Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Determinants of Pneumothorax Among Mechanically Ventilated COVID-19 Intensive Care Unit Patients, a Single Centre Study
Authors Hundie TG, Alemu ZA, Getachew LZ, Abera LA, Seyoum AB , Mogus LS, Admasu NM, Regassa GB , Tilahun YB, Bareamichael PI, Tessema AG, Derese TN
Received 24 September 2023
Accepted for publication 4 December 2023
Published 11 December 2023 Volume 2023:16 Pages 3977—3989
DOI https://doi.org/10.2147/JMDH.S441798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tsegaye Gebreyes Hundie,1,2,* Zewdie Aderaw Alemu,3 Lidiya Zenebe Getachew,4 Lidya Abebe Abera,4 Abebaw Bekele Seyoum,1 Lia Solomon Mogus,3 Nardos Mulu Admasu,5 Gadise Bekele Regassa,6 Yohannes Bayou Tilahun,7 Pineal Iyassu Bareamichael,8 Abel Girma Tessema,9 Tadios Niguss Derese1,*
1Department of Research and Training, Eka Kotebe General Hospital, Addis Ababa, Ethiopia; 2Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; 3Department of Public Health, Gamby Medical and Business College, Addis Ababa, Ethiopia; 4Department of Internal Medicine, Eka Kotebe General Hospital, Addis Ababa, Ethiopia; 5Department of Pediatric Surgery, Addis Ababa University, Addis Ababa, Ethiopia; 6Department of Epidemiology and Biostatistics, Addis Continental Institute of Public Health, Addis Ababa, Ethiopia; 7General Practitioner at HealthHub Specialty Clinics by Al-Futtaim, Dubai, United Arab Emirates; 8Department of Internal Medicine, Addis Ababa University, Addis Ababa, Ethiopia; 9Department of Immunology, University of Manchester, Manchester, UK
*These authors contributed equally to this work
Correspondence: Tsegaye Gebreyes Hundie, Email [email protected]
Introduction: Millions of deaths and co-morbidities have been brought on by the COVID-19 epidemic worldwide. Acute respiratory distress syndrome (ARDS), multiple organ failure, and death can result from the condition in some people. The disease’s course can range from a moderate upper respiratory tract infection to severe pneumonia. Numerous reports have been made on the occurrence of pneumothorax in COVID-19 ICU patients, particularly in those who are receiving invasive ventilation. This study assesses factors associated with pneumothorax among mechanically ventilated COVID-19 ICU patients in Addis Ababa, Ethiopia.
Methods: A case-control study design was employed from August 1, 2022, to August 31, 2022, GC, with a sample size of 281, where cases are 94 and controls are 187. A pre-tested structured quantitative tool was used to collect data on ODK and export it to SPSS version 26 for analysis. Descriptive statistics were presented using text and tables. The association between variables was analyzed with binary logistic regression. A statistical significance was declared at a p-value of 0.05 with a 95% confidence interval. Assumptions like model fitness and multicollinearity were checked to be satisfied.
Results: A total of 281 (94 cases and 187 controls) patient charts were carefully reviewed. After adjustment for possible confounders in multivariate analysis, ARDS (AOR = 0.214, 95% CI (0.088, 0.519), P value =0.001) and invasive ventilation (AOR = 0.311, 95% CI (0.121, 0.796), P value =0.015) had a significant association with pneumothorax.
Conclusion: Despite the introduction of preventive breathing methods, pneumothorax is still a frequent and deadly consequence in COVID-19 patients with ARDS. ARDS and invasive mechanical ventilation were found to be significantly associated with the development of pneumothorax. Health facilities should be well equipped with recent medical equipment in intensive care units and with well-trained and organized manpower.
Keywords: pneumothorax, mechanical ventilation, intensive care unit, Eka Kotebe General Hospital, COVID-19
Introduction
The SARS-CoV-2 virus, which can harm several human organs, is the origin of COVID-19. The respiratory system is the organ system that is affected the most frequently. The range of pulmonary complications includes pneumonia brought on by alveolar injury, acute respiratory distress syndrome (ARDS), and changes to the coagulation cascade that result in lung infarction via thrombi and emboli. Patients who are brought to the ICU after being diagnosed with COVID-19 pneumonia frequently have invasive mechanical ventilation, and in these situations, the mortality risk is substantial for patients with advanced disease. In critically ill patients, pneumothorax is a frequent side effect of invasive mechanical ventilation (IMV).1
Pneumothorax is a potentially fatal complication and a medical emergency and is described as the presence of air in the gap between the parietal and visceral pleura, with or without lung collapse. Pneumothoraxes can be categorized as spontaneous, which can be primary, secondary, or traumatic, depending on the origin. Diagnostic or therapeutic procedures, including central venous catheter implantation, thoracentesis, lung and/or pleural biopsy, barotrauma, and similar procedures, might result in traumatic pneumothorax.2–4
Pneumothorax occurs about 1% of the time in COVID-19 patients who need hospital admission and 2% of the time in ICU patients. IMV usage among COVID-19 hospitalized patients ranges from 17% to 42% overall, with non-survivors experiencing higher rates of usage (57% to 59%) compared to survivors (1–15%).2,3 Pneumothorax is more common in critically ill COVID-19 patients with ARDS. ARDS and pneumothorax together have led to a protracted hospital stay and a high fatality rate.3–5
The majority of COVID-19 patients have higher oxygen requirements, which raises the need for intensive care and invasive mechanical ventilation. This places a significant strain on healthcare systems. These patient populations have relatively high rates of pneumothorax.2,6,7
The occurrence of pneumothorax in COVID-19 ICU patients who were placed on invasive ventilation has been detailed in numerous papers.1,2 Advanced age, prior lung problems, disease stage, and comorbidities like hypertension, diabetes mellitus, and malignancy are additional risk factors.2,6
There is conflicting information regarding the link between pneumothorax and mortality in COVID-19 patients; previous studies reported it as a separate indicator of a bad prognosis, whereas newer research has tended to support the link between pneumothorax and high mortality. When IMV, septic shock, and tension physiology are present, the mortality and recovery rates for individuals who have pneumothorax are lower than for those who experience procedure-related pneumothorax.2,3,5
However, there is a scarcity of data on the complications associated with the use of invasive mechanical ventilation in the treatment of COVID-19. Previous research indicated that the prevalence of problems was rising internationally in critically ill patients and that clinical observations during the previous year in the ICU indicated a rise in the incidence of pneumothorax. The information gained on the magnitude and associated factors for the development of pneumothorax in COVID-19 patients will help clinicians and policymakers prevent the burden of the disease at some level.8,9 The study aimed to assess factors associated with pneumothorax among mechanically ventilated COVID-19 ICU patients at Eka Kotebe General Hospital, Addis Ababa, Ethiopia.
Methods
Study Area and Period
This study was conducted at Eka Kotebe General Hospital, the national COVID-19 treatment centre in Addis Ababa, Ethiopia. The date when data were accessed for research purposes was between August 1, and August 31, 2022, GC.
Study Design
A case-control study design was employed to conduct the study.
Study and Source Population
The source population for the cases was COVID-19 patients admitted to Eka Kotebe General Hospital ICU ward who were mechanically ventilated and diagnosed with pneumothorax.
The source population for the control group was COVID-19 patients admitted to Eka Kotebe General Hospital ICU ward who were mechanically ventilated and were not diagnosed with pneumothorax.
The study population for the cases was all selected COVID-19 patients who were admitted to Eka Kotebe General Hospital ICU Ward and who were on mechanical ventilation and diagnosed with pneumothorax during the study period.
The study population for the control group was all selected COVID-19 patients who were admitted to Eka Kotebe General Hospital ICU Ward and who were on mechanical ventilation and were not diagnosed with pneumothorax during the study period.
Inclusion and Exclusion Criteria
For cases, all medical records of COVID-19-confirmed ICU patients who were mechanically ventilated and diagnosed with pneumothorax were included, and patients with incomplete medical records were excluded from the study.
For controls, all medical records of COVID-19-confirmed ICU patients who were mechanically ventilated and were not diagnosed with pneumothorax were included, and patients with incomplete medical records were excluded from the study.
Sample Size Determination and Sampling Procedure
The sample size was calculated using Open Epi software based on factors associated with pneumothorax.3,5 Using an odds ratio of 2.20, 95% CI, 80% power, 35% of controls exposed, and the ratio of controls to cases 2, which yields a total sample size of 281 after adding 10% for chart loss.
A simple random sampling technique was used to select the study participants for both cases and controls. There were a total of 1200 mechanically ventilated (both invasive and non-invasive ventilation) patients admitted at Eka Kotebe General Hospital from March 2021 to April 2022. From this, a total of 281 mechanically ventilated patients were selected from the chart using a simple random sampling technique at Eka Kotebe General Hospital, of which 94 were cases and the rest 187 were controls.
Dependent Variable
- Pneumothorax
Independent Variables
- Demography: Age, Sex, Occupation, Residence
- Clinical conditions: Status of a patient at admission, Onset of dyspnea, Smoking history, History of chronic lung disease, Severity status and duration of ARDS, Need for tracheostomy, Duration of Invasive Mechanical ventilation
- Comorbidities: Asthma, COPD, Cardiovascular diseases, Diabetic Mellitus, Chronic kidney diseases, Malignancy, Pulmonary TB, HIV/AIDS
- Imaging and Lab Profile: Change in lung structure such as the presence of fibrosis, consolidation, emphysema-like change or pulmonary cyst, leukocyte count
- Change in MV reading: PEEP, FiO2, Peak pressure.
Data Collection Tools and Procedure
The data was collected by using a pretested, structured checklist that was adopted from reviewing different related literature.2,10,11 The checklist had five major sections: socio-demographic, comorbidities, clinical conditions, imaging and lab profiles, and changes in MV readings. A total of 281 mechanically ventilated patients’ charts were reviewed. The data was collected by an electronic data collection tool, ODK (Kobo Tool Box), by three general practitioners working in the ICU and supervised by a senior general practitioner working at the ICU from August 1, 2022, to August 31, 2022.
Data Management and Analysis
After training was given to data collectors and supervisors to ensure the validity and reliability of the data collection tool, a pre-test was done on 5% of the total sample size before the actual data collection, and the questionnaires were checked for clarity, understandability, and simplicity.
The principal investigator checked the collected data, and any incomplete documents were cleaned and checked for quality before being exported from the Kobo Toolbox to SPSS for analysis.
Descriptive analysis was done using simple frequencies and proportions, and the results were presented in tables, graphs, and words. A binary logistic regression model was used to assess the association between the independent variable and the outcome variables. Odds ratio, p-value, and 95% CI for odds ratio were used for testing significance and interpreting results. Variables with a p-value of =0.05 were considered to have statistical significance.
Ethical Consideration
An ethical clearance paper was obtained from the Eka Kotebe General Hospital institutional review board with a reference number of Eka/150/5/132. In each step, the data obtained from this study was kept confidential and secured not to be used for any other purpose except for this study, and patient consent to review their medical records was not required by the approving ethics committee. Additionally, authors did not have access to information that could identify individual participants during or after data collection. The study complies with the Declaration of Helsinki.
Result
Socio-Demographic Characteristics
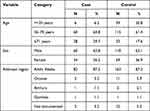
A total of 281 (94 cases and 187 controls) charts of admitted patients over the past two years in the ICU were carefully reviewed and data entered. The mean age of patients who were ventilated during the study period was 58.1 (SD = 16.7), where 94 years was the maximum and the minimum age recorded was 19 years. Sixty (63.8%) of the cases and 118 (63.1%) of the controls were males, while thirty-four (36.2%) of the cases and 69 (36.9%) of the controls were found to be females. Patients who came from the main city (Addis Ababa) constituted the largest portion (cases 82 (87.2%) and controls 163 (87.2%)); followed by the Oromia region (cases 5 (5.3%) and controls 11 (5.9%) (Table 1).
Presenting Symptoms, Comorbidity, and COVID-19 Severity Status
Cough was the dominant presenting symptom among admitted patients, contributing 92 (97.9%) for cases and 174 (93.0%) for controls, followed by shortness of breath, 85 (90.4%) for cases, and 166 (88.8%) for controls. The third and fourth presenting symptoms were fatigue and fever, which contributed 77 (81.9%) for cases, 149 (79.7%) for controls, and 72 (76.6%) for cases and 121 (64.7%) for controls, respectively. Loss of sense of smell and taste were more common among controls than cases (26.7%, 28.3%, 23.4%, and 22.3%, respectively). Controls were presented with symptoms of headache more commonly than cases (42.2%) and 40.4%, respectively. The onset of dyspnea or shortness of breath before hospital admission was more common among cases (92.6%) than controls (88.2%), while the onset after hospital admission was less common among cases (7.4%) than the controls (11.8%). On the other hand, COVID-19 severity status at admission was more likely to occur among cases (61.7%) than controls (60.4%), and patients with mild or moderate admission status were more common in the controls group (2.1%) than cases (1.1%). Patients with critical status during hospital admission had an almost equal occurrence rate among cases (37.2) and controls (37.3) (Table 2).
Vital Signs of the Study Participants During Admission
Normal pulse rate was more common among cases (67.0%) than controls (58.8%), while high pulse rate was recorded more commonly among controls than cases. A nearly equal number of patients with high respiratory rates were present among the cases (92.6%) and controls (95.7%) groups. The majority of patients maintain their oxygen saturation level above 89% among cases and controls during admission while they are on oxygen support. Patients who came with an oxygen requirement of 1–5 liters, 6–25 liters, and mechanical ventilation were more likely to develop pneumothorax as compared to the control group, whereas those who did not need any oxygen support during admission were more likely to belong to the control group (Table 3).
Comorbidity Status of the Respondents
Comorbid diseases were most likely to occur among both cases (69.1%) and controls (71.7%). Hypertension was more common among cases (38.3) than controls (33.7%), while diabetes mellitus was less common among patients with pneumothorax (27.7%) than patients with no pneumothorax (41.7%). Chronic obstructive pulmonary disease, malignancy, and pulmonary tuberculosis were the least common comorbid conditions among both cases and controls (Table 4).
ICU Admission Laboratory Profiles and Imaging
Most patients had a high WBC count when they were admitted to the ICU; the high count was more prevalent among cases (79.8%) than controls (77.5%). Patients who were categorized as cases were more likely to experience high BUN and creatinine counts during ICU admission than those who were”controls. Chest X-ray features of consolidation, GGO, and infiltration were more likely to happen among cases (Table 5).
Medication, Ventilation, and Tracheostomy
Almost all patients took antibiotics, steroids, and anticoagulants during their hospitalization. Remdesevir was found to be a rarely administered drug, but only 22.3% of cases and 25.7% of controls took it during their hospital stay. Most patients who were under control (82.9%) took noninvasive/CPAP ventilation compared to cases (74.5%) which resulted in a mean duration of 5.23 (SD = 3.90) days. Twenty and 100% were the maximum PEEP and FIO2 the patients were getting, respectively. On the other hand, nearly all patients were on invasive ventilation among cases (91.5%) compared to controls (61.5%). The maximum number of days a patient stayed on invasive ventilation was 36, while a day was the minimum period a patient was intubated (Table 6).
ARDS and Outcome
Acute respiratory distress syndrome (ARDS) was the dominant feature among cases that resulted in 87 (92.6%) patients experiencing the disease, while 60.4% of patients without pneumothorax developed the syndrome. Most of the patients who had pneumothorax were dead (94.3%) as compared to the control group (74.3%). However, patients who belonged to the control group had a higher chance of being discharged to their homes or transferred to another facility (Table 7).
 |
Table 7 ARDS and Outcome for Determinants of Pneumothorax Among Mechanically Ventilated COVID-19 Patients Who Were Admitted to the Eka Kotebe General Hospital ICU, Addis Ababa, Ethiopia, 2022 (n=287) |
Duration of Pneumothorax Diagnosed After Hospital Admission, Mechanical Ventilation, and Site of Pneumothorax the Patient Developed
Patients tended to develop pneumothorax on average 13.14 (SD = 7.6) days after hospital admission and 7.81 (SD = 6.13) days after invasive or noninvasive ventilation. 48.9% of patients developed pneumothorax in both lungs, followed by the right lung. Only 19 patients out of 94 were diagnosed with pneumothorax on the left side of their lungs.
Factors Associated with Pneumothorax
The following list of variables showed a significant association with pneumothorax in the bivariable analysis. Among socio-demographic factors included in the study, age <= 35 years, fever, cough, chest pain, oxygen requirement, DM, noninvasive ventilation, invasive ventilation, tracheostomy done for ARDS, and outcome (death) showed a significant association with pneumothorax in the bi-variable analysis.
After adjustment for possible confounders in multivariable binary logistic regression analysis, ARDS and invasive ventilation have a significant association with the outcome variable at 95% CI (p <0.05) (Table 8).
 |
Table 8 Logistic Regression Analysis on Determinants of Pneumothorax Among Mechanically Ventilated COVID-19 Patients at Eka Kotebe General Hospital ICU, Addis Ababa, Ethiopia, 2022 (n=281) |
Discussion
Pneumothorax has been a recognized complication among COVID-19 patients, with an increased incidence among mechanically ventilated patients.1,3,4,8,11 In our study, pneumothorax was observed in our patients on an average of 13 days after admission, while some studies reported pneumothorax in the post-COVID-19 phase.12,13 Pneumothorax develops more frequently on the right side, followed by the left, with a few having bilateral disease. This was not demonstrated in our study, where 48.9% had bilateral disease, followed by right-side disease, and lastly, left-side pneumothorax. Our study showed that 63.8% of the patients with pneumothorax were men, this could be explained by smoking status which is common in men, and this result agrees with findings in many papers.1,3,4,8,9,11,14,15 Cough (97.8%), shortness of breath (90.4%), fatigue (81.9%), and fever (76.6%) were the most common symptoms among this group. Laboratory parameters in these patients showed that they were more likely to have an elevated white count. A study from Spain, also demonstrated that COVID-19 patients developing spontaneous pneumothorax more frequently had an increased leukocyte count.2
Although 69.1% of the patients with pneumothorax had associated comorbidities, this did not reach statistical significance. Like the majority of the studies, the presence of comorbidities is not associated with an increased risk of developing pneumothorax, but some suggest patients with an underlying lung disease have an increased incidence.1,4,7,14–16 This study also found that those under the age of 35 had a lower incidence of the disease, unlike the conclusion made by other studies.
In this study, the odds of developing pneumothorax in patients who had not been on invasive ventilation were reduced by 69% compared to those patients who were on invasive ventilation. Similarly, a study conducted in the USA showed the overall incidence of pneumothorax in critically ill patients was 83/842 (10%), and in mechanically ventilated patients was 80/594 (13%). Retrospectively collected and analyzed medical records of COVID-19 patients complicated by pneumothorax in China showed a 56% prevalence rate of the disease in patients that require invasive mechanical ventilation as compared to other noninvasive ventilator support.7
The study also revealed that patients who have been diagnosed with ARDS have a 71% higher risk of developing pneumothorax than those who were not diagnosed with ARDS. A similar study from China showed that major factors contributing to the development of pneumothorax were the onset of dyspnea, chronic lung disease, smoking history, severity and duration of ARDS, and changes in lung structure during ARDS (fibrosis, consolidation, pulmonary cysts, and emphysema-like changes).7 This could be explained by the severity of COVID-19 infection with concomitant secondary bacterial infection that results in compromised lung function and features of acute respiratory distress syndrome, which leads to invasive or noninvasive ventilation requirements and the development of pneumothorax.
COVID-19 patients with pneumothorax are known to have a higher mortality rate compared to those without pneumothorax. The mortality rate has ranged from 47.2% to 86.3%.3,4,7,8,11,12,14–16 The association between in-hospital mortality and pneumothorax was also reported in another meta-analysis.17 This study found that 94.3% of COVID-19 patients with pneumothorax admitted to the ICU died, compared to 74.3% of those without pneumothorax. As a result, we can state that pneumothorax played a major role in deaths of cases compared to deaths of controls that had been invasively or noninvasively ventilated. Thus, it is unequivocally demonstrated that preventing pneumothorax and its associated complications in this specific patient group significantly enhances survival outcomes.
Conclusion and Recommendation
In this research, ARDS and invasive ventilation have been significantly associated with the development of pneumothorax. There is a high chance of developing pneumothorax when a patient is put on mechanical ventilation for respiratory support at the COVID-19 ICU and being diagnosed with acute respiratory distress syndrome may lead to the development of pneumothorax through the requirement of invasive or noninvasive ventilation.
Health facilities should be well equipped with recent medical equipment in the intensive care unit and with well-trained and organized manpower. All health professionals should follow their patients who are on invasive or noninvasive mechanical ventilation cautiously in the intensive care unit, high dependency unit, and other severe wards and look for any clinical signs and symptoms of ARDS, sepsis, or any respiratory disorders. Clinicians should look for any signs of pneumothorax, make a diagnosis, and treat it as early as possible.
There has to be further prospective research done to look for other variables, with further imaging and other COVID-19 treatment centres included.
Abbreviations
ARDS, acute respiratory distress syndrome; CLD, chronic lung disease; COPD, chronic obstructive pulmonary disease; FIO2, fraction of inspired oxygen; MERS, Middle East Respiratory Syndrome; PEEP, positive end-expiratory pressure; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus-2; SP, spontaneous pneumothorax.
Acknowledgments
We would like to thank Eka Kotebe General Hospital for giving the ethical clearance letter timely and we would like to pass our gratitude to GAMBY Medical and Business College, data collectors, and supervisors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Marciniak SJ, Farrell J, Rostron A, et al. COVID-19 pneumothorax in the UK: a prospective observational study using the ISARIC WHO clinical characterization protocol. Europ resp J. 2021;58:3.
2. Miró Ò, Llorens P, Jiménez S, et al. Frequency, risk factors, clinical characteristics, and outcomes of spontaneous pneumothorax in patients with coronavirus disease 2019: a case-control, emergency medicine-based multicenter study. Chest. 2021;159(3):1241–1255. doi:10.1016/j.chest.2020.11.013
3. Akram J, Yousaf Z, Alabbas Y, Almoyaaf MIA, Ibrahim ASS, Kharma N. Epidemiological and outcome analysis of COVID-19-associated pneumothorax: multicentre retrospective critical care experience from Qatar. BMJ open. 2022;12(2):e053398. doi:10.1136/bmjopen-2021-053398
4. Chong WH, Saha BK, Hu K, Chopra A. The incidence, clinical characteristics, and outcomes of pneumothorax in hospitalized COVID-19 patients: a systematic review. Heart Lung. 2021;50(5):599–608. doi:10.1016/j.hrtlng.2021.04.005
5. El-Nawawy AA, Al-Halawany AS, Antonios MA, Newegy RG. Prevalence and risk factors of pneumothorax among patients admitted to a pediatric intensive care unit. Indian J Crit Care Med. 2016;20(8):453–458. doi:10.4103/0972-5229.188191
6. McGraw-Hill Education. Harrison principle of internal medicine. McGraw-Hill Education; 2018.
7. Wang XH, Duan J, Han X, et al. High incidence and mortality of pneumothorax in critically Ill patients with COVID-19. Heart Lung. 2021;50(1):37–43. doi:10.1016/j.hrtlng.2020.10.002
8. Akboga SA, Gokce A, Hatipoglu M, et al. The relationship between mortality and inflammatory markers and the systemic immune inflammatory index in patients in the intensive care unit with a pneumothorax as a complication of COVID-19 disease. Ir J Med Sci. 2022;191(4):1931–1936. doi:10.1007/s11845-021-02740-x
9. Bobbio A, Dechartres A, Bouam S, et al. Epidemiology of spontaneous pneumothorax: gender-related differences. Thorax. 2015;70(7):653–658. doi:10.1136/thoraxjnl-2014-206577
10. Akdogan RE, Mohammed T, Syeda A, Jiwa N, Ibrahim O, Mutneja R. Pneumothorax in mechanically ventilated patients with COVID-19 infection. Case Rep Crit Care. 2021;2021:6657533. doi:10.1155/2021/6657533
11. Bonato M, Fraccaro A, Landini N, et al. Pneumothorax and/or pneumomediastinum worsens the prognosis of COVID-19 patients with severe acute respiratory failure: a multicenter retrospective case-control study in the North-East of Italy. J Clin Med. 2021;10(21):4835. doi:10.3390/jcm10214835
12. Chopra A, Al-Tarbsheh AH, Shah NJ, et al. Pneumothorax in critically ill patients with COVID-19 infection: incidence, clinical characteristics and outcomes in a case-control multicenter study. Respir Med. 2021;184:106464. doi:10.1016/j.rmed.2021.106464
13. Shah S, Pokhrel A, Chamlagain R, et al. Case report of a spontaneous pneumothorax after the recovery from COVID‐19 pneumonia: delayed complication. Clin Case Rep. 2021b;9(10). doi:10.1002/ccr3.4971
14. Geraci TC, Williams D, Chen S, et al. Incidence, management, and outcomes of patients with COVID-19 and pneumothorax. Ann Thorac Cardiovasc Surg. 2022;114(2):401–407. doi:10.1016/j.athoracsur.2021.07.097
15. Starshinova A, Guglielmetti L, Rzhepishevska O, Ekaterincheva O, Zinchenko Y, Kudlay D. Diagnostics and management of tuberculosis and COVID-19 in a patient with pneumothorax (clinical case). J Clin Tuberculosis Other Mycobacterial Dis. 2021;24:100259. doi:10.1016/j.jctube.2021.100259
16. Udwadia ZF, Toraskar KK, Pinto L, et al. Increased frequency of pneumothorax and pneumomediastinum in COVID-19 patients admitted in the ICU: a multicentre study from Mumbai, India. Clin Med. 2021;21(6):e615–e9. doi:10.7861/clinmed.2021-0220
17. Woo W, Kipkorir V, Marza AM, et al. Prognosis of spontaneous pneumothorax/pneumomediastinum in coronavirus disease 2019: the CoBiF score. J Clin Med. 2022;11(23):7132. doi:10.3390/jcm11237132
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.