-928-2275, Email [email protected]
Purpose: The aim of this prospective study was to develop a virtual reality simulator (VRS) for spinal cord stimulation (SCS) trials and establish its effectiveness.
Methods: We developed a VRS for SCS training by integrating patient imaging data analytics, creating artificial X-ray images, and using spatial alignment techniques and virtual reality technologies. The simulator was created by a physician with considerable experience in performing SCS, and can simulate the feeling of the procedure in a virtual environment. The efficacy of the simulator for SCS trials was assessed using a cohort of 20 novice trainees. The primary outcomes were duration of the procedure, checklist score, number of C-arm images captured, and overall trainee satisfaction.
Results: The cohort that utilized the VRS had better Zwisch scale scores (P < 0.001), completed the procedure in a shorter time (P < 0.001), took fewer C-arm images (P < 0.001), and reported better overall satisfaction (P = 0.011) than the cohort that did not.
Conclusion: We developed a realistic and efficient VRS for educating novice trainees on SCS trials, thereby eliminating the risk of radiation exposure associated with cadaver training. The results of this study indicate that our VRS has potential as an instrumental resource that can be integrated into the educational framework for SCS trials.
Keywords: surgical procedure, simulation training, spinal cord stimulator, virtual reality, virtual reality simulator
Introduction
Spinal cord stimulation (SCS) is an economically viable therapy for chronic pain caused by a spectrum of conditions, such as failed back surgery syndrome, neuropathic pain disorders, peripheral vascular disease, and angina pectoris.1,2 Implantable neurostimulators are used for the treatment of chronic pain, and over 14,000 devices are implanted globally per year. Implantation of neurotransmitter devices is increasingly gaining popularity owing to technological advancements and the expansion of their clinical utility.3,4 However, clinicians generally avoid implanting neuromodulation devices because of their limited experience and the absence of uniform training protocols in their academic pain fellowships.5,6 Simulation phantom models used in educational settings often fall short in replicating an SCS trial or implantation scenario.7 The use of human cadavers has its own challenges, including morbidity, expense, and rapid degradation.7
Virtual reality simulators (VRSs) were recently developed for use in several medical disciplines, including pain management, rehabilitation, and surgical procedure instructions.8–10 The superiority of virtual reality simulation over cadaver training lies in the avoidance of radiation exposure, particularly when teaching C-arm-guided spinal procedures.8–13
The development of a VRS requires not only the integration of software development technologies, but also experience and an accurate understanding of procedures. In this study, we introduce a virtual simulator developed by a doctor with experience in performing SCS and evaluate the effectiveness of the simulator as an educational tool for trainees with limited experience.
Materials and Methods
Study Design and Participants
This was a randomized controlled trial conducted to establish the effectiveness of a novel VRS for SCS trials. The study protocol was reviewed and approved by the Institutional Review Board of Korea University Anam Hospital (2023AN0092). This study was conducted rigorously in accordance with the principles of the 2013 Declaration of Helsinki and adhered to good clinical practice guidelines. The study population consisted of 20 who were undergoing anesthesiology and pain medicine training and had no prior experience with performing SCS procedures on their own. Exclusion criteria was as follows: (1) pregnancy (2) severe prior motion sickness in virtual reality (3) physical impairment at the time of the study. The participants were randomly assigned to either the VRS (group V, n = 10) or the control (group C, n = 10) group depending on whether they received training using VRS or audiovisual training only. Envelopes that contained the group allocations were numbered sequentially and sealed prior to group assignments. A researcher blinded to the trainees’ assessments opened the sealed envelopes. Written informed consent was obtained from all participants.
Development of the Simulator
The simulator was developed using a process similar to that used for our previous spinal procedure simulator.14 However, considering that the SCS procedure is usually performed on the cervical or thoracic spine, the CT data of the entire spine were used in this study. As with the previous simulator, real-time virtual X-rays could be generated by matching the patient’s Digital Imaging and Communications in Medicine data coordinates, Hounsfield unit values, and virtual objects in the virtual spaces.
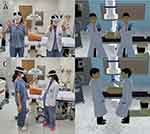
Several disadvantages of previous spinal simulators were overcome in our simulator. First, to help trainees who are unfamiliar with virtual simulators, the simulation was designed to be performed manually rather than with a controller. We used the Oculus integration SDK (version 55.0; https://developer.oculus.com/) to recognize and visualize the user’s hands. Additionally, avatars were created to reduce the sense of heterogeneity and to provide guidance from a tutor so that users could share experiences in the same virtual space as the avatars, not just in their hands. Considering that moving around in a virtual space when using your hand is difficult compared with using a controller, the simulator was designed to identify the user’s hand movements and navigate in the intended direction. This gesture recognition is useful for performing various functions, such as virtually moving in a small space or capturing and saving a C-arm image (Figure 1A and B). If a large space is available, it is also possible to select a simulation method by walking around and recognizing the surrounding space.
An Internet-based network was created, and clients connected to the network can share the positions of the 30 joints of both hands (three joints in each of the 10 fingers), both wrists, and the head, while wearing the device in real-time to share virtual experiences between users. These shared data are used to control each rigged avatar, and if the users are nearby, they can share a reference point to match their virtual and actual locations (Figure 1C and D). Moreover, the location and movement of the C-arm and the location and status of each surgical tool are shared in real-time through the network. In addition, users can efficiently operate the C-arm or surgical table by lightly touching a specific finger or triggering directional arrows. We also developed a software that allows mobile phones and computers to join the same virtual space, making it easier and more convenient for tutors and observers to be included in the virtual space.
The C-arm and surgical table were designed to be adjusted during the simulation using buttons that can be adjusted with the light beam emitted from the hand (Figure 2A). However, we had to create additional codes for SCS simulations. An epidural needle with a curved tip and a syringe were virtually created to simulate the epidural approach. The syringe was programmed to adjust the position of the piston when held with both hands and engage by approaching the back of the epidural needle. The syringe was programmed to remain engaged when attached to the needle, move while holding the needle, and disengage when disconnected from the needle. The tip of the epidural needle was set to be immovable with hand movements once it touches the skin of the virtual patient; instead, it was programmed to make fine forward and backward movements and rotations by through interactions with nearby virtual arrows (Figure 2B). When the epidural needle combined with the syringe enters the skin, the right thumb can be used to press the syringe piston, which was set to move along with the finger; however, the piston immediately returns to its original position when the hand is removed (Figure 2C). The epidural needle was programmed to stop advancing once it touches bone. When the needle enters the epidural space, the piston prevents it from returning, thereby simulating loss of resistance. We also simulated the appearance of the cerebrospinal fluid leaking when the needle advances further and punctures the dura (Figure 2D). A simulation was also created for insertion of the SCS lead when the tip of the needle enters the epidural space. When the SCS lead enters the end of the epidural needle, which is disconnected from the syringe, it first moves along the epidural needle; when the lead passes through the needle, it can travel along the virtual spinal cord in the epidural space (Figure 2E). The tip of the lead at the side of the epidural space can be finely adjusted by rotating the opposite end, similar to the actual procedure, and the placement of the entire lead can be simulated by moving forward and backward according to the direction of the tip. The transparency of the skin and bones after the procedure can be adjusted so that the positional relationship of the actual anatomical structure can be identified on the X-ray image (Figure 2F). (See the Supplementary Video for a full description of the simulator)
Evaluation of the Simulator
Curriculum and Tests
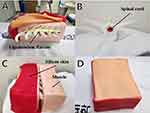
We developed a thoracic simulation phantom model that allows for the detection of loss of resistance when the needle tip is inserted into the epidural space. The phantoms were developed as previously described.15 For SCS, the ligamentum flavum was constructed using silicon rubber as a durable coating (Figure 3A). The spinal cord was inserted into the neural canal to allow the SCS lead travel along the posterior epidural space (Figure 3B). The surrounding muscles and skin were created using urethane and silicone, respectively, and were designed to be detachable (Figure 3C and D). A comprehensive flowchart of the curriculum is shown in Figure 4. Data on sex, age, duration of residency training, and previous experience in performing, observing, and assisting with SCS trials were collected for each participant. Subsequently, each participant performed a pretest SCS trial using the simulation phantom. The procedural assessments included needle insertion for lead placement into the epidural space at the T11 and T12 and lead placement at T11 and T12. After the initial test, the test group underwent 60 min of audiovisual education and VRS-integrated training. Conversely, after the control group underwent an initial test and an 8-min video lecture similar to that of the test group, they were granted a 60-min self-study period using audiovisual resources without VRS. After completing all training sessions, the participants underwent a posttest evaluation performed using a phantom. The posttest was similar to the pretest and was conducted after all training modules were completed. The assessment metrics included the checklist score, modified Zwisch scale score, duration of procedure (in seconds), number of C-arm shots taken, and satisfaction score (0–5). Each performance was evaluated by physicians specialized in pain assessment who were blinded to the group assignments.
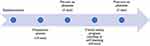 |
Figure 4 Study design. |
Outcome Measures
The primary outcome was change in the checklist score (posttest vs pretest) used to gauge proficiency in executing SCS trials, a metric that has been utilized in multiple studies.15–17 The validated checklist score17 used for evaluating C-arm-guided spinal procedures comprises seven task-specific questions, with the possible response for each question being either “yes” or “no”. The modified Zwisch scale18 comprises seven questions, with a maximum of five points for each question. The modified English versions of the abovementioned evaluation tools are presented in Appendix 1 (checklist score) and 2 (modified Zwisch scale). These tools were translated into Korean, and proficiency in their use for SCS trials was assessed accordingly. Supplementary metrics recorded included duration of the procedure and the number of C-arm shots taken. Duration of the procedure was defined as the time from the acquisition of the initial X-ray image to the completion of the procedure. Participant satisfaction was assessed upon completion of all the modules, and responses were graded on a scale of 1 to 5, with 1 and 5 indicating dissatisfaction and satisfaction, respectively.
Statistical Analysis
A previous study17 revealed a five-point difference in checklist ratings between a control group, which attended a didactic session, and a low-fidelity group, which learned using a foam-encased plastic spine. We cautiously assumed that half of this difference was an average discrepancy. Dividing the interquartile range by 1.35 yielded the standard deviation. The larger standard deviation of 1.5 determined the sample size of the test and control groups. G.powerⓇ (version 3.1.9.7., Düsseldorf, Germany)18 recommended a target of nine participants per group (power = 0.9; significance threshold = 0.05). Considering a 10% attrition rate, 10 patients were included in each group. The normality of the data distribution was evaluated using the Shapiro–Wilk test. Normally distributed data are presented as mean ± standard deviation, whereas skewed data are expressed as medians (interquartile range [IQR]). The independent Student’s t-test was used to compare the difference between the groups for normally distributed data, whereas the Mann–Whitney U-test was used to compare continuous variables with a skewed distribution. Categorical demographic variables are expressed as numerals and were analyzed using Pearson’s chi-square or Fisher’s exact test. The comparison of group outcomes before and following the educational intervention was conducted using either the paired t-test or the Wilcoxon signed-rank test, as appropriate. Statistical significance was set at P <0.05. Data were analyzed using SPSS Statistics for Windows (version 24.0; IBM Corp., Armonk, NY, USA).
Results
Twenty residents were enrolled in the study. Their demographic data are presented in Table 1. The extent of their experience in performing, observing, and assisting with C-arm-guided spinal procedures was analogous. There were noteworthy disparities between the demographic characteristics of the two cohorts.
 |
Table 1 Participant Demographic Data |
The average checklist and modified Zwisch scale scores are presented in Table 2. Both cohorts showed an increase in the mean post-test scores relative to their pre-test scores.
 |
Table 2 Pre-Test and Post-Test Results for Lumbar Transforaminal Epidural Block |
Specifically, both groups showed improved checklist scores compared to their pre-rest performance metrics (group V, P = 0.004; group C, P = 0.017). However, no significant difference was observed in the magnitude of change in post-training checklist scores between the groups. Both groups showed an increase in modified Zwisch scale scores following training (group V, P = 0.004; group C, P = 0.005). However, group V showed a more significant increase in modified Zwisch scale scores post-training than group C (P <0.001). In both groups, the post-test procedure durations and number of C-arm images captured were shorter and fewer, respectively, than the pre-test values. However, group V showed a significantly shorter procedure duration (P <0.001) and took fewer C-arm images (P <0.001) post-training than group C. In addition, group V reported significantly higher overall satisfaction scores than Group C (P = 0.011).
Discussion
In this study, we introduced a VRS for SCS developed by a pain medicine specialist and evaluated its value for training novice trainees. To our knowledge, this is the first VRS designed by a pain medicine specialist. The modified Zwisch scale scores of the participants in the VRS group indicated that VRS improved SCS performance compared with traditional training. In addition, the VRS group also required fewer C-arm pictures and completed the procedure faster than the control group.
SCS is a complex and challenging procedure. The treatment involves inserting a large needle at the gentlest angle into the cervical or thoracic epidural space, which is narrower than the lumbar space.19 Next, a relatively stiff but bendable electrode is inserted into the small space and placed in the desired location. The handle farthest from the lead must be rotated to insert the electrode. However, the central area between the lead and handle often bends or moves incorrectly. These intricacies in the procedure indicate that proper training for SCS is essential; however, educational support for SCS training is lacking for different reasons.20 A limited number of SCS cases, lack of a structured SCS curriculum, and few SCS-trained staff are the main issues.20
Some researchers introduced a simulator that could demonstrate SCS by generating virtual X-rays based on the application of needles, leading to the creation of a virtual simulation model.7 However, this simulator could not reproduce the feeling of the actual procedure, making it difficult to reflect actual patient data. Additionally, the system incurs significant costs, and the integrity of the puncture pad tends to diminish because of continuous usage. However, our simulator has the advantage of accessibility in that it can be used in an actual procedure room with only an inexpensive head-mounted display and the software installed in it. If an Internet connection is available, sharing the procedure on multiple devices such as computers and mobile phones, even in remote places, is possible. Another notable advantage is the tutor’s ability to access the educational environment virtually and guide the trainee via the internet connection. This eliminates the challenges trainees may face when undergoing VR education without the assistance of a tutor. Above all, actual patient data can be easily applied to our simulator; therefore, it can be used to perform difficult procedures in advance and train unskilled doctors.
Several efforts have been made to enhance the precision and ease of executing procedures using virtual simulations.10,21,22 Using virtual simulations to visualize intricate structures can assist clinicians in effectively performing procedures that are expected to be complex.23 Compared to other simulation tools, the VRS used in the present study has the unique advantage of being designed by a physician proficient in and dedicated to instructing trainees on the specific procedure.24,25 This is particularly important because the expertise of the physician enabled the development of a comprehensive simulator that includes all the relevant and nuanced aspects of the procedure.
Simulating real-world experiences through VR remains challenging despite several notable technological advances, particularly owing to constraints related to device capabilities and cost. It is still difficult to mirror tactile sensations or perform small movements in a VR environment.
Therefore, for VR to optimally serve educational purposes, it is essential for developers to receive extensive feedback from users and actively reexamine and adjust the methodology as appropriate. For example, developers may find it difficult to understand the exact process of operating the actual C-arm or receiving image guidance during the procedure. The simulation must allow for precise movement control and mimic the tactile feel of real procedures as closely as possible, even within the constraints of virtual reality. During the assessment process, feedback from the tutor or trainee should reflect information that can help the trainee obtain the maximum training benefits. Thus, only a specialist very well acquainted with the process can create a comprehensive and effective simulator.
In general, VR has problems such as fatigue and motion sickness. Motion sickness is especially common when a person’s sensory information about body movement and position does not match reality.26 In numerous prior virtual reality simulations, body positions and movements often deviated from real-life experiences, such as when encountering a roller coaster ride.27 Comparatively, in this simulation, although complete elimination was not possible due to the inherent limitations of virtual reality, the incidence of cybersickness was mitigated by aligning the user’s movement direction, behaviors, and hand placement. Further research on the effectiveness of this type of simulator in reducing motion sickness is encouraged for clearer conclusions.
We hypothesized that novice residents who undergo SCS training using our simulator will master the procedure more efficiently than those who trained using alternative methods. In line with our expectations, we observed a significant increase in the modified Zwisch scale scores of the trainees who used the simulator compared to the control group. In addition, we noted that the simulator training group (group V) completed the procedure in shorter time and required fewer C-arm images than the conventional training group (group C). We attributed these outcomes to the trainees’ access to the program and the opportunity to repeatedly hone their skills by practicing SCS using our simulator, which led to them becoming progressively more proficient in performing the procedure. The ability to scrutinize the 3D architecture of the vertebrae and confirm the relative placement of the needle tip upon completion of the procedure are distinct attributes that are not replicable, even in a clinical context involving an actual patient. However, in the learning process of a procedure like SCS, in addition to being familiar with the technical aspects, it is essential to learn experience and theoretical knowledge to cope with various situations. Simulating the procedure with a virtual phantom model poses challenges in replicating uncommon patient scenarios, unexpected complications, and pre-/post-procedure considerations. In contrast, this virtual reality simulator has the advantage of expanding training by allowing the incorporation of diverse situations. Although the evaluation of the development and application of related content was not described in this study, we believe that the application of such extensions will be of great help in activating the use of this simulator and proving its effectiveness.
This study had some limitations. This study had some limitations. First, this simulator cannot reproduce the exact tactile feedback experienced during a real procedure, which hinders training on the physical feedback encountered, significantly affecting the efficacy of training outcomes. However, despite this device limitation, the simulator offers easy accessibility with simple equipment and compensates for the lack of tactile sense through other audiovisual stimuli. Moreover, aligning with a real surgical space opens the possibility of future implementation of tactile feedback. Second, the study was conducted with participants of uniform experience levels and over a short duration. A more diverse range of expertise and a longer evaluation period would have allowed for a more thorough assessment of the simulator’s impact. In addition, this study only compared audiovisual training materials, and no comparison was made with other methods. We believe that a more reliable and meaningful verification would have been made if comparisons had been made with other previously developed training methods.
Finally, if we had had more systematic and refined feedback on each of the assessment tools we used, that would have been valuable.
Conclusion
We described a novel simulator that allows novice trainees to acquire SCS skills without the risk of radiation exposure and confirmed the effectiveness of the simulator in SCS training. The results of this study indicate that the VRS for SCS amplified the learning efficiency of the trainees compared with conventional training techniques. Further studies are needed to explore the potential of the VRS as a remotely accessible substitute for instructing novice trainees on a range of spinal procedures.
Data Sharing Statement
The datasets obtained in the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank Editage for their English language editing.
Funding
This research was supported by the Korea University Research (grant no: K2325441).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Eliasson T, Augustinsson LE, Mannheimer C. Spinal cord stimulation in severe angina pectoris--presentation of current studies, indications and clinical experience. Pain. 1996;65(2–3):169–179. doi:10.1016/0304-3959(95)00238-3
2. Kumar K, Toth C, Nath RK, Laing P. Epidural spinal cord stimulation for treatment of chronic pain--some predictors of success. A 15-year experience. Surg Neurol. 1998;50(2):110–120; discussion 120–111. doi:10.1016/s0090-3019(98)00012-3
3. Meyerson BA, Linderoth B. Mode of action of spinal cord stimulation in neuropathic pain. J Pain Symptom Manage. 2006;31(4 Suppl):S6–S12. doi:10.1016/j.jpainsymman.2005.12.009
4. Atkinson L, Sundaraj SR, Brooker C, et al. Recommendations for patient selection in spinal cord stimulation. J Clin Neurosci. 2011;18(10):1295–1302. doi:10.1016/j.jocn.2011.02.025
5. Fanciullo GJ, Rose RJ, Lunt PG, Whalen PK, Ross E. The state of implantable pain therapies in the United States: a nationwide survey of academic teaching programs. Anesth Analg. 1999;88(6):1311–1316. doi:10.1097/00000539-199906000-00021
6. Gharibo C, Laux G, Forzani BR, Sellars C, Kim E, Zou S. State of the field survey: spinal cord stimulator use by academic pain medicine practices. Pain Med. 2014;15(2):188–195. doi:10.1111/pme.12264
7. White WW, Jung MJ. Three-dimensional virtual reality spinal cord stimulator training improves trainee procedural confidence and performance. Neuromodulation. 2023;26(7):1381–1386. doi:10.1016/j.neurom.2022.03.005
8. Pourmand A, Davis S, Marchak A, Whiteside T, Sikka N. Virtual reality as a clinical tool for pain management. Curr Pain Headache Rep. 2018;22(8):53. doi:10.1007/s11916-018-0708-2
9. Perez-Marcos D. Virtual reality experiences, embodiment, videogames and their dimensions in neurorehabilitation. J Neuroeng Rehabil. 2018;15(1):113. doi:10.1186/s12984-018-0461-0
10. Bernardo A. Virtual Reality and Simulation in Neurosurgical Training. World Neurosurg. 2017;106:1015–1029. doi:10.1016/j.wneu.2017.06.140
11. Vozenilek J, Huff JS, Reznek M, Gordon JA. See one, do one, teach one: advanced technology in medical education. Acad Emerg Med. 2004;11(11):1149–1154. doi:10.1197/j.aem.2004.08.003
12. Choi EJ, Go G, Han WK, Lee PB. Radiation exposure to the eyes and thyroid during C-arm fluoroscopy-guided cervical epidural injections is far below the safety limit. Korean J Pain. 2020;33(1):73–80. doi:10.3344/kjp.2020.33.1.73
13. Kim TH, Hong SW, Woo NS, Kim HK, Kim JH. The radiation safety education and the pain physicians’ efforts to reduce radiation exposure. Korean J Pain. 2017;30(2):104–115. doi:10.3344/kjp.2017.30.2.104
14. Kim JY, Lee JS, Lee JH, Park YS, Cho J, Koh JC. Virtual reality simulator’s effectiveness on the spine procedure education for trainee: a randomized controlled trial. Korean J Anesthesiol. 2023;76(3):213–226. doi:10.4097/kja.22491
15. Koh JC, Jang YK, Seong H, Lee KH, Jun S, Choi JB. Creation of a three-dimensional printed spine model for training in pain procedures. J Int Med Res. 2021;49(11):3000605211053281. doi:10.1177/03000605211053281
16. Park J, MacRae H, Musselman LJ, et al. Randomized controlled trial of virtual reality simulator training: transfer to live patients. Am J Surg. 2007;194(2):205–211. doi:10.1016/j.amjsurg.2006.11.032
17. Gonzalez-Cota A, Chiravuri S, Stansfield RB, Brummett CM, Hamstra SJ. The effect of bench model fidelity on fluoroscopy-guided transforaminal epidural injection training: a randomized control study. Reg Anesth Pain Med. 2013;38(2):155–160. doi:10.1097/AAP.0b013e318283f5bc
18. Hagedorn JM, Moeschler S, Goree J, Weisbein J, Deer TR. Diversity and inclusion in pain medicine. Reg Anesth Pain Med. 2020;45(10):839. doi:10.1136/rapm-2020-101284
19. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi:10.3758/bf03193146
20. Visser WA, Lee RA, Gielen MJ. Factors affecting the distribution of neural blockade by local anesthetics in epidural anesthesia and a comparison of lumbar versus thoracic epidural anesthesia. Anesth Analg. 2008;107(2):708–721. doi:10.1213/ane.0b013e31817e7065
21. Pak DJ, Gruber J, Deer T, et al. Spinal cord stimulator education during pain fellowship: unmet training needs and factors that impact future practice. Reg Anesth Pain Med. 2019;44(3):407–414. doi:10.1136/rapm-2018-100065
22. Kockro RA, Serra L, Tseng-Tsai Y, et al. Planning and simulation of neurosurgery in a virtual reality environment. Neurosurgery. 2000;46(1):118–135; discussion 135–117.
23. Mutter D, Dallemagne B, Bailey C, Soler L, Marescaux J. 3D virtual reality and selective vascular control for laparoscopic left hepatic lobectomy. Surg Endosc. 2009;23(2):432–435. doi:10.1007/s00464-008-9931-y
24. Seong H, Yun D, Yoon KS, Kwak JS, Koh JC. Development of pre-procedure virtual simulation for challenging interventional procedures: an experimental study with clinical application. Korean J Pain. 2022;35(4):403–412. doi:10.3344/kjp.2022.35.4.403
25. Pires F, Costa C, Dias P. On the Use of Virtual Reality for Medical Imaging Visualization. J Digit Imaging. 2021;34(4):1034–1048. doi:10.1007/s10278-021-00480-z
26. Koch A, Cascorbi I, Westhofen M, Dafotakis M, Klapa S, Kuhtz-Buschbeck JP. The neurophysiology and treatment of motion sickness. Dtsch Arztebl Int. 2018;115(41):687–696. doi:10.3238/arztebl.2018.0687
27. Gui C, Venema DM, Chien JH, Cochran TM, Siu KC. Quantifying fear of falling by utilizing objective body sway measures: a 360° virtual video study. Gait Posture. 2022;93:160–165.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.