Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Determinants of Activity Phenotype in Patients with Chronic Obstructive Pulmonary Disease
Authors Murakami Y, Minakata Y , Kato M, Sasaki S, Azuma Y, Kawabe K, Ono H
Received 19 May 2023
Accepted for publication 15 August 2023
Published 31 August 2023 Volume 2023:18 Pages 1919—1929
DOI https://doi.org/10.2147/COPD.S421755
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jill Ohar
Yusuke Murakami, Yoshiaki Minakata, Mai Kato, Seigo Sasaki, Yuichiro Azuma, Kazumi Kawabe, Hideya Ono
Department of Respiratory Medicine, National Hospital Organization Wakayama Hospital, Wakayama, 644-0044, Japan
Correspondence: Yoshiaki Minakata, Department of Respiratory Medicine, National Hospital Organization Wakayama Hospital, 1138 Wada, Mihama-cho, Hidaka-gun, Wakayama, 644-0044, Japan, Tel +81-738-22-3256, Email [email protected]
Introduction: Physical activity (PA) and sedentary behavior (SB) have attracted attention in chronic obstructive pulmonary disease (COPD), and there have been efforts to evaluate PA and SB separately. The factors associated with the characteristics of the four activity phenotypes defined by the durations of PA and SB are largely unknown. The aim of this study was to clarify the factors that could differentiate each activity phenotype.
Materials and Methods: Study subjects were outpatients with stable COPD who were ≥ 40 years of age. We investigated the influence of 26 different factors on the activity phenotypes of COPD and extracted the factors that showed significant differences among the four activity phenotypes.
Results: Two hundred sixteen patients were included in the analysis. Exercise capacity and dyspnea were determinants that distinguished the low PA groups from the high PA groups. The pulmonary function and desaturation during exercise were factors that distinguished the high PA with low SB group from the low PA with high SB group. BMI, grip strength, upper arm circumference and HbA1c were higher in the low PA and low SB group than in the low PA and high SB group.
Conclusion: These factors could be the determinants discriminating activity phenotypes of patients with COPD.
Keywords: COPD, physical activity, sedentary behavior, nutritional status, muscle strength
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease mainly caused by smoking. It caused approximately 3.23 million deaths worldwide in 2019.1 It is characterized by chronic respiratory symptoms due to inflammation and remodeling of the airways and/or alveoli that cause persistent, often progressive, air flow obstruction.2,3 Patients with COPD have reduced respiratory function compared to healthy individuals, and the level of respiratory function decline has been associated with decreased physical activity (PA).4–6 In addition, a low level of PA has been found associated with increased exacerbations and mortality.7 Therefore, the maintenance or improvement of PA is an important target for management, similar to smoking cessation in patients with COPD.
Recently, similar to PA, sedentary behavior (SB) has attracted attention in COPD. SB can be defined as an energy expenditure of ≤1.5 metabolic equivalents (METs) in a sitting or reclining position. On the other hand, PA is defined as any body movement produced by skeletal muscles that results in energy expenditure but is usually evaluated with the duration of the intensity of PA at ≥3 METs (moderate to vigorous intensity).8–10 The daily duration of PA and that of SB have been reported to be negatively correlated in patients with COPD.11 The mortality among patients with COPD is associated with both a decrease in PA and an increase in SB, both of which are important indicators.7,12
In recent years, there have been efforts to evaluate PA and SB separately. Bakrania et al, categorized individuals into four activity groups based on their levels of PA and SB: (1) “Busy Bees”, those who engage in high levels of PA and low levels of SB; (2) “Sedentary Exercisers”, those who engage in low levels of both PA and SB; (3) “Light Movers”, those who engage in high levels of both PA and SB; and (4) “Couch Potatoes”, those who engage in low levels of PA but high levels of SB.13 They examined participants cardiometabolic markers by combined categories of quantified PA and SB. McKeough et al called these four groups as “activity phenotype” and examined mortality and cardiometabolic risk with a similar classification in patients with COPD.14 However, the factors associated with the characteristics of each activity phenotype are still largely unknown.
The aim of this study was to perform a detailed comparison of the four activity phenotypes mentioned above, which were defined by the durations of PA and SB in patients with COPD and to clarify the factors that could differentiate each activity phenotype.
Materials and Methods
Subjects
Patients were recruited from the enrollment date of a previous study in which a detailed reference equation for PA in Japanese patients with COPD was created.15 Patients were recruited from 21 institutes belonging to the National Hospital Organization of Japan from January 2017 to February 2020. Study subjects were outpatients with stable COPD diagnosed with postbronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) <0.7 and ≥40 years of age. The following exclusion criteria were applied: 1) clinically relevant bronchial asthma, 2) receiving oxygen therapy, 3) a history of lung resection, 4) a history of an exacerbation within three months, 5) PA extremely suppressed by other diseases (including neuromuscular disease, bone and joint disease, active malignant disease, myocardial infarction, etc.), and 6) participation in this study deemed inappropriate by the attending physician.
This study was conducted in accordance with the provisions of the Declaration of Helsinki, was approved by the ethics committee of the National Hospital Organization Wakayama Hospital (approval number: 04-3; approval date: 21 February 2023), and has been registered with the University Hospital Medical Information Network (UMIN 000050745, 1 April 2023). We explained the research details on the National Hospital Organization Wakayama Hospital’s website and provided the opportunity to refuse participation in the study.
Variables
This was a prospective cross-sectional observational study. Observational duration was from day 0 to day 14 (or up to day 28). Day 0 was defined as the day when the subject patient gives consent to participate in the study. On day 0, the following variables were collected: age, sex, smoking history, modified Medical Research Council (mMRC) dyspnea score, Hospital Anxiety and Depression Scale (HADS), medical history, body mass index (BMI), nutritional status, grip strength, pulmonary function tests after bronchodilator administration and blood tests. The medical history included a history of COPD medication, whether or not pulmonary rehabilitation was performed, and the presence of comorbidities. The nutritional status was assessed by measuring upper arm circumference and triceps subcutaneous fat thickness. Blood tests included assessments of red blood cells (RBCs), hemoglobin (Hb), fasting blood glucose (FBG), hemoglobin A1c (HbA1c), albumin (Alb), and brain natriuretic peptide (BNP). A 6-minute walk test (6MWT) was performed on day 14 (or up to day 28).
Accelerometric Evaluation
From day 0 to day 14 (up to day 28), PA was measured with a triaxial accelerometer, Active Style Pro HJA-750C® (Omron Healthcare, Kyoto, Japan) for 24 hours except for bathing or water activities, and the information was recorded in the patient’s dairy. Duration at ≥3.0 METs were employed as an indicator of PA and duration at 1.0–1.5 METs were employed as an indicator of SB.
Data Cleaning
First, from the 15 obtained days (up to 29 days), rainy days, days with special activity, and days when the average temperature was <2.5°C were excluded because they were considered to affect the patients’ physical status. Next, the accelerometric data between 07:00 and 20:00 were extracted from the data measured throughout the day, and the days with less than 10 hours of accelerometer wearing time were excluded. The accelerometer nonwear time was defined as 90 minutes of consecutive zeros with allowance for 2 minutes of interruptions in accordance with Choi’s method.16 Finally, to obtain reproducibility of the data, data from patients who had at least three valid days were analyzed.17 Information about weather and special activity days was obtained from the content of the diary. The mean temperature was detected from the records of the nearest weather station to each institution where the patient was recruited.
Activity Phenotype
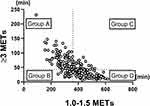
In accordance with Bakrania’s classification,13 the patients were divided into four groups based on their PA (duration at ≥3 METs) and SB (duration at 1.0–1.5 METs): Group A consisted of patients with greater than or equal to the median duration at ≥3 METs and less than the median duration at 1.0–1.5 METs, which corresponded to “busy bees”; Group B consisted of patients with less than the median duration at ≥3 METs and less than the median duration at 1.0–1.5 METs which corresponded to “light movers”; Group C consisted of patients with greater than or equal to the duration at ≥3 METs and greater than or equal to the duration at 1.0–1.5 METs, which corresponded to “sedentary exercisers”; and Group D consisted of patients with less than the median duration at ≥3 METs and greater than or equal to the median duration at 1.0–1.5 METs which corresponded to “couch potatoes”. We expressed these four groups as activity phenotypes, according to the study conducted by McKeough et al.14
Statistical Analyses
The differences in the median changes in features observed in the four groups were compared using the Kruskal–Wallis test. Categorical variables were tested with the chi-square test. The features that were significantly different in a Kruskal–Wallis test were subjected to multiple comparisons between independent groups using Dunn’s test. All tests were two-tailed and the significance level was set at p<0.05.
Results
Two hundred fifty-three patients were recruited, and 14 were excluded for the following reasons: accelerometer trouble (N=6), postbronchodilator FEV1.0/FVC ≥0.7 (N=3), other diseases (N=2), spirometry performed without a bronchodilator (N=1), withdrawal of consent (N=1), and duplicate registration (N=1). Among these patients, 23 were excluded because the number of valid days was <3. Finally, 216 patients were registered in the analysis (Figure 1).
 |
Figure 1 Flow diagram. Abbreviations: FEV1.0, forced expiratory volume in one second; FVC, forced vital capacity; BD, bronchodilator. |
The average age of the patients was 73.1 years, 202 (94%) patients were male, and the average FEV1.0% of predicted value (FEV 1.0%pred) was 62.6%. The average duration at ≥3.0 METs per day was 52 minutes, and the average duration at 1.0–1.5 METs per day was 371 minutes (Table 1).
 |
Table 1 Characteristics of All Patients |
The median values of duration at ≥3 METs per day and at 1.0–1.5 METs per day were 43 minutes and 362 minutes, respectively. The values in each group were as follows: Group A, ≥43 minutes of duration at ≥3.0 METs and <362 minutes of duration at 1.0–1.5 METs; Group B, <43 minutes at ≥3.0 METs and <362 minutes at 1.0–1.5 METs; Group C, ≥43 minutes at ≥3.0 METs and ≥362 minutes at 1.0–1.5 METs; and Group D, <43 minutes at ≥3.0 METs and ≥362 minutes at 1.0–1.5 METs (Figure 2). The numbers of patients in Groups A, B, C, and D were 83 (38.4%), 25 (11.6%), 25 (11.6%), and 83 (38.4%), respectively. Factors that showed significant differences among groups were BMI, IC, FEV1.0%pred, FEV1.0/FVC, 6-minute walk distance (6MWD), lowest SpO2 during the 6MWT, upper arm circumference, grip strength, HbA1c, mMRC, PA and SB status (Table 2).
 |
Table 2 Differences Among Groups |
The factors that differed significantly among groups were divided into three major distinctive characteristics. The first characteristic was that Groups B and D tended to be inferior to Groups A and C, which included the 6MWD and mMRC (Figure 3). The second was that Group D was inferior to Group A, which included IC, FEV1.0%pred, FEV1.0/FVC and lowest SpO2 during the 6MWT (Figure 4). The third was that Group B had higher values than Group D, which included BMI, upper arm circumference, grip strength and HbA1c (Figure 5).
Discussion
The study examined the determinants of activity phenotypes of patients with COPD, which were classified by PA and SB. Patients in Groups B or D had a shorter 6MWD and higher mMRC than those in Groups A or C. Patients in Group D had lower levels of respiratory function and SpO2 during the 6MWT than in Group A. Patients in Group B had higher BMI, grip strength, upper arm circumference, and HbA1c than those in Group D.
The 6MWD was one of the determinants distinguishing Groups B or D from Groups A or C, which indicated that PA but not SB could affect the 6MWD. An official systematic review of the European Respiratory Society/American Thoracic Society showed that the 6MWD was moderately or strongly correlated with objective measures of PA in patients with COPD.18 Furthermore, their correlation was stronger than the correlations between the 6MWD and pulmonary function or quality of life. On the other hand, Schneider et al showed that the patients in the active + nonsedentary group (corresponding to Group A) had longer 6MWD than those in the active + sedentary group (corresponding to Group C), which is not compatible with our results in Groups A and C.11 One possible reason for this discrepancy is that the number of patients in the active + sedentary group in Schneider’s report was small (n=9, 6%), which may have led to less reliable results. Alternatively, cultural differences between study populations could account for distinct outcomes.
mMRC was another determinant that distinguished Group B or D from Group A or C, which indicated that mMRC could be associated with PA but not SB. A higher mMRC score was associated with lower PA levels in patients with COPD,19,20 which was compatible with our results. Munari et al reported that mMRC was associated with not only PA but also SB in patients with COPD,21 which was not compatible with our results. This is presumably because the median value of mMRC (2 points) and the mean value of FEV1.0%pred (35.1%) of the participants were worse than those in our study. In other words, in the population with a poor pulmonary function and severe dyspnea, mMRC may also be associated with the duration of SB.
IC, FEV1.0%pred and FEV1.0/FVC were the determinants distinguishing Group D from Group A. Previous studies showed that patients with a better pulmonary function have longer periods of PA, but the correlation was not as strong.6,22 These reports only evaluated the PA aspect. The relationship between the pulmonary function and SB in patients with COPD was also reported.23 An increase in SB duration can affect the development of COPD and is a risk factor for a decreased pulmonary function, similar to smoking.24,25 These reports suggest that both PA and SB are important factors for the pulmonary function in patients with COPD, rather than one or the other. In this study, there was significant difference in respiratory function only between Groups A and D, while no differences were found between Groups A and B, Groups A and C, Groups B and D, or Groups C and D. This result suggests that the decline in respiratory function in patients with COPD could be associated with both the decrease in PA duration and the increase in SB duration, rather than a decrease in PA duration alone or an increase in SB duration alone.
The lowest SpO2 during the 6MWT was significantly reduced in Group D in comparison to Group A. Some studies have shown that exercise-induced oxygen desaturation in patients with COPD is associated with FEV1.0.26,27 The lowest SpO2 during the 6MWT might be a determinant discriminating Group D from Group A, similar to the pulmonary function.
BMI, upper arm circumference, grip strength, and HbA1c were determinants discriminating Group B from D, which indicated that these factors were associated with SB among patients with low PA. BMI and upper arm circumference are commonly used as indicators of the nutritional status and are frequently assessed in patients with COPD.28
Among the population with a low level of PA, BMI was high in patients with a low level of SB (Group B). Mesquita et al reported that BMI was higher in “couch potatoes” (a low level of PA with a high level of SB) than other activity phenotypes,29 which was not compatible with our results. The difference might be attributed by difference in BMI of the respective patient populations. The mean BMI in the current study was approximately within the normal range of 22.4±3.3. However, in Mesquita’s study, the median BMI was 25.8, which indicated that more than half of the patients were overweight. Other reports have demonstrated a relationship between obesity and an increase in SB,30,31 although these reports were not specifically in studies of patients with COPD. Our results suggest that if the BMI of patients with COPD is within the normal range, such as Japanese patients,32,33 a higher BMI might be associated with a low level of SB.
The upper arm circumference of patients in Group B was significantly longer than that of patients in Group D, suggesting that it could influence SB for patients with decreased PA. Although shorter upper arm circumference has been reported to be associated with the severity of COPD,34,35 no studies have demonstrated a direct correlation between upper arm circumference in patients with COPD and the durations of PA or SB. Benítez et al reported that upper arm circumference in inpatients was positively correlated with BMI.36 Upper arm circumference may be affected by SB to reflect the nutritional status, similar to BMI.
Muscle strength is an important factor in assessing sarcopenia in patients with COPD, similar to the nutritional status. Hand grip strength is a muscle strength parameter that can be easily measured. Our study found that hand grip strength was significantly higher in patients in Group B than those in Group D, which indicated that hand grip strength was associated with SB for the patients with decreased PA. However, high hand grip strength was reported to be related to an increase in PA and a decrease in SB in meta-analysis.37 One possible reason for this discrepancy is that the participants in these reports included many healthy individuals; in fact, in this meta-analysis, only 12 of the 112 papers were related to patients with COPD. These differences in registrant characteristics might have led to different results regarding the association between hand grip strength and PA.
Although BMI, upper arm circumference, and grip strength were significantly different between patients in Groups B and D, no significant difference was found between Groups A and C. This result suggests that if PA is maintained to some extent, the effects of nutrition and muscle strength on SB might be small. The lack of difference between Groups C and D suggests that the negative effects of these factors may have more impact on SB than on PA.
Patients in Group B had higher HbA1c values than those in Group D. This means that an increase in HbA1c is associated with a decrease of SB, which is difficult to explain. McCarthy et al reported that in high-risk patients with type 2 diabetes, a decrease in HbA1c was associated with an increase in PA, while no relationship was observed between HbA1c and SB.38 In addition, they revealed a relationship between an increase in HbA1c and weight gain. The effects of HbA1c on SB might be similar to those of BMI. Furthermore, most of the HbA1c values of the patients were in the normal range in the current study (mean ± SD: 5.95±0.65), which suggests that HbA1c might have a greater impact on SB than on PA among patients whose HbA1c value is in the normal range.
The present study is associated with several limitations. First, it is unclear whether the sample size was sufficient to draw this conclusion, especially, the sample sizes of Groups B and C were small, which might have led to inaccuracies in the analysis. Second, patients with severely reduced PA by other diseases or with oxygen therapy were excluded from this study. Therefore, the number of patients with very severe disease was relatively small. Third, we could not evaluate the effects of osteoporosis, the presence or absence of work or hobbies, and household environment (whether the participant lived alone or with others), although these factors were reported to be associated with PA.39–41 These factors should be evaluated in future studies. Fourth, due to the cross-sectional design of the study, the relationship between the four activity phenotypes and mortality could not be examined. Fifth, this study was a Japanese predominantly male population and therefore generalizability to a broader COPD may be limited.
Conclusion
We identified the characteristics of activity phenotypes defined by PA and SB in patients with COPD. Exercise capacity, dyspnea, pulmonary function, desaturation during exercise, nutritional status, muscle strength, and HbA1c could be the determinants discriminating activity phenotypes.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the local ethics committee (IRB Committee of National Hospital Organization Wakayama Hospital; approval number: 04-3; approval date: 23 February 2023).
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Informed Consent Statement
The contents of this study and the opportunity to decline participation were explained on the website of the National Hospital Organization Wakayama Hospital. URL: http://wakayama.hosp.go.jp (accessed on 11 April 2023).
Acknowledgments
Patient recruitment was performed at the following institutes belonging to the National Hospital Organization (NHO): NHO Asahikawa Medical Center, NHO Takasaki General Medical Center, NHO Yokohama Medical Center, NHO Kanazawa Medical Center, NHO Tenryu Hospital, NHO Minami Kyoto Hospital, NHO Kinki-chuo Chest Medical Center, NHO Osaka Toneyama Medical Center, NHO Osaka Minami Medical Center, NHO Himeji Medical Center, NHO Nara Medical Center, NHO Minami Wakayama Medical Center, NHO Wakayama Hospital, NHO Minami-Okayama Medical Center, NHO Yamaguchi-Ube Medical Center, NHO Ehime Medical Center, NHO Kochi National Hospital, NHO Fukuoka Hospital. The authors thank Brian Quinn for reading the manuscript.
Author Contributions
All authors have read and agreed to the published version of the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by the Environmental Restoration and Conservation Agency of Japan. URL: http://www.erca.go.jp/erca/english/index.html (accessed on 1 April 2023).
Disclosure
Y Minakata received lecture fees from Nippon Boehringer Ingelheim. The authors report no other conflicts of interest in this work.
References
1. World Health Organization. Chronic obstructive pulmonary disease. Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)/.
2. Global initiative for chronic obstructive lung disease: global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease 2023 report. Available from: http://www.goldcopd.com/.
3. Celli B, Fabbri L, Criner G, et al. Definition and nomenclature of chronic obstructive pulmonary disease: time for its revision. Am J Respir Crit Care Med. 2022;206(11):1317–1325. doi:10.1164/rccm.202204-0671PP
4. Minakata Y, Sugino A, Kanda M, et al. Reduced level of physical activity in Japanese patients with chronic obstructive pulmonary disease. Respir Investig. 2014;52(1):41–48. doi:10.1016/j.resinv.2013.06.002
5. Vorrink SN, Kort HS, Troosters T, et al. Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res. 2011;12(1):33. doi:10.1186/1465-9921-12-33
6. Pitta F, Takaki MY, Oliveira NH, et al. Relationship between pulmonary function and physical activity in daily life in patients with COPD. Respir Med. 2008;102(8):1203–1207. doi:10.1016/j.rmed.2008.03.004
7. Gimeno-Santos E, Frei A, Steurer-Stey C, et al.; PROactive consortium. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax. 2014;69(8):731–739. doi:10.1136/thoraxjnl-2013-204763
8. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131.
9. Sedentary Behaviour Research Network. Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37(3):540–542. doi:10.1139/h2012-024
10. World Health Organization. WHO guidelines on physical activity and sedentary behavior. Available from: https://www.who.int/publications/i/item/9789240015128/.
11. Schneider LP, Furlanetto KC, Rodrigues A, et al. Sedentary behaviour and physical inactivity in patients with chronic obstructive pulmonary disease: two sides of the same coin? COPD. 2018;15(5):432–438. doi:10.1080/15412555.2018.1548587
12. Furlanetto KC, Donária L, Schneider LP, et al. Sedentary behavior is an independent predictor of mortality in subjects with COPD. Respir Care. 2017;62(5):579–587. doi:10.4187/respcare.05306
13. Bakrania K, Edwardson CL, Bodicoat DH, et al. Associations of mutually exclusive categories of physical activity and sedentary time with markers of cardiometabolic health in english adults: a cross-sectional analysis of the health survey for England. BMC Public Health. 2016;16:1–10. doi:10.1186/s12889-015-2639-8
14. McKeough Z, Cheng SWM, Alison J, et al. Low leisure-based sitting time and being physically active were associated with reduced odds of death and diabetes in people with chronic obstructive pulmonary disease: a cohort study. J Physiother. 2018;64(2):114–120. doi:10.1016/j.jphys.2018.02.007
15. Minakata Y, Sasaki S, Azuma Y, et al. Reference equations for assessing the physical activity of Japanese patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2021;16:3041–3053. doi:10.2147/COPD.S336670
16. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–364. doi:10.1249/MSS.0b013e3181ed61a3
17. Miyamoto S, Minakata Y, Azuma Y, et al. Verification of a motion sensor for evaluating physical activity in COPD patients. Can Respir J. 2018;2018:8343705. doi:10.1155/2018/8343705
18. Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European RESPIRATORY Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–1478. doi:10.1183/09031936.00150414
19. Hayata A, Minakata Y, Matsunaga K, et al. Differences in physical activity according to mMRC grade in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2203–2208. doi:10.2147/COPD.S109694
20. Albarrati AM, Gale NS, Munnery MM, et al. Daily physical activity and related risk factors in COPD. BMC Pulm Med. 2020;20(1):1–8. doi:10.1186/s12890-020-1097-y
21. Munari AB, Gulart AA, Dos Santos K, et al. Modified medical research council dyspnea scale in GOLD classification better reflects physical activities of daily living. Respir Care. 2018;63(1):77–85. doi:10.4187/respcare.05636
22. Watz H, Waschki B, Meyer T, et al. Physical activity in patients with COPD. Eur Respir J. 2009;33(2):262–272. doi:10.1183/09031936.00024608
23. Geidl W, Carl J, Cassar S, et al. Physical activity and sedentary behaviour patterns in 326 persons with COPD before starting a pulmonary rehabilitation: a cluster analysis. J Clin Med. 2019;8(9):1346. doi:10.3390/jcm8091346
24. Lei Y, Zou K, Xin J, et al. Sedentary behavior is associated with chronic obstructive pulmonary disease: a generalized propensity score-weighted analysis. Medicine. 2021;100(18):e25336. doi:10.1097/MD.0000000000025336
25. Campbell Jenkins BW, Sarpong DF, Addison C, et al. Joint effects of smoking and sedentary lifestyle on lung function in African Americans: the Jackson heart study cohort. Int J Environ Res Public Health. 2014;11(2):1500–1519. doi:10.3390/ijerph110201500
26. Andrianopoulos V, Franssen FM, Peeters JP, et al. Exercise-induced oxygen desaturation in COPD patients without resting hypoxemia. Respir Physiol Neurobiol. 2014;190:40–46. doi:10.1016/j.resp.2013.10.002
27. van Gestel AJ, Clarenbach CF, Stöwhas AC, et al. Prevalence and prediction of exercise-induced oxygen desaturation in patients with chronic obstructive pulmonary disease. Respiration. 2012;84(5):353–359. doi:10.1159/000332833
28. World Health Organization. Physical status: the use of and interpretation of anthropometry, report of a WHO expert committee. Available from: https://www.who.int/publications/i/item/9241208546/.
29. Mesquita R, Spina G, Pitta F, et al. Physical activity patterns and clusters in 1001 patients with COPD. Chron Respir Dis. 2017;14(3):256–269. doi:10.1177/1479972316687207
30. Weinsier RL, Hunter GR, Heini AF, et al. The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. Am J Med. 1998;105(2):145–150. doi:10.1016/S0002-9343(98)00190-9
31. Silveira EA, Mendonça CR, Delpino FM, et al. Sedentary behavior, physical inactivity, abdominal obesity and obesity in adults and older adults: a systematic review and meta-analysis. Clin Nutr ESPEN. 2022;50:63–73. doi:10.1016/j.clnesp.2022.06.001
32. Makita H, Suzuki M, Konno S, et al. Unique mortality profile in Japanese patients with COPD: an analysis from the Hokkaido COPD cohort study. Int J Chron Obstruct Pulmon Dis. 2020;15:2081–2090. doi:10.2147/COPD.S264437
33. Motegi T, Jones RC, Ishii T, et al. A comparison of three multidimensional indices of COPD severity as predictors of future exacerbations. Int J Chron Obstruct Pulmon Dis. 2013;8:259–271. doi:10.2147/COPD.S42769
34. Baig MMA, Hashmat N, Adnan M, et al. The relationship of dyspnea and disease severity with anthropometric indicators of malnutrition among patients with chronic obstructive pulmonary disease. Pak J Med Sci. 2018;34(6):1408–1411. doi:10.12669/pjms.346.15769
35. Arora S, Madan K, Mohan A, et al. Serum inflammatory markers and nutritional status in patients with stable chronic obstructive pulmonary disease. Lung India. 2019;36(5):393–398. doi:10.4103/lungindia.lungindia_494_18
36. Benítez Brito N, Suárez Llanos JP, Fuentes Ferrer M, et al. Relationship between mid-upper arm circumference and body mass index in inpatients. PLoS One. 2016;11(8):e0160480. doi:10.1371/journal.pone.0160480
37. Ramsey KA, Rojer AGM, D’Andrea L, et al. The association of objectively measured physical activity and sedentary behavior with skeletal muscle strength and muscle power in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2021;67:101266. doi:10.1016/j.arr.2021.101266
38. McCarthy M, Edwardson CL, Davies MJ, et al. Change in sedentary time, physical activity, bodyweight, and HbA1c in high-risk adults. Med Sci Sports Exerc. 2017;49(6):1120–1125. doi:10.1249/MSS.0000000000001218
39. Li Y, Gao H, Zhao L, et al. Osteoporosis in COPD patients: risk factors and pulmonary rehabilitation. Clin Respir J. 2022;16(7):487–496. doi:10.1111/crj.13514
40. Liu WT, Kuo HP, Liao TH, et al. Low bone mineral density in COPD patients with osteoporosis is related to low daily physical activity and high COPD assessment test scores. Int J Chron Obstruct Pulmon Dis. 2015;10:1737–1744. doi:10.2147/COPD.S87110
41. Ichinose M, Minakata Y, Motegi T, et al. A non-interventional, cross-sectional study to evaluate factors relating to daily step counts and physical activity in Japanese patients with chronic obstructive pulmonary disease: STEP COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:3385–3396. doi:10.2147/COPD.S277782
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.