Back to Journals » Drug Design, Development and Therapy » Volume 16
Determining the Minimum Effective Concentration of Ropivacaine in Epidural Anesthesia for Tolerable Pain in Transforaminal Percutaneous Endoscopic Lumbar Discectomy to Avoid Nerve Injury: A Double-Blind Study Using a Biased-Coin Design
Authors Hu B , Li L , Wang H, Ma T, Fu Z , Kang X , Feng Z
Received 17 August 2021
Accepted for publication 21 December 2021
Published 9 February 2022 Volume 2022:16 Pages 315—323
DOI https://doi.org/10.2147/DDDT.S334605
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Bingwei Hu,1,2 Liang Li,2 Hongwei Wang,2 Tingting Ma,2 Zhimei Fu,2 Xianhui Kang,3 Zhiying Feng1
1Department of Pain, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Anesthesiology, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang, People’s Republic of China; 3Department of Anesthesiology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Zhiying Feng; Xianhui Kang
The First Affiliated Hospital, Zhejiang University School of Medicine, No. 79 Qingchun Road, Hangzhou, 310003, Zhejiang, People’s Republic of China
, Email [email protected]; [email protected]
Purpose: Epidural anesthesia (EA) is the main anesthesia method for transforaminal percutaneous endoscopic lumbar discectomy (PELD). Reducing the concentration of ropivacaine can help preserve tactile sensation, allowing patients to provide timely feedback to the surgeons when a nerve root is contacted to avoid nerve injury. Therefore, a 90% effective concentration (EC90) that allows for mild pain [visual analog scale (VAS) score ≤ 3] while maximizing tactile sensation must be identified.
Methods: The concentration of ropivacaine for EA was varied for consecutive patients in this study using a two-stage biased-coin design (BCD) according to the response of the previous patient; the concentration used for the first patient was 0.2%. When the previous patient had a negative response (VAS score > 3), the concentration used for the next one was increased by 0.015%. When the previous patient had a positive response (VAS score ≤ 3), the concentration used for the next one had an 89% probability of remaining the same and an 11% probability of being reduced by 0.015%. The EC90 of ropivacaine was estimated using isotonic regression, and the 95% confidence interval (CI) was estimated using the bootstrapping method in R.
Results: A total of 58 patients were included in the study. The calculated EC90 was 0.294% [95% CI (0.271%, 0.303%)]. Among 13 patients who reported unintended nerve root contact during the operation, none were found to have irreversible nerve injury after the operation.
Conclusion: To preserve maximum tactile sensation, the EC90 of ropivacaine was 0.294% for patients with allowed mild pain. This concentration could allow for timely feedback when the nerve root is contacted, to avoid nerve injury.
Keywords: minimum effective concentration, epidural anesthesia, ropivacaine, transforaminal percutaneous endoscopic lumbar discectomy, biased-coin design, isotonic regression
Introduction
Lumbar disc herniation (LDH) is a common orthopedic disease. Traditional open surgery can effectively remove diseased nucleus pulposus tissue. With the development of modern microsurgery, percutaneous endoscopic lumbar discectomy (PELD) has been gradually applied in the treatment of LDH. Of particular concern is the tight operative space and the proximity of the endoscope to the spinal cord and nerve roots; the likelihood of irreversible spinal cord nerve injury is extremely high.
Local anesthesia (LA) has been recommended in PELD to avoid nerve injury1–4 because it allows surgeons to determine whether nerve roots are injured based on timely feedback from patients.5,6 However, some patients receiving LA cannot tolerate pain during surgery. One study7 showed that 20% of the patients who underwent LA suffered unbearable pain, and 50% of the patients developed a fear of surgery. These results indicate that LA cannot adequately relieve pain, which could lead to fatal cardiovascular events.8 Patients have even been reported experiencing shock due to severe pain.9 Therefore, some surgeons recommend general anesthesia (GA) for patients undergoing percutaneous endoscopic lumbar discectomy.7 Although patients who receive GA cannot feel pain, they are unconscious and cannot perceive nerve injury. A study reported10 that among patients receiving GA, 2 (4.8%) had transient motor weakness, 3 (7.4%) had lower limb numbness, and 1 (2.4%) had cauda equina syndrome.
Epidural anesthesia (EA) is another anesthesia method that allows patients to remain conscious.11 However, nerve injury occurred in PELD using EA as before. A single-center retrospective analysis found that the incidence of nerve root injury in PELD using EA was 1.2% (5/426);12 another study reported, an 8.6% (30/233) incidence.13 EA with a low concentration of ropivacaine, the most commonly used anesthetic in recent studies, not only has a good analgesic effect but can also preserve lower extremity motor function. Compared with LA, low concentration EA with 0.3% or 0.375% ropivacaine can achieve a better analgesic effect.10,14 The incidence of nerve injury was much lower in patients receiving low concentration EA than in patients receiving GA.10 Because low concentrations of ropivacaine do not completely block lower extremity motor function, the surgeon can detect nerve injury by observing the movement of a patient’s toe during surgery.5,10,15 However, this approach has shortcomings: patients often receive motor feedback after nerve injury has occurred. Because low concentrations of ropivacaine preserve lower extremity motor function but completely block the sensory fibers (including pain and tactile sensation), patients cannot immediately perceive nerve contact. Nerve injury has been reported to occur under EA with 0.375% ropivacaine.10 Therefore, preserving tactile sensation while blocking pain is a better choice to avoid nerve injury.
To minimize intraoperative nerve injury, the concentration of local anesthetics should be reduced as much as possible to preserve maximal tactile sensation. Our study, undertaken in a tertiary teaching hospital, used the biased-coin design (BCD) method to explore EC90, estimated as the minimum effective concentration (MEC) of EA in PELD, when the patient’s intraoperative pain could be tolerated. This EC90 could preserve maximal tactile sensation to provide timely feedback to avoid nerve injury.
Materials and Methods
This study was conducted in accordance with the Declaration of Helsinki. All of the participants were informed about the purpose of the trial. It was approved by the Medical Ethics Committee of Tongde Hospital of Zhejiang Province (Zhe Tongde Expedited Review No. [2020]013); and was registered with the Chinese Clinical Trial Registry (ChiCTR2000031593). After obtaining written informed consent, consecutive patients undergoing PELD at our hospital from 1 April 2020 to 31 October 2020 were recruited.
The inclusion criteria were as follows: American Society of Anesthesiologists (ASA) physical status I to III, age between 18 and 80 years, and elective PELD. The exclusion criteria were as follows: local anesthetic drug allergy, contraindications to EA, severe systemic disease, and mental retardation or an inability to communicate. The withdrawal criteria were as follows: patient withdrawal from the study; unintended nerve root contact during epidural puncture; the occurrence of total spinal anesthesia; erroneous insertion of the epidural catheter into the blood vessel or subarachnoid space; an EA level not covering the surgical site; severe drug allergy; and manifestations of local anesthetic poisoning.
All patients were fasted from food and water and were not given any medication before the operation. The puncture site for EA was the fourth intervertebral space (counting towards the head) from the intervertebral space that required surgery, and the catheter was inserted 3 cm into the epidural space in the caudal direction. Ensuring that the catheter was not placed in the subarachnoid space or any blood vessel was important. Each patient’s level of anesthesia was determined 15 minutes after an injection of a 12-mL split-dose of ropivacaine at the preestablished concentration (Naropin®, AstraZeneca AB, Sodertalje, Sweden). The investigator prepared the ropivacaine injections at the preestablished concentrations and gave them to the anesthesiologist. After 15 minutes of anesthesia level measurement, the operation was started. All patients were asked to report intraoperative pain of VAS score > 3 or when there was sudden pain in the lower legs during the operation. Patients whose pain was VAS>3 at any time during the operation would receive 4 mg of oxycodone for salvage analgesia. If the pain was VAS>3 after 5 minutes, salvage analgesia was repeated with 2 mg of oxycodone until the total does reached 10 mg.
Patients were anesthetized by three experienced anesthesiologists, and none of the anesthesiologist, follow-up nurse, or patients were aware of the concentrations. Each patient was visited on the ward on the first post-operative day (24 h after surgery) by the same nurse. On each occasion, complications as pain, lower extremity muscle strength, and paresthesia were recorded. One week after surgery, each patient received the same inquiry through telephone interviews by the same nurse.
This study used a two-stage BCD,16 and the concentration of ropivacaine given to each patient depended on the response of the previous patient. A negative response was defined as VAS score > 3 of the patient’s intraoperative pain at any time, while a positive response was defined as VAS score ≤ 3. In the first stage (BCD I), the initial concentration of ropivacaine was 0.2%. If the previous patient had a negative response, the concentration of ropivacaine used for the next patient was increased by 0.015%. The BCD I continued until a patient had a positive response, at which point the second stage (BCD II) started. In BCD II, if the previous patient had a negative response, the concentration of ropivacaine used for the next patient was increased by 0.015%. If the previous patient had a positive response, the concentration of ropivacaine used for the next patient was determined by biased-coin randomization using the statistical software R (Γ=0.9). The concentration of ropivacaine used for the next patient had an 11% [b=(1-Γ)/Γ=0.11] probability of being reduced by 0.015% and an 89% (1-b=0.89) probability of remaining the same. To estimate the EC90, at least 45 patients with positive pain responses were required.17,18 After a patient had a positive response, an envelope marked with 1 or 0 (obtained randomly by the BCD; 1 indicated that the concentration of ropivacaine used for the next patient remained unchanged, and 0 indicated a decrease of 0.015%) was opened. The study was terminated when 44 envelopes had been opened, that is, a total of 45 patients had a positive response. If a patient withdrew, the concentration used for the next patient remained unchanged.
Statistical Analysis
Statistical analyses were performed using R for Windows (R ver. 4.0.3), and graphing was performed using GraphPad Prism 8. Continuous data were tested for normality using the Shapiro–Wilk test. Normally distributed data are presented as the means and standard deviations (mean ±SD), while skewed data are reported as the medians (M) and interquartile ranges (IQR). Count data are presented as numbers and percentages (%). The concentration set Cx = { X1, X2, . . . Xi, . . . XN} obtained in BCD II included N objects, and Xi was the ropivacaine concentration used for the i th patient. The concentration level set  included K objects, and
included K objects, and 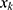 was the kth level of ropivacaine concentration. The positive rate set Px= {
was the kth level of ropivacaine concentration. The positive rate set Px= {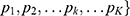 included K objects, and
included K objects, and 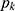 was the true positive rate at the concentration level
was the true positive rate at the concentration level 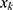 . Due to individual differences among patients, the positive rate did not increase monotonically with the ropivacaine concentration, which contradicted the quantal dose-response curve. Therefore, an adjusted positive rate set Px*=
. Due to individual differences among patients, the positive rate did not increase monotonically with the ropivacaine concentration, which contradicted the quantal dose-response curve. Therefore, an adjusted positive rate set Px*=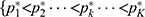 } was obtained by using the pooled-adjacent-violators algorithm (PAVA) for isotonic regression, and
} was obtained by using the pooled-adjacent-violators algorithm (PAVA) for isotonic regression, and 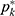 was the adjusted positive rate at
was the adjusted positive rate at 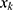 19. The PAVA has three estimators, and
19. The PAVA has three estimators, and 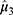 is more accurate than the other two.20 If
is more accurate than the other two.20 If 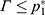 , then
, then 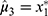 . If
. If 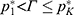 , then
, then 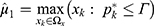 ,
, 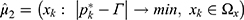 , and
, and 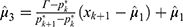 . If
. If 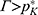 , then
, then 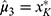 .
. 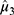 was calculated using the R program. Objects in
was calculated using the R program. Objects in 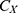 were sampled with replacement 2000 times (M=2000) to generate the bootstrap samples. The positive set
were sampled with replacement 2000 times (M=2000) to generate the bootstrap samples. The positive set 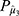 = {
= {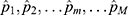 } was obtained by the isotonic regression of all bootstrap samples, and
} was obtained by the isotonic regression of all bootstrap samples, and 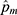 was the adjusted positive rate determined by isotonic regression of the mth bootstrap sample. The 95% confidence interval (CI) of
was the adjusted positive rate determined by isotonic regression of the mth bootstrap sample. The 95% confidence interval (CI) of 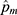 was obtained using the bias-corrected percentile method. The 95% CI of
was obtained using the bias-corrected percentile method. The 95% CI of 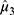 was calculated
was calculated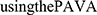 based on Px* and the 95% CI of
based on Px* and the 95% CI of 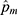 . All of these parameters were automatically computed using an R-script that was programmed by the investigator.
. All of these parameters were automatically computed using an R-script that was programmed by the investigator.
Results
A total of 60 patients were recruited, and two patients withdrew from the study because of nerve root contact (electrical sensation or reflex pain in the lower limbs) during epidural puncture. Fifty-eight patients were included in this study (Table 1), with an average age of 57 years and a female proportion of 43.1%. Anesthesia lasted for the duration of the surgery in all of the patients, and no anesthesia complications such as systemic local epidural hematoma, paraplegia and cauda equina syndrome occurred.
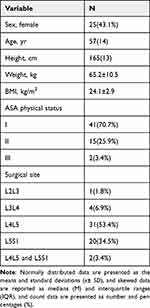 |
Table 1 Baseline Characteristics |
In BCD II, the concentration set C, composed of 55 objects (Figure 1), and the concentration level set Ω= {0.245%, 0.26%, 0.275%, 0.29%, 0.305%} were obtained. The true positive rates of the five concentration levels were calculated to generate the true positive rate set P= {50%, 66.7%, 0.75%, 86.4%, 100%}. Isotonic regression of P was used to generate the adjusted positive rate set P*= {50%, 66.7%, 0.75%, 86.4%, 100%}; however, P* could be obtained by P*=P without isotonic regression because the latter increased monotonically in this study. Nonetheless, the PAVA still needed to be determined as the bootstrap samples inevitably had some violations of true positive rates. The calculated 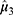 was 0.294%, which is the EC90 of ropivacaine for EA that could avoid nerve injury in PELD. The 95% CI of the positive rate
was 0.294%, which is the EC90 of ropivacaine for EA that could avoid nerve injury in PELD. The 95% CI of the positive rate 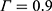 at the level of
at the level of 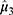 was 72.6% to 98.5% using the 2000 bootstrap samples, and the 95% CI of EC90 (
was 72.6% to 98.5% using the 2000 bootstrap samples, and the 95% CI of EC90 (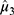 ) was 0.271% to 0.303% using the PAVA.
) was 0.271% to 0.303% using the PAVA.
Among the 45 positive-response patients, eight patients reported sudden soreness or radiating pain in the lower leg during the operation, and three patients reported sudden soreness in the lower back during the operation; these signs disappeared after the surgeon adjusted the position of the device. All patients could move the toes of both feet as instructed at the end of the surgery. At the follow-up 24 hours after the operation, one patient experienced motor weakness in the right lower limb that had subsided before the phone follow-up one week later, and the rest of the patients had motor weakness or paresthesia in the lower limbs (Table 2).
 |
Table 2 Positive Response, VAS Score, Unintended Contact and Nerve Injury at Different Concentration Levels |
Among the 13 patients with a negative response, the maximum VAS score was 7. The VAS score of these patients was less than 3 points after oxycodone was administered for rescue analgesia, and the maximum dose of oxycodone was 6 mg (Table 3). Moreover, three patients reported sudden pain in the lower leg during the operation, which disappeared after the surgeon adjusted the position of the device.
 |
Table 3 Average Oxycodone Dose in Patients with Different Pain Scores |
Discussions
Nerve injury is the most unwanted complication of PELD. It causes considerable pain to patients, reflecting the urgent need to avoid nerve injury. In this study, we believe there is an MEC of ropivacaine for EA that allows mild pain during the operation while preserving maximal tactile sensation of the nerve roots such that patients can provide timely feedback to the surgeon when a nerve root is contacted, thereby avoiding nerve injury. By isotonic regression, the EC90 of ropivacaine for EA was calculated to be 0.294% in patients with a VAS score ≤3.
Our study focused on preserving the tactile sensation of nerves under EA. This view of blocking pain and tactile sensation separately has never been reported previously. Previous studies have focused on the use of EA with low concentrations of ropivacaine to separate motor sensation such that nerve injury can be detected by observing the movement of patients’ toes during surgery. However, motor feedback often occurs after nerve injury; thus, separation of tactile sensation and pain may be a better choice. Blocking pain and tactile sensation separately is theoretically achievable. According to pharmacodynamics, as the concentration of a local anesthetic increases, its nerve blocking effect gradually increases, resulting in the weakening or even loss of pain sensation, tactile sensation and motor function. Sensory nerves can be divided into four types: Aα, Aβ, Aδ and C nerve fibers. Pain sensation is mainly transmitted through thin myelinated Aδ nerve fibers (2–6 μm) and unmyelinated C nerve fibers (0.3–3 μm). Tactile sensation is mainly transmitted through thick myelinated Aβ nerve fibers (6–12 μm). Different nerve fibers have different susceptibilities to local anesthetic blocks. Aδ nerve fibers are more susceptible than Aβ nerve fibers to local anesthetics.21 Therefore, pain sensation is often blocked with lower concentrations of LA than are needed to block tactile sensation.
It has been demonstrated that blocking the pain sensation without blocking the tactile sensation was achievable. In the preliminary study, we applied EA with 0.4% ropivacaine in six patients for PELD to confirm our conjecture. During the operation, when a nerve root was intentionally contacted by the surgeon, all six patients reported soreness in the lower leg, demonstrating that tactile sensation can be preserved with suitable concentrations of ropivacaine. In this study, the five concentrations of local anesthetics were all lower than 0.4%, and we therefore speculated that all patients had tactile sensation. Based on the nonmaleficence principle, the surgeon was not required to use an instrument to lightly touch a nerve root after incision to confirm whether all patients had tactile sensation, but 14 patients (24.1%) provided intraoperative feedbacks that indicated nerve root contact. Sudden swelling or pain in the lower limbs or lower back during the operation is regarded as a sign of nerve root contact, which can subside after changing the position of the device. We speculate that when the nervi nervorum surrounding the nerve sheath of the nerve root is contacted, the patient feels slight pain or soreness in the lower back, while a stronger touch or squeeze can stimulate nerve fibers in the nerve sheath, resulting in radiating pain or soreness in the innervated area.
Preserving tactile sensation effectively avoids nerve injury in PELD with low-concentration EA using ropivacaine. In this study, 14 patients showed signs of nerve root contact during the operation, and the signs disappeared after the surgeon adjusted the device in a timely manner. Only one patient had motor weakness the next day; the rest of the patients did not have motor weakness or paresthesia the next day. After receiving EA with 0.305% ropivacaine, the patient with postoperative motor weakness provided intraoperative feedback indicating soreness in the lower leg. The follow-up on the day after the operation showed that the patient’s right lower extremity muscle strength was grade 4, but it completely recovered by the time of the telephone follow-up one week later.
Finding a lower effective concentration; seems necessary because a low-concentration EA could not completely prevent nerve root injury. In previous studies, nerve injury occurred despite the concentrations of ropivacaine used in EA. In a study using 0.375% ropivacaine for EA,10 one patient (2.2%) had motor weakness, and three patients (6.7%) had lower extremity numbness, which was completely relieved at the last follow-up. Therefore, a patient’s tactile sensation should be preserved as much as possible to improve the patient’s sensitivity to nerve root contact, which helps reduce the incidence or severity of nerve root injury. As the concentration of ropivacaine decreases, pain becomes increasingly significant. Therefore, the concentration that allows tolerable mild pain is the MEC to obtain maximal tactile sensation.
Although MEC with a VAS score = 0 can be considered a better choice, we chose MEC at a VAS score ≤ 3 because different nerve fibers are not blocked sequentially and may be blocked simultaneously (Figure 2). Preserving sufficient tactile sensation while completely blocking pain is difficult. Patients are generally believed to be able to tolerate mild pain with VAS score ≤3. Therefore, the intraoperative local anesthetic concentration when the VAS score ≤3 is the lowest clinically acceptable concentration that preserves maximal tactile sensation. This concentration is the MEC that allows for mild pain while maximizing tactile sensation in PELD.
In this study, MEC was defined as the EC90. The MEC for each individual patient is often difficult to estimate due to individual differences; therefore, it is measured clinically using the quantal dose-response curve. Many studies on anesthetics define the median effective concentration (EC50) as the MEC. However, higher quantiles of effective concentrations, such as EC90 can better meet clinical requirements (Figure 3). Although the EC50 obtained by the up-and-down method (UMD) proposed by Dixon and Mood is acceptable, higher quantiles of concentrations obtained with this method have large bias. Therefore, statisticians recommend using the BCD to calculate higher quantiles of effective concentrations. The EC90 of ropivacaine that allows patients to have a VAS score ≤3 can be obtained through the BCD, which can be used to determine the MEC that allows tolerable mild pain while maximizing tactile sensation such that the patient can provide timely feedback to the surgeon when a nerve root is contacted, thereby maximally avoiding nerve root injury.
Our study showed that a ropivacaine concentration of 0.294% can result in an intraoperative VAS score ≤3 in 90% of patients, which can meet clinical needs. However, using the EC90 indicates that 10% of patients will have a VAS score >3. To solve this problem, anesthesiologists can use intravenous analgesics to enhance analgesia to further reduce or completely block pain in all patients. Among the 13 patients with negative responses, three patients (receiving ropivacaine concentrations of 0.2%, 0.215%, and 0.245%) required 6 mg of oxycodone for rescue analgesia, and the remaining patients required only 4 mg of oxycodone for rescue analgesia, suggesting that if EA with 0.294% ropivacaine combined with 4 mg of oxycodone is applied, more than 94.8% of the patients will have mild pain or less and that if EA with 0.294% ropivacaine combined with 6 mg of oxycodone is applied, 100% of the patients will have mild pain or less. In conclusion, 0.294% ropivacaine EA can preserve sufficient nerve tactile to prevent nerve injury during PELD. Although patients who received 0.294% EA had mild pain during the operation to maximize tactile sensation, the pain could be tolerated and even relieved with other analgesic drugs.
Limitations
Because whether the haptic efficacy obtained at the EC90 and a VAS score=0 is sufficient to allow patients to perceive nerve root contact is unclear, whether the maximum tactile sensation obtained at the EC90 and VAS score ≤3 is most beneficial to the patient is not certain. In this study, oxycodone was used to reduce the VAS scores of 13 patients with negative responses to below 4, which does not indicate that the pain score can be reduced to 0 within a reasonable range of oxycodone doses. Moreover, for patients with contraindications to intravenous drugs, the concentration of ropivacaine must be increased to obtain a satisfactory analgesic effect. However, increasing the concentration of ropivacaine also indicates that tactile sensation may be completely blocked. Therefore, the upper limit of the ropivacaine concentration that can retain sufficient tactile sensation must be further clarified.
Conclusions
This study demonstrated that patients undergoing PELD could preserve sufficient tactile sensation to avoid nerve injury using EA consisting of low-concentration ropivacaine. Tolerable mild pain was allowed, and the tactile sensation was maintained maximally at an EA concentration of 0.294%.
Data Sharing Statement
Data will not be shared because a further study based on the data will be subsequently carried out.
Acknowledgments
This research was supported by a grant from Projects of medical and health technology program of Zhejiang, China(WKJ-ZJ-2025).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Henmi T, Terai T, Hibino N, et al. Percutaneous endoscopic lumbar discectomy utilizing ventral epiduroscopic observation technique and foraminoplasty for transligamentous extruded nucleus pulposus: technical note. J Neurosurg Spine. 2016;24(2):275–280. doi:10.3171/2015.4.SPINE141305
2. Fan Y, Gu G, Fan G, et al. The effect of preoperative administration of morphine in alleviating intraoperative pain of percutaneous transforaminal endoscopic discectomy under local anesthesia A STROBE compliant study. Med. 2017;96(43):1–5. doi:10.1097/MD.0000000000008427
3. Gibson JNA, Cowie JG, Iprenburg M. Transforaminal endoscopic spinal surgery: the future “gold standard” for discectomy? - A review. Surgeon. 2012;10(5):290–296. doi:10.1016/j.surge.2012.05.001
4. Sairyo K, Egawa H, Matsuura T, et al. State of the art: transforaminal approach for percutaneous endoscopic lumbar discectomy under local anesthesia. J Med Investig. 2014;61(3–4). doi:10.2152/jmi.61.217
5. Zhu Y, Zhao Y, Fan G, et al. Comparison of the effects of local anesthesia and epidural anesthesia for percutaneous transforaminal endoscopic discectomy in elderly patients over 65 years old. Int J Surg. 2017;48:260–263. doi:10.1016/j.ijsu.2017.11.029
6. Wu K, Zhao Y, Feng Z, Hu X, Chen Z, Wang Y. Stepwise local anesthesia for percutaneous endoscopic interlaminar discectomy: technique strategy and clinical outcomes. World Neurosurg. 2020;134:e346–e352. doi:10.1016/j.wneu.2019.10.061
7. Ye XF, Wang S, Wu AM, et al. Comparison of the effects of general and local anesthesia in lumbar interlaminar endoscopic surgery. Ann Cardiothorac Surg. 2020;9(3):1103–1108. doi:10.21037/apm-20-623
8. Pan M, Li Q, Li S, et al. Percutaneous endoscopic lumbar discectomy: indications and complications. Pain Physician. 2020;23(1):49–56. doi:10.36076/ppj.2020/23/49
9. Ahn S, Kim S, Kim D, Ph D. Learning curve of percutaneous endoscopic lumbar discectomy based on the period (Early vs. Late) and technique (in-and-out vs. in-and-out-and-in): … - PubMed - NCBI. Korean Neurosurg Soc. 2019;58(6):539–546. doi:10.3340/jkns.2015.58.6.539
10. Ren Z, He S, Li J, et al. Comparison of the safety and effectiveness of percutaneous endoscopic lumbar discectomy for treating lumbar disc herniation under epidural anesthesia and general anesthesia. Neurospine. 2020;17(1):254–259. doi:10.14245/ns.1938366.183
11. Akakin A, Yilmaz B, Akay A, Sahin S, Eksi MS, Konya D. Epidural anesthesia in elective lumbar microdiscectomy surgery: is it safe and effective? Turk Neurosurg. 2015;25(1):117–120. doi:10.5137/1019-5149.JTN.11549-14.0
12. Zhou C, Zhang G, Panchal RR, et al. Unique complications of percutaneous endoscopic lumbar discectomy and percutaneous endoscopic interlaminar discectomy. Pain Physician. 2018;21(2):E105–E112. doi:10.36076/ppj.2018.2.e105
13. Choi I, Ahn JO, So WS, Lee SJ, Choi IJ, Kim H. Exiting root injury in transforaminal endoscopic discectomy: preoperative image considerations for safety. Eur Spine J. 2013;22(11):2481–2487. doi:10.1007/s00586-013-2849-7
14. Xu T, Tian R, Qiao P, Han Z, Shen Q, Jia Y. Application of continuous epidural anesthesia in transforaminal lumbar endoscopic surgery: a prospective randomized controlled trial. J Int Med Res. 2019;47(3):1146–1153. doi:10.1177/0300060518817218
15. Fang G, Ding Z, Song Z. Comparison of the effects of epidural anesthesia and local anesthesia in lumbar transforaminal endoscopic surgery. Pain Physician. 2016;19(7):E1001–E1004.
16. Stylianou M, Proschan M, Flournoy N. Estimating the probability of toxicity at the target dose following an up-and-down design. Stat Med. 2003;22(4):535–543. doi:10.1002/sim.1351
17. Tran DQH, Dugani S, Dyachenko A, Correa JA, Finlayson RJ. Minimum effective volume of lidocaine for ultrasound-guided infraclavicular block. Reg Anesth Pain Med. 2011;36(2):190–194. doi:10.1097/AAP.0b013e31820d4266
18. Durham SD, Flournoy N, Rosenberger WF. A random walk rule for Phase I clinical trials. Biometrics. 2006;53(2):745. doi:10.2307/2533975
19. Oron AP, Flournoy N. Centered isotonic regression: point and interval estimation for dose–response studies. Stat Biopharm Res. 2017;9(3):258–267. doi:10.1080/19466315.2017.1286256
20. Saranteas T, Finlayson R, Tran DQ. Dose-finding methodology for peripheral nerve blocks. Reg Anesth Pain Med. 2014;39(6):550–555. doi:10.1097/AAP.0000000000000157
21. Groopper MA, Cohen NH, Eriksson LI, Fleisher LA, Wiener-Kronish JP. Miller’s Anesthesia.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



