Back to Journals » Patient Preference and Adherence » Volume 17
Determinants of Non-Adherence to Exercise or Physical Activity in People with Metabolic Syndrome: A Mixed Methods Review
Authors El Haddad L , Peiris CL , Taylor NF , McLean S
Received 23 July 2022
Accepted for publication 18 October 2022
Published 3 February 2023 Volume 2023:17 Pages 311—329
DOI https://doi.org/10.2147/PPA.S383482
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Laila El Haddad,1 Casey L Peiris,2 Nicholas F Taylor,2,3 Sionnadh McLean1
1Department of Allied Health Professions, Sheffield Hallam University, Sheffield, UK; 2School of Allied Health, Human Services and Sport, La Trobe University, Melbourne, Victoria, Australia; 3Allied Health Clinical Research Office, Eastern Health, Box Hill, Victoria, Australia
Correspondence: Sionnadh McLean, Collegiate Campus, Sheffield Hallam University, L108, 36 Collegiate Crescent, Sheffield, S10 2BP, UK, Tel +447342 092 340, Email [email protected]
Background: Long-term adherence to exercise or physical activity (EPA) is necessary for effective first-line management of metabolic syndrome (MetS). Little is known about the determinants of adherence in this population. This systematic review aims to identify the determinants of adherence to EPA in people with MetS.
Methods: Six databases (MEDLINE, CINAHL Complete, PubMed, PsycINFO, SPORTDiscus, and Cochrane Central Register of Controlled Trials (CENTRAL)) were searched for studies published before April 26, 2021. Primary research studies investigating factors affecting EPA adherence in adults with MetS in outpatient settings were included. Risk of bias was assessed using the QUIPS (Quality in Prognostic Factor Studies) and CASP (Critical Appraisal Skills Program) tools, for quantitative and qualitative methodologies, respectively.
Results: Four quantitative studies (n = 766) and one qualitative (n = 21) study were included in the review, evaluating 34 determinants of adherence to EPA in MetS. Limited evidence was found for an association between ten determinants and non-adherence to EPA: lower self-rated health, lower baseline EPA, lower high-density lipoprotein cholesterol (HDL-C), fewer walk-friendly routes within 1 km, less consciousness raising, lower self-re-evaluation, lower self-liberation, reporting more arguments against EPA (cons), lower social support, and fewer positive psychological constructs. There was limited evidence of no association or conflicting evidence for the remaining 24 determinants.
Conclusion: A small number of included studies, most of low methodological quality, resulted in limited confidence in the findings for all determinants. The identified determinants associated with non-adherence are all potentially modifiable, thus further high-quality studies are required to increase confidence in the determinants of EPA in people with MetS, and test interventions.
Keywords: adherence, long-term condition, behavior change
Introduction
Metabolic Syndrome (MetS) is characterized by a cluster of five risk factors; raised triglycerides, lowered high-density lipoprotein cholesterol (HDL-C), abdominal obesity, hypertension, and impaired glucose tolerance.1 According to the International Diabetes Foundation (IDF), abdominal obesity plus any two risk factors constitute MetS, while the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria define MetS as any three of the five risk factors.2 The global prevalence of MetS is increasing as a likely consequence of rising levels of obesity and sedentary lifestyles,1 with an estimated 25% of adults affected worldwide. MetS results in a doubling of the risk of atherosclerotic cardiovascular disease and a five-fold increase in the risk of type 2 diabetes mellitus.2,3
Lifestyle modification is the first-line management strategy for MetS, with pharmacology used as a second-line intervention or to supplement lifestyle changes.4,5 Physical inactivity, obesity, and an atherogenic diet are targeted with exercise and a reduced calorie and saturated and trans-fat diet, using principles of behavior change.2 Exercise has acknowledged benefits on cardiovascular health,6 and on MetS parameters. Both resistance and endurance training can reduce blood pressure in MetS populations,7,8 while moderate-intensity aerobic exercise may improve HDL-C, triglyceride levels, and glucose tolerance/insulin sensitivity, in people with MetS.9,10 A systematic review of supervised exercise coupled with dietary interventions found they significantly reduced waist circumference, blood pressure, fasting blood glucose, and triglycerides in adults with MetS.11 However, a systematic review of unsupervised exercise coupled with dietary intervention found smaller reductions in waist circumference and blood pressure and no significant improvements in other components of MetS.12
Poor adherence is one possible explanation for the difference in effect of supervised versus unsupervised exercise.12 In studies investigating exercise alone in MetS populations, there is a consistent lack of reporting of adherence to exercise interventions.9,10 However, poor adherence to exercise recommendations is a common problem in many health conditions,13–16 which may diminish the effectiveness of EPA interventions. It seems likely that improvements in MetS are only achieved and maintained if lifestyle modifications such as exercise are maintained in the long term.17 Adherence is defined by the World Health Organization as the extent to which a person’s behavior corresponds with agreed recommendations from a health care professional.18 Increased adherence to EPA recommendations is associated with better outcomes in chronic conditions including those with musculoskeletal conditions and MetS.17,19 However, adherence is reported to be low in populations with chronic conditions.20,21 Bullard et al21 found that across cancer, cardiovascular disease and type 2 diabetes mellitus populations, the average adherence rate to prescribed exercise was 77%. In another study, 90% of individuals at risk of MetS were non-adherent to lifestyle changes,20 highlighting the need to consider adherence in this population.
To identify individuals at risk of non-adherence and design appropriate interventions to target this, we should first be able to identify the determinants of adherence.14 A mixed methods review investigating determinants of adherence to EPA in patients with musculoskeletal disorders identified strong or moderate evidence for 38 determinants under seven overarching themes: individual internal characteristics; individual’s knowledge and experience of health problem; social influences; therapeutic relationship; characteristics of exercise program; support for exercise, and effect of exercise programme.22 Sociodemographic factors and socioeconomic status (SES) may also affect exercise adherence during cancer treatment23 and among older people.24 For people with obesity, healthier eating and physical activity behaviors, higher initial weight loss, and older age predict greater adherence to lifestyle interventions.13,14 For the MetS population, who have high rates of obesity and sedentary behavior,25 the determinants of adherence to EPA recommendations are likely to be equally multi-dimensional and therefore complex to manage.
To the best of our knowledge, no previous systematic review has investigated the determinants of adherence to EPA in people with MetS. The aim of this review is to identify the determinants of adherence to home, outpatient, or community-based EPA in adults with MetS. Understanding determinants will help healthcare professionals identify individuals at risk of non-adherence and thus those most likely to benefit from targeted behavior change interventions, with the goal of improving health outcomes in this population.
Methods
This mixed methods systematic review was designed in accordance with the Cochrane Prognosis Methods Group guidelines,26 the Cochrane Qualitative and Implementation Methods Group (CQIMG) Guidance Series27 and PRISMA reporting guidelines recommended for use in mixed-method reviews.28 The inclusion of a qualitative evidence synthesis can provide insight into how the environments in which people live and experience healthcare, and their attitudes and beliefs, may impact their behavior.29 This is of particular importance when investigating adherence to EPA, a concept with the behavior of individuals at its core.
Data Sources and Search Strategy
Six electronic databases, MEDLINE, CINAHL Complete, PubMed, PsycINFO, SPORTDiscus, and Cochrane Central Register of Controlled Trials (CENTRAL), were searched from their inception to April 26, 2021. Search terms synonymous with determinants, exercise/physical activity, adherence, and metabolic syndrome, along with MeSH terms, were used to search for relevant studies (Supplementary Table 1).
Eligibility Criteria
We included studies that (1) were peer-reviewed primary quantitative, qualitative or mixed methods studies published in the English language; (2) investigated adult populations with MetS as defined by either the IDF or NCEP-ATP III criteria or by other closely aligned criteria, i.e. presence of 3 out of the 5 risk factors associated with MetS: impaired glucose tolerance, hypertension, raised triglycerides, low high-density lipoprotein, and abdominal obesity ; (3) investigated factors that affect adherence, related to participants (eg, age, gender, self-efficacy), healthcare providers (eg, aspects of therapeutic relationship such as communication), or healthcare organizations (eg, facilities, reputation); (4) investigated programs of EPA delivered for therapeutic benefit, by a healthcare professional, trained lay representative, or as part of a multi-disciplinary package of management, performed in any outpatient setting; and (5) investigated adherence to EPA or physical activity levels.
We excluded studies if they investigated determinants of adherence to diet and/or medication only, if adherence to diet and exercise was reported as combined data, or if adherence was measured by attendance to appointments or clinics only.
Screening
Covidence, a web-based software program for systematic review management (covidence.org), was used to import references from searches, remove duplicates, complete the screening process, and generate the PRISMA flowchart of study selection. Title and abstracts were independently screened by two authors, with LE reviewing all entries and the remaining three authors reviewing a third each. The same process was followed for screening of full-text articles remaining for review. Conflicts were resolved through discussion between all four reviewers. Agreement between reviewers was assessed using Cohen’s kappa statistic. A kappa value between 0.00 and 0.20 represents slight agreement, 0.21–0.40 represents fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement, and 0.81–1.00 almost perfect agreement.30 Reference lists of included full-text articles were screened for additional relevant studies.
Assessment of Study Quality
The methodological quality of quantitative studies was assessed using the QUIPS (Quality In Prognostic factor Studies) checklist, which assesses risk of bias over six domains: study participation, study attrition, prognostic factor management, outcome measurement, study confounding, statistical analysis, and reporting.31 Each domain was rated as having “high”, “moderate”, or “low” risk of bias based on responses to a list of prompts, then an overall risk of bias was determined based on all domains.31
Methodological quality of qualitative studies was assessed using the modified Critical Appraisal Skills Program.32 This tool consists of 10 items which assess three aspects of the study: quality of reporting of the rationale, aims and context of the study; rigor of the methods used; and credibility of the methods used. Studies were assessed for the fulfilment of each criterion (‘yes’, “no” or “unclear”), and a judgement was made on the overall assessment of limitations, as calculating total quality scores is not recommended.27 Methodological quality assessment was conducted independently by two reviewers (LE and NT) and consensus was reached through discussion. Agreement between the two reviewers was assessed using Cohen’s kappa statistic.
Data Extraction
The CHARMS-PF checklist (checklist for critical appraisal and data extraction for systematic reviews of prediction modelling studies-prognostic factors) was used to guide extraction of key data items across 11 domains from each quantitative study included in the review.26 Data extraction from qualitative studies was completed based on a template designed by the National Institute for Health and Care and Excellence (NICE),33 recommended by the CQIMG.27 Information was extracted about themes that were evaluated by included studies. This process was completed by one reviewer (LE) and checked for accuracy by a second reviewer (CP), with full extraction tables available (Supplementary Tables 2 and 3).
Certainty of Evidence
Assessing the certainty of evidence from quantitative studies was completed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for prognostic factor studies.34 Six domains were taken into consideration: phase of investigation, study limitations, inconsistency, indirectness, imprecision, and publication bias, which determined if the certainty rating was downgraded.34 An initial rating of “moderate” was given to each determinant, as studies were Phase I explanatory studies. Where the majority (≥75%) of studies evaluating a determinant were of low methodological quality, the rating was downgraded.22 If only one study evaluated a determinant, it was not possible to assess inconsistency, thus the score was downgraded. Imprecision is difficult to evaluate in the absence of a meta-analysis, but the presence of rationale for sample sizes and sample sizes themselves were considered.34 Huguet et al34 recommend downgrading for publication bias unless a determinant has been investigated in a large number of cohort studies, thus all determinants were downgraded for publication bias. Moderate or large effect sizes, and an exposure-response gradient were reasons for upgrading. This process was conducted by one reviewer (LE) and checked by a second reviewer (SMc).
Assessment of certainty of evidence from qualitative studies was done using the GRADE-CERQual (Confidence in the Evidence from Reviews of Qualitative Research) method.27 This approach evaluates four domains: methodological limitations, coherence, adequacy of data, and relevance. An initial rating of “very high” was given to each determinant, as advised by the protocol.35 Both approaches produce a certainty rating of high, moderate, low, or very low.34,35
Data Synthesis
A triangulation protocol described by Farmer et al36 was used to synthesise data from quantitative and qualitative studies and to evaluate the extent of alignment or non-alignment between determinants of EPA adherence. It followed four stages as described below. Triangulation was completed by one reviewer (LE) with a second reviewer (CP) involved for cross-checking, to increase reliability.
- Sorting. Findings from each data set (quantitative and qualitative) were sorted into categories based on identified determinants or themes.
- Certainty of evidence for qualitative and quantitative data sets. This information was obtained through the GRADE and GRADE-CERQual protocols.
- Convergence coding. Determinants or themes from each data set were compared to assess the degree of convergence, using the coding scheme in Table 1.
- Evaluating overall strength of evidence. Assessing overall certainty of evidence for the combined qualitative and quantitative data sets for each determinant based on risk of bias, quantity of studies, consistency of findings across the studies, and impact, using pre-determined criteria according to the Dietary Guidelines Advisory Committee in Table 2.37 All determinants were evaluated in relation to non-adherence.
 |
Table 1 Convergence Coding scheme |
 |
Table 2 Criteria for Assessing Overall Strength of evidence |
Results
Study Selection
Database and reference list searches yielded 1952 results. Following removal of duplicates, 1272 titles and abstracts were screened. Of these, 1243 were excluded and the remaining 29 full-text articles were assessed for eligibility. Five studies met the inclusion criteria, one qualitative and four quantitative studies, and were included in the review (Figure 1). There was moderate agreement between reviewers when evaluating titles and abstracts (kappa = 0.41, 95% CI 0.28 to 0.56) and substantial agreement between reviewers when evaluating full-text articles for inclusion (kappa = 0.70, 95% CI 0.38 to 1).
 |
Figure 1 PRISMA flow chart of screening and study selection. Notes: Adapted from Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;(372):n160.28 |
Quality Assessment
QUIPS assessment found that three quantitative studies had a high risk of bias, and the remaining quantitative study had a low risk of bias (Figure 2). Each of the three studies with a high risk of bias scored “high” for the domain of study attrition, due to inadequate response rates,38 inadequate reasons provided for participant drop-out,39 reported differences in participant characteristics between those who completed the study and those who did not,38 or a lack of reporting on potential differences between those who completed the study and those who did not.39,40 In assessing methodological quality, agreement between the two reviewers was fair (kappa = 0.29, 95% CI −0.07 to 0.65), with independent ratings and final consensus by domain recorded.
 |
Figure 2 Quality assessment of quantitative studies. |
The qualitative study43 adequately described nine out of ten items in the CASP tool, but the relationship between researcher and participants was deemed to have not been adequately considered (Figure 3). In line with use of the CASP tool in a Cochrane mixed methods systematic review,41 an answer of “no” to any of the ten items in the tool is interpreted as the study having major limitations. Agreement between the two reviewers was perfect.
 |
Figure 3 Quality assessment of qualitative studies. |
Study Characteristics
The five included studies evaluated determinants of adherence in 787 adults diagnosed with MetS. Two of the quantitative studies were cross-sectional studies, and two were longitudinal studies (Table 3). The qualitative study used general qualitative methods with directed content analysis and inductive and deductive coding methods (Table 4).
 |
Table 3 Summary of Quantitative Results |
 |
Table 4 Summary of Qualitative Results |
The four quantitative studies used self-report measures to record either adherence to EPA recommendations or overall physical activity levels, and one study39 also measured physical activity using an accelerometer (Table 3).
Determinants of Adherence to EPA
During the triangulation process, 34 determinants were identified and evaluated for their relationship with adherence to EPA. The adapted GRADE approach, as described by Huguet et al,34 was used to evaluate strength of the quantitative evidence for each determinant (Supplementary Table 4). As only Kim et al42 was of high methodological quality, most determinants were downgraded. Twenty-nine of 34 determinants were only evaluated in one quantitative study, therefore these were downgraded for inconsistency. Where there was an absence of rationale for sample sizes,38–40 sample sizes were considered adequate; thus, no determinants were downgraded for imprecision.34 No determinants were upgraded as there was no evidence of moderate or large effect sizes or exposure-response gradients.
The GRADE-CERQual approach was applied to evaluate the strength of the qualitative evidence (Supplementary Table 5).35 As the only qualitative study43 involved had major methodological limitations, there were serious concerns in the methodological limitations and adequacy domains.44,45 There were no concerns in the domain of coherence, with clear and cogent study findings, and minor concerns in the relevance domain due to very limited ethnic diversity in the study population.46,47 Given the serious concerns in two out of four domains, and minor concerns in another, the confidence in the findings was rated as “low” for each of the 6 determinants identified in this study.
Table 5 shows the summary of the triangulation process with quantitative and qualitative results combined, convergence level, and the overall certainty of evidence (strong, moderate, limited, unassignable) for each determinant. The full breakdown of results from all quantitative and qualitative studies can be found in Supplementary Table 6. Determinants were categorised into five themes: Demographic Characteristics, General Health Parameters, MetS-specific Characteristics, Geographical Factors, and Psychosocial Factors.
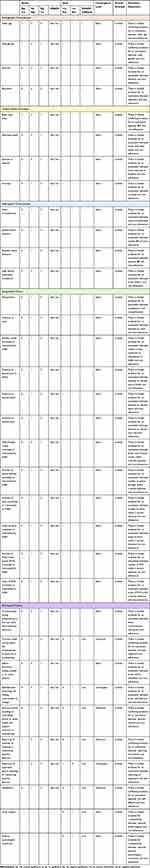 |
Table 5 Summary of Triangulation for Determinants of Adherence to PA |
Demographic Characteristics
There is limited conflicting evidence that older age and male gender are associated with lower adherence to EPA. Neither ethnicity nor educational level is associated with non-adherence (limited evidence).
General Health Parameters
Lower self-rated health and lower baseline EPA are associated with lower adherence (limited evidence). There is limited but conflicting evidence that body mass index is associated with lower adherence to EPA.
MetS-Specific Characteristics
There is limited evidence for an association between lower HDL-C and non-adherence to EPA. No association was found between waist circumference, systolic or diastolic blood pressure and non-adherence (limited evidence).
Geographical Factors
There is limited evidence for an association between fewer walk-friendly routes within a 1 km distance and non-adherence to EPA. No associations were found between the other 11 geographical factors and non-adherence (Table 5) (limited evidence).
Psychosocial Factors
There is limited evidence for an association between the following psychosocial factors and non-adherence to EPA: lower consciousness raising (reduced undertaking to find out more about exercise behavior), lower self-re-evaluation (seeing oneself as a less active person), lower self-liberation (lesser ability to choose and make a commitment to change), reporting more arguments against adopting or maintaining exercise behavior, lower social support, and lower positive psychological constructs.
There is limited conflicting evidence for an association between the following psychosocial factors and non-adherence to EPA: dramatic relief (caring about the consequences of inactivity or non-exercise), stimulus control (avoiding or controlling stimuli or other causes that support inactivity or non-exercise), reporting more positives of adopting or maintaining exercise behavior, and self-efficacy.
Discussion
To the best of our knowledge, this systematic review is the first to investigate the determinants of adherence to EPA in people with MetS. Based on one high quality and four low quality studies, this review identified 34 potential determinants and found limited evidence of an association between ten determinants and non-adherence to EPA: lower self-rated health, lower baseline EPA, lower high-density lipoprotein cholesterol (HDL-C), fewer walk-friendly routes within 1km, lower consciousness raising, lower self-re-evaluation, lower self-liberation, reporting more arguments against EPA, lower social support, and fewer positive psychological constructs. There was limited evidence of no association or conflicting evidence for the remaining 24 determinants, potentially due to the lack of high-quality studies available for this review.
The existing literature supports our finding that lower self-rated health is associated with non-adherence to EPA. Studies in populations at risk of MetS and a systematic review in populations of older adults found associations, or results to suggest associations, between better self-rated health and greater adherence to EPA.20,24,48 Self-rated health is an individual’s perception of their biological, psychological, and social health. Some evidence suggests a link between low self-rated health and psychological distress.49 Evidence in MetS populations and in older people suggests that those who experience psychological distress show reduced adherence to healthy behaviors, including physical activity.24,50 Therefore, those with greater self-rated health may suffer less psychological distress, which may contribute to better adherence to EPA in these groups.
Our finding that higher baseline physical activity is associated with greater adherence to EPA is reflected by studies in those at risk of MetS, in people with type 2 diabetes mellitus, and in people with cancer.23,51–53 Considering the transtheoretical model of behavior change, individuals with higher baseline physical activity are in the “action” or “maintenance” stages of change. Here, individuals may perceive more benefits of physical activity than costs.42 Enjoyment of exercise and previous positive adherence behavior are positively associated with adherence in musculoskeletal populations.22 Compared to those who enjoy exercise, those who do not may lack intrinsic motivation and therefore have no intention to initiate or continue EPA.54 Enjoyment has been shown to predict physical activity more than cognitive intentions to engage in physical activity.55
We found conflicting evidence for an association between self-efficacy and adherence in people with MetS. However, existing evidence points towards an association between greater self-efficacy and better adherence in those at risk of MetS,48,56 in type 2 diabetes mellitus populations,52,57 in people with Parkinson’s disease,58 people with cancer,23 and in older people.59 However, Susin et al53 found no association between self-efficacy and adherence to a diet and exercise intervention for people with MetS, although the data for diet and exercise was combined. Although Kim et al42 did not find self-efficacy to be a significant predictor of exercise behavior in people with MetS, levels of self-efficacy did increase as individuals progressed through stages of change, equating to increasing levels of exercise behavior. Self-efficacy is an individual’s confidence in their ability to achieve a set goal,60 and is the central construct of Bandura’s social cognitive theory, which hypothesises that health behaviors are driven by personal, behavioral, and environmental factors.52 Self-efficacy is thought to increase as individuals progress through an exercise or physical activity intervention.42 EPA adherence requires skills related to self-regulation, such as goal setting, self-monitoring, time management, and relapse prevention.59
Social and spousal support were identified as facilitators to physical activity in this review. Existing qualitative research supports this finding, with social support, often including healthcare professionals, regular follow-ups, and exercising in groups, required for EPA adherence in populations similar to MetS,61–63 in sedentary women,64 and in people with cancer.23 Social support, therapeutic support, and regular follow-ups are associated with greater exercise adherence in musculoskeletal populations,22 and supervised exercise appears more effective than unsupervised exercise to improve metabolic risk factors in people with MetS.12 Social support is thought to enhance EPA adherence by increasing self-efficacy, or by increasing positive emotions experienced while participating in physical activity, leading to enjoyment.60 Group exercise is often cited as a preference by participants.61–63 This could be driven by the opportunity to share experiences with other people going through similar health and behavior challenges.22 Providing, as well as receiving, social support may have beneficial effects on health,65 another possible driver of the desire for group exercise. Out of four effective interventions for increasing EPA adherence in older adults, three involved some form of feedback and/or monitoring from programme leaders.66 Regular follow-ups from healthcare professionals also increased adherence to exercise in a type 2 diabetes mellitus population.67 This supports the notion that support from a healthcare professional, or perhaps from anyone perceived as knowledgeable and able to offer guidance, feedback, and motivation, such as the leader of an exercise programme, is important in EPA adherence.
Limitations
This review was designed and conducted in accordance with current guidelines produced by the Cochrane Prognosis Methods Group, the CQIMG, and PRISMA. Multiple databases were used, and multiple reviewers were involved throughout the review, and any conflicts were resolved through discussion. These processes are considered gold standard for increasing the rigour of systematic reviews.68,69
One limitation of this review is that only studies in the English language were included, and search methods were limited to database searching and reference list searching of the eligible studies. Additional supplementary search methods, such as hand searching and citation tracking, may have led to the inclusion of additional studies.70 Additionally, publication bias cannot be excluded and may lead to the exclusion of unpublished data, leading to overestimation of the importance of each determinant.71 However, this is a potential bias in all systematic reviews.
A further limitation relates to using physical activity measures as proxy markers of adherence. Each included quantitative study reported physical activity levels, an increase of which was interpreted as adherence to the purpose of this review. Application of a measure for a purpose other than for which it was designed may affect the validity of the results.72 In patient populations, EPA is often prescribed in accordance with physical activity guidelines in order to increase overall physical activity levels and improve self-management of the health condition, therefore any significant difference in physical activity might be considered an acceptable indicator of adherence to a prescribed therapeutic recommendation.
One final limitation of this review is the lack of high-quality literature, leading to a low number of included studies. This leads to a low level of confidence that the findings in this review are associated with non-adherence to EPA. However, multiple other reviews support the idea that a wide range of factors are likely to be associated with non-adherence in various patient populations,22–24 and therefore there is a need to undertake further research that strengthens our understanding of these factors for people with MetS.
Clinical Implications
Physiotherapists and other healthcare professionals must consider a broad, complex range of factors affecting patients’ adherence to EPA, and help patients seek solutions to overcome these barriers using an individualized and holistic approach.
Patients with poorer self-rated health may need more support to initiate and maintain EPA.50 For those with low baseline EPA, identifying enjoyable ways to engage in EPA may facilitate greater uptake and continuation of EPA in patients with MetS.54 For patients with low self-efficacy, physiotherapists may need to help patients identify potential EPA strategies that would fit with the patient’s lifestyle, support goal setting, and discuss relapse management until the patient has sufficiently increased their ability to engage with EPA independently.60 For patients with smaller social support systems, increased guidance, feedback and motivation may be provided by physiotherapists or other professionals.66,67
Implications for Research
The small number of eligible studies and very low certainty of evidence in this review suggests that the existing research into the determinants of EPA in MetS is limited and that further high-quality quantitative, qualitative or mixed-methods research in this area is warranted. Identification of determinants also creates the opportunity to begin identifying behaviour change interventions that might be used to increase EPA adherence and to begin testing the effectiveness of these behaviour change techniques in populations with MetS.
Finally, existing research in other health populations, such as those at risk of MetS, and those with musculoskeletal conditions, has reached comparable conclusions that there are multiple determinants of EPA adherence. It is possible that determinants of EPA across all clinical populations may be similar. However, research is required to confirm or reject this hypothesis.
Conclusion
This review found evidence of limited certainty for associations between several factors and adherence to EPA. Taking the existing literature into consideration, lower baseline EPA, lower self-rated health, and a number of psychosocial factors may be the most significant determinants of reduced EPA adherence. The findings of this review highlight the lack of research into the determinants of EPA adherence in MetS populations. While it is plausible that factors affecting EPA adherence are similar across all clinical populations, it is imperative to establish this through further research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alberti KG, Eckel RH, Grundy SM., et al. Harmonizing metabolic syndrome: a Joint interim statement of the International and Blood Institute; American Heart Association; World Heart Federation; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;1(120):1640–1645.
2. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi:10.1161/CIRCULATIONAHA.105.169404
3. Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–414. doi:10.1016/j.jacc.2006.09.032
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–237. doi:10.1242/dmm.001180
5. Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Diabetes Care. 2005;28(9):2289–2304. doi:10.2337/diacare.28.9.2289
6. Saladini F, Palatini P. Arterial distensibility, physical activity, and the metabolic syndrome. Curr Hypertens Rep. 2018;20(5):39. doi:10.1007/s11906-018-0837-3
7. Lemes ÍR, Ferreira PH, Linares SN, Machado AF, Pastre CM, Netto J. Resistance training reduces systolic blood pressure in metabolic syndrome: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2016;50(23):1438–1442. doi:10.1136/bjsports-2015-094715
8. Pattyn N, Cornelissen VA, Toghi Eshghi SR, Vanhees L. The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome. Sports Med. 2013;43(2):121–133.
9. Ostman C, Smart NA, Morcos D, Duller A, Ridley W, Jewiss D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16(1):110. doi:10.1186/s12933-017-0590-y
10. Wewege MA, Thom JM, Rye KA, Parmenter BJ. Aerobic, resistance or combined training: a systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis. 2018;274(274):162–171. doi:10.1016/j.atherosclerosis.2018.05.002
11. van Namen M, Prendergast L, Peiris CL. Supervised lifestyle intervention for people with metabolic syndrome improves outcomes and reduces individual risk factors of metabolic syndrome: a systematic review and meta-analysis. Metabolism. 2019;101(101):153988. doi:10.1016/j.metabol.2019.153988
12. Peiris CL, van Namen M, O’Donoghue G. Education-based, lifestyle intervention programs with unsupervised exercise improve outcomes in adults with metabolic syndrome. A systematic review and meta-analysis. Rev Endocr Metab Disord. 2021;22(22):877–890. doi:10.1007/s11154-021-09644-2
13. Burgess E, Hassmén P, Pumpa KL. Determinants of adherence to lifestyle intervention in adults with obesity: a systematic review. Clin Obes. 2017;7(3):123–135. doi:10.1111/cob.12183
14. Leung AW, Chan RS, Sea MM, Woo J. An overview of factors associated with adherence to lifestyle modification programs for weight management in adults. Int J Environ Res Public Health. 2017;14(8):922. doi:10.3390/ijerph14080922
15. Martin BJ, Hauer T, Arena R, et al. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation. 2012;126(6):677–687. doi:10.1161/CIRCULATIONAHA.111.066738
16. Wallis JA, Webster KE, Levinger P, Taylor NF. What proportion of people with Hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta-analysis. Osteoarthritis Cartilage. 2013;21(11):1648–1659. doi:10.1016/j.joca.2013.08.003
17. Fappa E, Yannakoulia M, Pitsavos C, Skoumas I, Valourdou S, Stefanadis C. Lifestyle intervention in the management of metabolic syndrome: could we improve adherence issues? Nutrition. 2008;24(3):286–291. doi:10.1016/j.nut.2007.11.008
18. World Health Organisation. Adherence to long-term therapies, Evidence for action; 2003. Available from: https://apps.who.int/iris/handle/10665/42682.
19. Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142(9):776–785. doi:10.7326/0003-4819-142-9-200505030-00014
20. Alefishat EA, Farha RKA, Al-Debei MM. Self-reported adherence among individuals at high risk of metabolic syndrome: effect of knowledge and attitude. Med Principles Practice. 2017;26(2):157–163. doi:10.1159/000453037
21. Bullard T, Ji M, An R, Trinh L, Mackenzie M, Mullen SP. A systematic review and meta-analysis of adherence to physical activity interventions among three chronic conditions: cancer, cardiovascular disease, and diabetes. BMC Public Health. 2019;19(1):636. doi:10.1186/s12889-019-6877-z
22. Ahuja D. Determinants of Rehabilitation Adherence in Outpatient Musculoskeletal Physiotherapy: A Mixed Methods Project. Sheffield, UK: Sheffield Hallam University; 2015.
23. Ormel HL, Van Der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psycho‐Oncology. 2018;27(3):713–724. doi:10.1002/pon.4612
24. Picorelli AMA, Pereira LSM, Pereira DS, Felício D, Sherrington C. Adherence to exercise programs for older people is influenced by program characteristics and personal factors: a systematic review. J Physiother. 2014;60(3):151–156. doi:10.1016/j.jphys.2014.06.012
25. Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2016;26(4):364–373. doi:10.1016/j.tcm.2015.10.004
26. Riley RD, Moons KGM, Snell KIE, et al.A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;(364):k4597. doi:10.1136/bmj.k4597
27. Noyes J, Booth A, Flemming K, et al. Cochrane Qualitative and Implementation Methods Group guidance series—paper 3: methods for assessing methodological limitations, data extraction and synthesis, and confidence in synthesized qualitative findings. J Clin Epidemiol. 2018;97(97):49–58. doi:10.1016/j.jclinepi.2017.06.020
28. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(372):n160. doi:10.1136/bmj.n160
29. Noyes J, Booth A, Cargo M, et al. Chapter 21: qualitative evidence. Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions (Version 6.2., Part 3). Cochrane; 2021. Available from http://www.training.cochrane.org/handbook.
30. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(33):159–174. doi:10.2307/2529310
31. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(158):280–286. doi:10.7326/0003-4819-158-4-201302190-00009
32. Critical Appraisal Skills Programme. CASP Checklists; 2018. Available from: https://casp-uk.net/casp-tools-checklists/.
33. National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance (third edition); 2012. Available from: https://www.nice.org.uk/process/pmg4/chapter/reviewing-the-scientific-evidence.
34. Huguet A, Hayden JA, Stinson J, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Syst Rev. 2013;2(1):1–12. doi:10.1186/2046-4053-2-71
35. Lewin S, Bohren M, Rashidian A, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings—paper 2: how to make an overall CERQual assessment of confidence and create a Summary of Qualitative Findings table. Implementation Sci. 2018;13(1):11–23. doi:10.1186/s13012-017-0689-2
36. Farmer T, Robinson K, Elliott SJ, Eyles J. Developing and implementing a triangulation protocol for qualitative health research. Qual Health Res. 2006;16(3):377–394. doi:10.1177/1049732305285708
37. United States Department of Agriculture. 2015 Dietary Guidelines Advisory Committee (DGAC) Nutrition Evidence Library Methodology; 2015. Available from: https://usdasearch.usda.gov/search?utf8=%E2%9C%93&affiliate=usda&query=dietary+guidelines+advisory+committee&commit=Search.
38. Abuissa H, Khanna A, Spertus J. Low rates of exercise in patients with metabolic syndrome after an acute coronary syndrome. Clin Cardiol. 2005;28(11):530–533. doi:10.1002/clc.4960281108
39. Jansen H, den Engelsen C, Rutten GE. Physical activity in patients with metabolic syndrome: at screening and three years thereafter. Metab Syndr Relat Disord. 2013;11(3):163–168. doi:10.1089/met.2012.0110
40. Colom A, Ruiz M, Wärnberg J, et al. Mediterranean built environment and precipitation as modulator factors on physical activity in obese mid-age and old-age adults with metabolic syndrome: cross-sectional study. Int J Environ Res Public Health. 2019;16(5):854. doi:10.3390/ijerph16050854
41. Pollock A, Campbell P, Cheyne J, et al. Interventions to support the resilience and mental health of frontline health and social care professionals during and after a disease outbreak, epidemic or pandemic: a mixed methods systematic review. Cochrane Database Sys Rev. 2020;11(11). doi:10.1002/14651858.CD013779
42. Kim CJ, Kim BT, Chae SM. Application of the transtheoretical model: exercise behavior in Korean adults with metabolic syndrome. J Cardiovascular Nursing. 2010;25(4):323–331. doi:10.1097/JCN.0b013e3181c8a3e8
43. Millstein RA, Huffman JC, Thorndike AN, et al. How do positive psychological constructs affect physical activity engagement among individuals at high risk for chronic health conditions? A qualitative study. J Phys Act Health. 2020;17(10):977–986. doi:10.1123/jpah.2019-0295
44. Munthe-Kaas H, Bohren MA, Glenton C, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings—paper 3: how to assess methodological limitations. Implementation Sci. 2018;13(1):25–32. doi:10.1186/s13012-017-0690-9
45. Glenton C, Carlsen B, Lewin S, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings—paper 5: how to assess adequacy of data. Implementation Sci. 2018;13(1):43–50. doi:10.1186/s13012-017-0692-7
46. Colvin CJ, Garside R, Wainwright M, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings—paper 4: how to assess coherence. Implementation Sci. 2018;13(1):33–41. doi:10.1186/s13012-017-0691-8
47. Noyes J, Booth A, Lewin S, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings–paper 6: how to assess relevance of the data. Implementation Sci. 2018;13(1):51–61. doi:10.1186/s13012-017-0693-6
48. Lundqvist S, Börjesson M, Larsson ME, Cider Å, Hagberg L. Which patients benefit from physical activity on prescription (PAP)? A prospective observational analysis of factors that predict increased physical activity. BMC Public Health. 2019;19(1):1–13. doi:10.1186/s12889-019-6830-1
49. Williams G, Di Nardo F, Verma A. The relationship between self-reported health status and signs of psychological distress within European urban contexts. Eur J Public Health. 2017;27(Suppl 2):68–73. doi:10.1093/eurpub/ckx008
50. Lee G, Yang SJ, Chee YK. Assessment of healthy behaviors for metabolic syndrome among Korean adults: a modified information-motivation-behavioral skills with psychological distress. BMC Public Health. 2016;16(1):1–8. doi:10.1186/s12889-016-3185-8
51. Eaglehouse YL, Venditti EM, Kramer MK, et al. Factors related to lifestyle goal achievement in a diabetes prevention program dissemination study. Transl Behav Med. 2017;7(4):873–880. doi:10.1007/s13142-017-0494-0
52. Olson EA, Mullen SP, Raine LB, Kramer AF, Hillman CH, McAuley E. Integrated social-and neurocognitive model of physical activity behavior in older adults with metabolic disease. Ann Behav Med. 2017;51(2):272–281. doi:10.1007/s12160-016-9850-4
53. Susin N, de Melo Boff R, Ludwig MWB, et al. Predictors of adherence in a prevention program for patients with metabolic syndrome. J Health Psychol. 2016;21(10):2156–2167. doi:10.1177/1359105315572451
54. Oman R, McAuley E. Intrinsic motivation and exercise behavior. J Health Educ. 1993;24(4):232–238. doi:10.1080/10556699.1993.10610052
55. Lawton R, Conner M, McEachan R. Desire or reason: predicting health behaviors from affective and cognitive attitudes. Health Psychol. 2009;28(1):56. doi:10.1037/a0013424
56. Chen CN, Chuang LM, Korivi M, Wu YT. Home-based exercise may not decrease the insulin resistance in individuals with metabolic syndrome. J Phys Act Health. 2015;12(1):74–79. doi:10.1123/jpah.2013-0284
57. Ji M, Ren D, Dunbar-Jacob J, Gary-Webb TL, Erlen JA. Self-management behaviors, glycemic control, and metabolic syndrome in type 2 diabetes. Nurs Res. 2020;69(2):E9–E17. doi:10.1097/NNR.0000000000000401
58. Ellis T, Cavanaugh JT, Earhart GM, et al. Factors associated with exercise behavior in people with Parkinson disease. Phys Ther. 2011;91(12):1838–1848. doi:10.2522/ptj.20100390
59. McAuley E, Mullen SP, Szabo AN, et al. Self-regulatory processes and exercise adherence in older adults: executive function and self-efficacy effects. Am J Prev Med. 2011;41(3):284–290. doi:10.1016/j.amepre.2011.04.014
60. McAuley E, Jerome GJ, Marquez DX, Elavsky S, Blissmer B. Exercise self-efficacy in older adults: social, affective, and behavioral influences. Ann Behav Med. 2003;25(1):1–7. doi:10.1207/S15324796ABM2501_01
61. Joelsson M, Lundqvist S, Larsson ME. Tailored physical activity on prescription with follow-ups improved motivation and physical activity levels. A qualitative study of a 5-year Swedish primary care intervention. Scand J Prim Health Care. 2020;38(4):399–410. doi:10.1080/02813432.2020.1842965
62. Johnson CC, Taylor AG, Anderson JG, Jones RA, Whaley DE. Feasibility and acceptability of an Internet-based, African dance-modified yoga program for African-American women with or at risk for metabolic syndrome. J Yoga Phys Ther. 2014;4:548.
63. Vafa FS, Mahmoodabad SSM, Vaezi AA, Karimi H, Fallahzadeh H. A survey on the enablers and nurturers of physical activity in women with prediabetes. J Family Med Primary Care. 2020;9(6):2940. doi:10.4103/jfmpc.jfmpc_583_19
64. Huberty JL, Ransdell LB, Sidman C, et al. Explaining long-term exercise adherence in women who complete a structured exercise program. Res Q Exerc Sport. 2008;79(3):374–384. doi:10.1080/02701367.2008.10599501
65. Thoits PA. Mechanisms linking social ties and support to physical and mental health. J Health Soc Behav. 2011;52(2):145–161. doi:10.1177/0022146510395592
66. Room J, Hannink E, Dawes H, Barker K. What interventions are used to improve exercise adherence in older people and what behavioural techniques are they based on? A systematic review. BMJ Open. 2017;7(12):e019221. doi:10.1136/bmjopen-2017-019221
67. Nesari M, Zakerimoghadam M, Rajab A, Bassampour S, Faghihzadeh S. Effect of telephone follow‐up on adherence to a diabetes therapeutic regimen. Japan J Nursing Sci. 2010;7(2):121–128. doi:10.1111/j.1742-7924.2010.00146.x
68. Brackett A, Batten J. Ensuring the rigor in systematic reviews: part 1, the overview. Heart Lung. 2020;49(5):660–661. doi:10.1016/j.hrtlng.2020.03.015
69. Belur J, Tompson L, Thornton A, Simon M. Interrater reliability in systematic review methodology: exploring variation in coder decision-making. Sociol Methods Res. 2021;50(2):837–865. doi:10.1177/0049124118799372
70. Boulos L, Ogilvie R, Hayden JA. Search methods for prognostic factor systematic reviews: a methodologic investigation. J Med Lib Assoc. 2021;109(1):23. doi:10.5195/jmla.2021.939
71. Tsuji S, Cristia A, Frank MC, Bergmann C. Addressing publication bias in meta-analysis. Zeitschrift für Psychologie. 2020;228(1):50–61. doi:10.1027/2151-2604/a000393
72. McLean S, Holden MA, Potia T, et al. Quality and acceptability of measures of exercise adherence in musculoskeletal settings: a systematic review. Rheumatology. 2017;56(3):426–438. doi:10.1093/rheumatology/kew422
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
