Back to Journals » Drug Design, Development and Therapy » Volume 16
Determination of the Dose-Response Relationship of Epidural Dexmedetomidine Combined with Ropivacaine for Labor Analgesia
Authors Ni JX, Feng JL, Yao SJ, Ni LF, Song SB, Song CZ, Qian XW, Mei Z , Yu J
Received 30 October 2021
Accepted for publication 19 February 2022
Published 6 March 2022 Volume 2022:16 Pages 609—618
DOI https://doi.org/10.2147/DDDT.S346842
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Jian-Xin Ni,1 Jia-Li Feng,2 Sheng-Jie Yao,1 Li-Feng Ni,1 Shao-Bo Song,1 Cong-Zhong Song,1 Xiao-Wei Qian,3 Zhong Mei,1,3,* Jing Yu1,*
1Department of Anesthesiology, Affiliated Xiaoshan Hospital, Hangzhou Normal University (Zhejiang Xiaoshan Hospital), Hangzhou, People’s Republic of China; 2Department of Obstetrics, Affiliated Xiaoshan Hospital, Hangzhou Normal University (Zhejiang Xiaoshan Hospital), Hangzhou, People’s Republic of China; 3Department of Anesthesiology, Women’s Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhong Mei; Jing Yu, Department of Anesthesiology, Affiliated Xiaoshan Hospital, Hangzhou Normal University (Zhejiang Xiaoshan Hospital), Yucai North Road 728, Xiaoshan, Hangzhou, 311202, People’s Republic of China, Tel +86-571-83865707, Email [email protected]; [email protected]
Background: The safety and efficacy of dexmedetomidine for epidural labor analgesia have been reported in numerous literatures, but the optimal dose has not been fully determined. The objective of this study was to determine the dose-response relationship of epidural dexmedetomidine (combined with ropivacaine) for labor analgesia.
Methods: A total of 120 full-term laboring parturients requesting epidural labor analgesia were enrolled in the study from July 5, 2020 to September 22, 2021. The parturients were randomly assigned to receive 0, 0.1, 0.2, 0.3, 0.4 or 0.5 μg/mL dexmedetomidine combined with 0.075% ropivacaine epidurally. An effective dose was defined as numerical rating scale (NRS) pain score ≤ 3 at 30-minutes of epidural drug injection. The dose-response relationship of dexmedetomidine (with ropivacaine) for epidural labor analgesia was performed using probit regression. The median effective dose (ED50) and the 95% effective dose (ED95) values for epidural dexmedetomidine combined with 0.075% ropivacaine with 95% confidence intervals (CIs) were derived by interpolation.
Results: The estimated values of ED50 and ED95 with 95% CIs for epidural dexmedetomidine (combined with 0.075% ropivacaine) were 0.085 (0.015 to 0.133) μg/mL and 0.357 (0.287 to 0.493) μg/mL, respectively. No differences were found among groups for sensory block level, number of parturients with Bromage score > 0, total dosage of analgesics, cesarean delivery rate, fetal birth weight, Apgar score at 1-minute, Apgar score at 5-minutes and adverse effects. Compared with other groups, group dexmedetomidine 0.5 μg/mL had a longer duration of the first stage of labor.
Conclusion: The ED50 and ED95 values of dexmedetomidine for epidural labor analgesia was 0.085 and 0.357 μg/mL under the conditions of this study. Dexmedetomidine is a suitable adjuvant for epidural labor analgesia.
Keywords: epidural, labor analgesia, dexmedetomidine, the dose-response relationship, ED50, ED95
Introduction
Epidural opioids added to local anesthetics are a commonly means of providing labor analgesia.1–3 Nonetheless, opioid-related adverse effects (eg, nausea, vomiting and pruritus) may lead to maternal intolerable.4,5 In contrast to opioids, dexmedetomidine has been used safely and effectively for epidural labor analgesia without increasing the incidence of adverse effects.6–12 Consequently, it can be recommended that dexmedetomidine is used as an epidural adjuvant instead of opioids in some clinical circumstances, especially for parturients with opioid intolerance. Previous studies have reported the appropriate doses of epidural dexmedetomidine used for labor analgesia, whereas the optimal dose is unknown.13,14 Before it is widely used in clinical practice, the epidural dose of dexmedetomidine should be fully determined.
The objective of this study was to determine the ED50 and ED95 of epidural dexmedetomidine for labor analgesia using probit regression.
Materials and Methods
Design and Study Subjects
This prospective, randomized and dose-finding study was approved by the Ethical Committee of Affiliated Xiaoshan Hospital, Hangzhou Normal University (Hangzhou, China) and was registered at the Chinese Clinical Trials (Registration number: ChiCTR2000034367). All parturients provided written informed consent.
120 full-term (37 ≥ weeks) laboring parturients requesting epidural labor analgesia were enrolled in the study from July 5, 2020 to September 22, 2021. Inclusion criteria were American Society of Anesthesiologists physical status (ASA) I or II, age 18 to 40 years, height 150 cm or greater, weight 100 kg or less, and baseline NRS pain scores > 3 (0 = no pain, 10 = worst pain). Exclusion criteria were as follows: allergy to amide-type local anesthetics or dexmedetomidine, bradycardia, multiple gestation pregnancies, pregnancy-induced hypertension or preeclampsia, known fetal abnormality and lack of informed consent.
Study Protocol
After arrival in the delivery room, each parturient was inserted an intravenous catheter. Subsequently, standard monitoring, including pulse oximetry, electrocardiography, non-invasive blood pressure and fetal heart rate was performed in each parturient.
A randomization code sequence was generated by an assistant who did not participate the following study using MedCalc 18.2.1 (MedCalc Software BV, Ostend, Belgium). The codes, were placed in numbered, opaque, sealed envelopes, randomly assigned parturients to one of six different doses of dexmedetomidine (Aibeining; Jiangsu Hengrui Co., Ltd.; 200 μg/ 2mL) with 0.075% ropivacaine (Naropin; AstraZeneca Co., Ltd.; 75 mg/ 10mL): 0, 0.1, 0.2, 0.3, 0.4 or 0.5 μg/mL dexmedetomidine.
A certified registered nurse anesthesia (CRNA) who was not involved in subsequent study performed the study solution preparation. Each dexmedetomidine dose, 75-mg ropivacaine and normal saline were mixed to a total volume of 100-mL injectate, then 10-mL loading dose was drawn into the identical 10-mL syringe and the rest 90-mL study solution was added into the infusion pump of patient controlled epidural analgesia (PCEA).
Parturients were placed in the left lateral position. After accomplishing aseptic precautions, skin and subcutaneous tissue was injected with 1% lidocaine. Epidural space was administered at the L2-3 vertebral interspace using an 18-gauge Tuohy needle and positioned using the loss-of-resistance-to-air technique. A reinforced epidural catheter was placed 3–4 cm into the epidural space and secured. The parturient was moved to the supine position, with left lateral uterine displacement. 10-mL study solution was then administered epidurally as a loading dose. The attending anesthesiologist who was blinded to the dose of the dexmedetomidine performed the epidural puncture and catheterization and injected the study solution.
The primary outcome was the effective rates of labor analgesia for different doses of dexmedetomidine with 0.075% ropivacaine at 30-minutes of epidural drug injection. An effective dose was defined as NRS pain score ≤ 3 at 30-minutes of epidural drug injection. Then the PCEA infusion pump was connected to maternal epidural catheter and switched on after 30-minutes of epidural drug injection. The background infusion rate was 3 mL/h, a bolus of 10-mL was administered when NRS pain score > 3 with a lockout interval of 20-minutes. An ineffective dose was defined as NRS pain score > 3 at 30-minutes of epidural drug injection. If pain relief was inadequate at 30-minutes of epidural drug injection, a 10-mL bolus of 1% lidocaine was administered, repeated at 15-minutes as required, and then the PCEA infusion pump was connected to epidural catheter.
NRS pain scores, non-invasive blood pressure, oxygen saturation, heart rate, and fetal heart rate was continuously monitored during this study. Sensory block (evaluated by pinprick), motor block (assessed using the Bromage scale), onset of analgesia (was defined as the duration from the end of the epidural drug injection to NRS pain scores ≤ 3), duration of stage of labor, total dosage of analgesics, fetal birth weight and Apgar scores, were logged. Adverse effects included nausea, vomiting, pruritus, bradycardia, maternal fever (was defined as a tympanic temperature of ≥ 38 °C), and respiratory depression (a decrease of SpO2 to < 95%) were recorded. Excessive sedation was measured using Ramsay Sedation Scale. Hypotension (a decrease of mean systolic blood pressure to < 90 mmHg or to ≤ 80% of baseline), was treated with intravenous fluids and 6 µg norepinephrine as required.
Statistical Analysis
Sample size was determined using PASS 11 (NSCC, LCC, Kaysville, UT) (the Cochran-Armitage test for trend in proportions) and based on our initial pre-experiment in which the proportions of parturients with effective labor analgesia were 0.4, 0.5, 0.7, 0.8, 0.9 and 0.99 in parturients who received epidural dexmedetomidine at doses of 0, 0.1, 0.2, 0.3, 0.4 and 0.5 μg/mL combined with 0.075% ropivacaine, respectively. 13 parturients were required per group (Power 1-Beta: 0.99, Alpha Significance Level: 0.05), and in order to improve power the number of subjects was increased to 20 per dose group.15
SPSS 25.0 (IBM Corp, Armonk, NY) was used for statistical analysis. The Shapiro–Wilk test was used to assess data normality. Normally distributed data were assessed using one-way analysis of variance (ANOVA) and least significant difference (LSD) post hoc test for pairwise comparisons was performed to evaluate significant differences within or among groups. Non-normally distributed data were calculated using the Kruskal–Wallis test and Dunn-Bonferroni post hoc test was conducted. Categorical data were analyzed by χ2 test or Fisher’s exact test. P < 0.05 was deemed to be statistically significant.
Probit regression was used to perform dose-response analysis. The primary endpoint was the effective rates of labor analgesia for different doses of dexmedetomidine with 0.075% ropivacaine at 30-minutes of epidural drug injection. An effective dose was defined as NRS pain score ≤ 3 at 30-minutes of epidural drug injection. ED50 and ED95 values for epidural dexmedetomidine combined with 0.075% ropivacaine with 95% CIs were derived by interpolation.
Results
Parturient recruitment is shown in Figure 1. 142 parturients were assessed for eligibility. Finally, a total of 120 parturients were randomized, allocated, received, and included in the data analysis. There were no statistically differences in cervical dilation before epidural drug injection, maternal age, height, weight, parity, proportion of nulliparous parturients and gestational age among groups (Table 1).
 |
Table 1 Demographic Data |
 |
Figure 1 CONSORT showing flow of laboring parturients. |
The dose-response relationship of dexmedetomidine with ropivacaine for epidural labor analgesia were performed using probit regression. The probit regression curve is shown in Figure 2. The estimated values of ED50 and ED95 with 95% CIs for epidural dexmedetomidine combined with 0.075% ropivacaine were 0.085 (0.015 to 0.133) μg/mL and 0.357 (0.287 to 0.493) μg/mL, respectively.
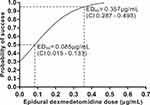 |
Figure 2 Dose-response curve for epidural dexmedetomidine (with 0.075% ropivacaine) for labor analgesia derived from probit analysis. |
According to our definition, there were 12, 11, 6, 2, 0 and 0 patients with inadequate analgesia in the 0-, 0.1-, 0.2-, 0.3-, 0.4- and 0.5-μg/mL groups (20 cases per group), respectively. Epidural labor analgesia was effective in 40, 45, 70, 90, 100 and 100% of the 0-, 0.1-, 0.2-, 0.3-, 0.4- and 0.5-μg/mL groups, respectively. No differences were found among groups for sensory block level, number of parturients with Bromage score > 0, total dosage of analgesics, cesarean delivery rate, fetal birth weight, Apgar score at 1-minute and Apgar score at 5-minutes. Compared with other groups, Group Dexmedetomidine 0.5 μg/mL had a longer duration of the first stage of labor (P < 0.05) (Table 2).
 |
Table 2 Labor Analgesia Characteristics and Neonatal Outcomes |
No statistical differences were noted for adverse effects including hypotension, nausea and vomiting, pruritus, bradycardia, maternal fever, respiratory depression, and excessive sedation (Table 3).
 |
Table 3 Adverse Effects |
Discussion
In this study, we have derived the dose-response curve for dexmedetomidine with ropivacaine given epidurally for labor analgesia. The values of ED50 and ED95 with 95% CIs for epidural dexmedetomidine with 0.075% ropivacaine for labor analgesia, in the setting of the conditions of the present study, were 0.085 (0.015 to 0.133) and 0.357 (0.287 to 0.493) μg/mL, respectively.
Previously, there is uncertainty regarding the optimal dose of epidural dexmedetomidine for labor analgesia, with doses ranging from 0.4 to 0.5 μg/mL published in the articles.13,14 One earlier study showed that when comparing four different doses (0.25, 0.5, 0.75, and 1 μg/mL), the optimal dose of epidural dexmedetomidine was 0.5 μg/mL. However, we found that 0.5 μg/mL dexmedetomidine for epidural labor analgesia will prolong the labor process.13 In another study, it has been reported that 0.4 μg/mL dexmedetomidine was the lowest concentration for optimal clinical efficacy in 5 different doses of dexmedetomidine (0, 0.3, 0.4, 0.5 and 0.6 μg/mL).14 It is obvious that their optimal dose of epidural dexmedetomidine is only the best dose between groups, not the best dose at the lowest concentration.
Our optimal dose (ED95) was lower than those reported in literatures of “minidose” epidural dexmedetomidine for labor analgesia.13,14 The ED95 of epidural dexmedetomidine for labor analgesia was 0.357 μg/mL. To the best of our knowledge, this study was the first to use probit regression to illuminate an analgesic dose-response relationship for epidural dexmedetomidine combined with ropivacaine for labor analgesia, and a more accurate ED95 value for epidural dexmedetomidine was calculated.
Our findings are consistent with previous studies showing dexmedetomidine can be safely and effectively used for epidural labor analgesia.6–12 Opioids are the most common epidural adjuvants for labor analgesia.1–3 However, opioids can lead to dose-related adverse effects such as nausea, vomiting and pruritus.4,5 Dexmedetomidine can effectively enhance epidural labor analgesia by taking effect on α-2 receptors in the spinal dorsal horn and decrease the incidence of opioid-related adverse effects.6,8,9,16 It has been reported that dexmedetomidine has better analgesic effect than sufentanil in the first labor process and analgesic effect.9,17 A low dose dexmedetomidine (≤ 0.5 μg/mL) resulted in dose-sparing effects of local anesthetic, which may decrease the incidence of motor block and assisted vaginal delivery.11,18 Our results also indicated that low-dose dexmedetomidine (≤ 0.5 μg/mL) was safe for epidural labor analgesia, as there were no significant differences in Apgar scores and adverse effects between the dexmedetomidine-free group and other groups.
In this study, ropivacaine was chosen for epidural labor analgesia because of its less neurotoxicity and cardiotoxicity. A number of animal experiments have confirmed that ropivacaine has less neurotoxicity and inhibitory effect on the heart than bupivacaine.19,20 In addition, numerous published studies found that ropivacaine was safe and effective in a variety of clinical settings during epidural labor analgesia.21,22
Various epidural adjuvants have been routinely added to local anesthetics when used for labor analgesia. First, lipophilic opioids are widely used for epidural labor analgesia, which have synergistic and additive analgesic effects with local anesthetics, leading to the dose-sparing effect of local anesthetics.1–3 Second, neostigmine has been administered epidurally with local anesthetics in the setting of labor analgesia. However, severe nausea and vomiting limit its use in obstetrics especially intrathecal administration.23,24 Third, clonidine is a lipophilic α-2 receptors agonist to provide analgesia for parturients with opioid intolerance. However, large doses of clonidine may cause adverse effects such as maternal bradycardia and excessive sedation, which limits its popularization and application in clinic.25–27 Fourth, pregabalin is also considered as epidural adjuvant for labor analgesia, showing that the regulation of emotional components of pain and visceral sensitivity is a new way for the treatment of labor pain.28
We also had some limitations. First, dexmedetomidine has not been consented by Food and Drug Administration (FDA) used as epidural adjuvant for labor analgesia, but numerous clinical studies have shown that epidural dexmedetomidine was safe and effective for labor analgesia. Second, we investigated the dose-response of dexmedetomidine combined with 0.075% ropivacaine, whereas the results may not be generalized to other concentrations of ropivacaine. Further trials are needed to explore this question. Third, we did not perform blood gas analysis on the umbilical cord because there was no blood gas analyzer in the delivery room. However, there were no significant differences in Apgar scores between the dexmedetomidine-free group and other groups, indicating there may be no significant differences in cord blood gas between groups. Forth, this was a single-center clinical trial, whereas the clinical promotion of dexmedetomidine needs a multi-center study.
Conclusion
In conclusion, under the conditions of this study, the ED50 and ED95 value of dexmedetomidine for epidural labor analgesia was 0.085 and 0.357 μg/mL. Dexmedetomidine is a suitable adjuvant for epidural labor analgesia.
Article Highlights
- The optimal dose of dexmedetomidine for epidural labor analgesia has not been fully determined.
- The dose-response relationship of dexmedetomidine for epidural labor analgesia was performed using probit regression.
- The ED50 and ED95 values of dexmedetomidine for epidural labor analgesia was 0.085 and 0.357 μg/mL under the conditions of this study.
- Dexmedetomidine is a suitable adjuvant for epidural labor analgesia.
Abbreviations
NRS pain scores, numerical rating scale pain scores; ED50, the median effective dose; ED95, the 95% effective dose; CIs, confidence intervals; ASA, American Society of Anesthesiologists; CRNA, certified registered nurse anesthesia; PCEA, patient controlled epidural analgesia; ANOVA, analysis of variance; FDA, Food and Drug Administration; CONSORT, Consolidated Standards of Reporting Trials.
Data Sharing Statement
The data supporting the study findings are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethical Committee of Affiliated Xiaoshan Hospital, Hangzhou Normal University (Hangzhou, China) and was registered at the Chinese Clinical Trials (Registration number: ChiCTR2000034367). All parturients provided written informed consent. We confirm our study complies with the Declaration of Helsinki.
Consent for Publication
All authors have read and approved the manuscript, and agree to submit to your journal.
Acknowledgments
All the authors thank staff of the maternity and Department of Anesthesia, Affiliated Xiaoshan Hospital, Hangzhou Normal University for their hard work and generous help. This study was supported by Science and technology plan project Xiaoshan District (2020212).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Lee AI, McCarthy RJ, Toledo P, Jones MJ, White N, Wong CA. Epidural Labor analgesia-fentanyl dose and breastfeeding success: a randomized clinical trial. Anesthesiology. 2017;127(4):614–624. doi:10.1097/ALN.0000000000001793
2. Väänänen A, Kuukasjärvi M, Tekay A, Ahonen J. Spinal and epidural sufentanil and fentanyl in early labour. Acta Anaesthesiol Scand. 2019;63(10):1413–1418. doi:10.1111/aas.13450
3. Vedagiri Sai R, Singh SI, Qasem F, et al. Onset of labour epidural analgesia with low-dose bupivacaine and different doses of fentanyl. Anaesthesia. 2017;72(11):1371–1378. doi:10.1111/anae.14000
4. Grangier L, Martinez de Tejada B, Savoldelli GL, Irion O, Haller G. Adverse side effects and route of administration of opioids in combined spinal-epidural analgesia for labour: a meta-analysis of randomised trials. Int J Obstet Anesth. 2020;41:83–103. doi:10.1016/j.ijoa.2019.09.004.
5. Wong CA, Scavone BM, Slavenas JP, Vidovich MI, Peaceman AM, Ganchiff JN. Efficacy and side effect profile of varying doses of intrathecal fentanyl added to bupivacaine for labor analgesia. Int J Obstet Anesth. 2004;13(1):19–24. doi:10.1016/S0959-289X(03)00106-7
6. Li G, Xiao Y, Qi X, et al. Combination of sufentanil, dexmedetomidine and ropivacaine to improve epidural labor analgesia effect: a randomized controlled trial. Exp Ther Med. 2020;20(1):454–460. doi:10.3892/etm.2020.8730
7. Sun S, Wang J, Bao N, Chen Y, Wang J. Comparison of dexmedetomidine and fentanyl as local anesthetic adjuvants in spinal anesthesia: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2017;11:3413–3424. doi:10.2147/DDDT.S146092
8. Li N, Hu L, Li C, Pan X, Tang Y. Effect of Epidural Dexmedetomidine as an Adjuvant to Local Anesthetics for Labor Analgesia: a Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med. 2021;2021:4886970. doi:10.1155/2021/4886970
9. Cheng Q, Bi X, Zhang W, Lu Y, Tian H. Dexmedetomidine versus sufentanil with high- or low-concentration ropivacaine for labor epidural analgesia: a randomized trial. J Obstet Gynaecol Res. 2019;45(11):2193–2201. doi:10.1111/jog.14104
10. Li G, Wang H, Qi X, Huang X, Li Y. Intrathecal dexmedetomidine improves epidural labor analgesia effects: a randomized controlled trial. J Int Med Res. 2021;49(4):300060521999534. doi:10.1177/0300060521999534
11. Zhang W, Li C. EC50 of Epidural Ropivacaine Combined With Dexmedetomidine for Labor Analgesia. Clin J Pain. 2018;34(10):950–953. doi:10.1097/AJP.0000000000000613
12. Li L, Yang Z, Zhang W. Epidural Dexmedetomidine for Prevention of Intrapartum Fever During Labor Analgesia: a Randomized Controlled Trial. Pain Ther. 2021;10(1):391–400. doi:10.1007/s40122-020-00215-y
13. Wangping Z, Ming R. Optimal Dose of Epidural Dexmedetomidine Added to Ropivacaine for Epidural Labor Analgesia: a Pilot Study. Evid Based Complement Alternat Med. 2017;2017:7924148. doi:10.1155/2017/7924148
14. Liu L, Drzymalski D, Xu W, Zhang W, Wang L, Xiao F. Dose dependent reduction in median effective concentration (EC(50)) of ropivacaine with adjuvant dexmedetomidine in labor epidural analgesia: an up-down sequential allocation study. J Clin Anesth. 2021;68:110115. doi:10.1016/j.jclinane.2020.110115
15. Mei Z, Ngan Kee WD, Sheng ZM, et al. Comparative dose-response study of hyperbaric ropivacaine for spinal anesthesia for cesarean delivery in singleton versus twin pregnancies. J Clin Anesth. 2020;67:110068. doi:10.1016/j.jclinane.2020.110068
16. Chen X, Cai M, Lei X, Jin Y. Obesity decreases the EC50 of epidural ropivacaine when combined with dexmedetomidine for labor analgesia. Expert Rev Clin Pharmacol. 2021;14(8):1051–1056. doi:10.1080/17512433.2021.1929924
17. Zhang T, Yu Y, Zhang W, Zhu J. Comparison of dexmedetomidine and sufentanil as adjuvants to local anesthetic for epidural labor analgesia: a randomized controlled trial. Drug Des Devel Ther. 2019;13:1171–1175. doi:10.2147/DDDT.S197431
18. Zhao Y, Xin Y, Liu Y, Yi X, Liu Y. Effect of Epidural Dexmedetomidine Combined With Ropivacaine in Labor Analgesia: a Randomized Double-Blinded Controlled Study. Clin J Pain. 2017;33(4):319–324. doi:10.1097/AJP.0000000000000411
19. Mio Y, Fukuda N, Kusakari Y, Amaki Y, Tanifuji Y, Kurihara S. Comparative effects of bupivacaine and ropivacaine on intracellular calcium transients and tension in ferret ventricular muscle. Anesthesiology. 2004;101(4):888–894. doi:10.1097/00000542-200410000-00013
20. Wang S, Lin Q, Wang Z, Pan X. Ropivacaine induces neurotoxicity by activating MAPK/p38 signal to upregulate Fas expression in neurogliocyte. Neurosci Lett. 2019;706:7–11. doi:10.1016/j.neulet.2019.04.048
21. Bolukbasi D, Sener EB, Sarihasan B, Kocamanoglu S, Tur A. Comparison of maternal and neonatal outcomes with epidural bupivacaine plus fentanyl and ropivacaine plus fentanyl for labor analgesia. Int J Obstet Anesth. 2005;14(4):288–293. doi:10.1016/j.ijoa.2005.04.007
22. Bawdane KD, Magar JS, Tendolkar BA. Double blind comparison of combination of 0.1% ropivacaine and fentanyl to combination of 0.1% bupivacaine and fentanyl for extradural analgesia in labour. J Anaesthesiol Clin Pharmacol. 2016;32(1):38–43. doi:10.4103/0970-9185.173350
23. Booth JL, Ross VH, Nelson KE, Harris L, Eisenach JC, Pan PH. Epidural Neostigmine versus Fentanyl to Decrease Bupivacaine Use in Patient-controlled Epidural Analgesia during Labor: a Randomized, Double-blind, Controlled Study. Anesthesiology. 2017;127(1):50–57. doi:10.1097/ALN.0000000000001669
24. Chaurasia M, Saxena AK, Chilkoti GT. Comparison of Epidural Butorphanol with Neostigmine and Epidural Sufentanyl with Neostigmine for First Stage of Labor Analgesia: a Randomized Controlled Trial. Anesth Essays Res. 2017;11(2):365–371. doi:10.4103/0259-1162.206271
25. Hemlata. Use of ropivacaine 0.2% with or without clonidine 1 μg/kg for epidural labor analgesia. J Anaesthesiol Clin Pharmacol. 2019;35(2):276. doi:10.4103/joacp.JOACP_122_18
26. Paech MJ, Pavy TJ, Orlikowski CE, Evans SF. Patient-controlled epidural analgesia in labor: the addition of clonidine to bupivacaine-fentanyl. Reg Anesth Pain Med. 2000;25(1):34–40. doi:10.1016/s1098-7339(00)80008-5
27. Kabi S, Verma R, Singh D, et al. A Comparison Between Dexmedetomidine and Clonidine as Adjuvants to Levobupivacaine in Labour Analgesia. Cureus. 2021;13(12):e20237. doi:10.7759/cureus.20237
28. Lavand’homme PM, Roelants F. Evaluation of pregabalin as an adjuvant to patient-controlled epidural analgesia during late termination of pregnancy. Anesthesiology. 2010;113(5):1186–1191. doi:10.1097/ALN.0b013e3181f5d32d
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
