Back to Journals » Infection and Drug Resistance » Volume 15
Deterioration of Cycloserine in Drug Susceptibility Testing of Mycobacterium
Authors Wang R , Zhao X, Wan K
Received 8 November 2021
Accepted for publication 23 December 2021
Published 13 January 2022 Volume 2022:15 Pages 135—140
DOI https://doi.org/10.2147/IDR.S348428
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ruibai Wang, Xiuqin Zhao, Kanglin Wan
State Key Laboratory for Infectious Diseases Prevention and Control, Tuberculosis Department, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, People’s Republic of China
Correspondence: Ruibai Wang
State Key Laboratory for Infectious Diseases Prevention and Control, Tuberculosis Department, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Changbai Road 155, Changping, Beijing, 102206, People’s Republic of China
Tel +86-10-58900779
Email [email protected]
Purpose: Cycloserine is an effective group C anti-tuberculosis drug. But the reliability and reproducibility of drug susceptibility tests (DST) for this drug cannot be guaranteed and provide poor clinical predictive values. However, DST of cycloserine in practice provides rough estimate of the drug resistance of Mycobacterium strains, there is practical need to clarify the problem of cycloserine in in vitro DST, and to explore solutions to overcome these limitations.
Methods: The effectiveness of serial cycloserine solutions incubated at 37°C for 1 to 29 days was tested using the Alamar Blue assay, and cycloserine in culture medium was analyzed by UPLC-MS.
Results: The data revealed that cycloserine itself continuously degraded in culture medium. This amount of degradation was sufficient to alter the minimum inhibitory concentration (MIC) value of Mycobacterium strains and therefore could not be ignored, although it was more stable than in phosphoric acid buffer.
Conclusion: The different test times and the degradation of cycloserine were responsible for the lack of agreements between the cycloserine DST methods and the low reliability of this in vitro test. By adjusting with the incubation time depended degradation ratio of cycloserine, more accurate MIC values may be obtained allowing for improved coincidence between in vitro experiment and clinic use. Furthermore, it can guide clinicians to carry out this anti-tuberculosis treatment more effectively and reliably.
Keywords: Mycobacterium, tuberculosis, drug susceptibility testing, cycloserine, deterioration
Introduction
Tuberculosis (TB) is an infectious disease that poses serious threat to human health. In 2020, the number of people newly diagnosed with TB and reported was 5.8 million globally and 1.5 TB deaths.1 TB still is the leading cause of death from a single infectious agent. TB treatment depends entirely on anti-TB drugs, which have been classified into four groups for the treatment of rifampicin-resistant TB and multidrug-resistant tuberculosis (MDR-TB).2 Cycloserine is one of the group C anti-TB drugs (other core second-line agents). According to meta-analyses of adult individual patient data (aIPD) and pediatric individual patient data (pIPD), the probability of successful anti-TB treatment increased with marginal statistical significance when cycloserine was included in the MDR-TB treatment regimen. Cycloserine also has advantages over other group C anti-TB drugs, ethionamide/prothionamide, which only showed statistical significance in adults. Furthermore, since it is inexpensive and less likely to induce drug resistance, cycloserine has been widely used to treat TB and non-tuberculous mycobacteria (NTM) for several years.3
Most of mycobacteria, including Mycobacterium tuberculosis and slow-growth non-tuberculous mycobacteria (NTM), grow slowly and drug susceptibility testing (DST) takes 2–4 weeks, so alternative testing methods are needed. The development of genotypic methods to detect drug resistance sites in Mycobacterium offered a more rapid testing option. However, the lack of complete data on the genetic basis for many anti-TB drugs mechanisms and the uncertain linkage between mutations and drug resistance means that traditional in vitro DST remains the primary testing method for Mycobacterium, DST also allows for qualitative data collection based on the drug critical concentrations of drugs and quantitative data collection based on the minimum inhibitory concentrations (MICs). Unfortunately, for cycloserine, DST was not recommended in the Clinical and Laboratory Standards Institute (CLSI) guideline for technical reasons4–6 namely that the reliability and reproducibility of the tests could not be guaranteed.7 For example, regarding the reproducibility of data from Sensititre MYCOTB MIC plate, the level of agreement for resistance determination was 84.1%, for exact MIC was 56.5% and for MIC ±1 doubling dilution was 92.8%.8 The interpretation results obtained using different DST methods were also different. The agreement in resistance determination between MYCOTB plate and agar proportional method (APM) for cycloserine was 96.4%, for exact MIC was 81.5% and for MIC ±1 doubling dilution was 99.6%.8 These three values between MYCOTB plates and the resazurin microtiter assay (REMA) were 100%, 53.2% and 91.3% respectively.9
Although it is not recommended and has poor clinical predictive value, in vitro DST to detect the effectiveness of cycloserine in practice for anti-TB and NTM3 therapy provides rough estimate of the drug resistance of Mycobacterium strains. Therefore, there is a practical need to clarify the reasons for the instability and inconsistency of cycloserine in in vitro DST, and to explore solutions to overcome these limitations.
Materials and Methods
Strains
The DST time of M. tuberculosis and other slow-growth Mycobacterium is at least 7 days and even cycloserine solution freshly prepared at the time of use will undergo a long 37 °C incubation. To reduce the proportion of bacterial culture time within the whole incubation time of cycloserine solution at 37°C and to distinguish the role of bacteria and the degradation of cycloserine itself, Mycobacterium hassiacum 95085 (DSM44199) was used in this study, which is a rapid-growth Mycobacterium species that is reported to be sensitive to cycloserine.10
Incubation of Cycloserine Solution
Eight concentrations of cycloserine (Sigma-Aldrich Co., St Louis, MO, USA) from 2 μg/mL to 512 μg/mL in MH medium were prepared by two-fold serial dilution and were sub-packed into 29 8-strip PCR tubes (cap attached) with 100 μL/tube. At the same time, 256 μg/mL cycloserine solution was also placed into 29 1.5mL Eppendorf (EP) tubes at 1 mL/tube. All tubes were stored at ˗20 °C. Every day, one strip and one EP tube were taken out and placed in the 37°C incubator. After 29 days, the solutions in the PCR tubes were used for DST and the solutions in the EP tubes were used for ultra-performance liquid chromatography (UPLC) and liquid chromatography tandem mass spectrometry (LC-MS).
DST
The broth microdilution method based on the Alamar Blue assay (MABA) was used for MIC determination. Briefly, fresh cultures were dispersed in saline using ultrasound (BACspreader 1100, TB Healthcare Group, Guangdong, China) and adjusted to 0.5 McFarland (1.5×108 cells/mL). The suspension was 1:200 diluted with Mueller–Hinton broth (Oxoid Ltd, Basingstoke, Hampshire, England). Then, 100 μL of bacteria suspension was added to the 8-strip PCR tubes containing the serial dilutions of cycloserine prepared in Incubation of Cycloserine Solution. The tubes were capped and incubated at 37°C. The indicator (20µL of Alamar Blue mixed with 50µL of 5% Tween-80) was added daily to the drug-free growth control and to all of the test tubes when the control showed a color change from blue to pink. The MIC was defined as the lowest drug concentration that inhibited the growth of the strain and prevented a color change. The whole test was repeated three times.
UPLC and LC-MS
UPLC was performed on Waters-ACQUITY H-class equipment (Waters Corporation, MA, USA) with an Acquity UPLC Beh Shield RP18 (2.1×100 mm, 1.7μm) column. The mobile phase A:B = 5%: 95% (A was methanol and B was 0.05% formic acid in water with 5mM ammonium formate) was applied. Other parameters were Isometric elution, 0.2mL/min flow rate, 3 μL injection volume and 226nm detection wavelength. The PDA detector collected chromatographic data at 210–400 nm.
LC-MS was performed on an Agilent Ultivo triple Quad LC-MS system (1290–6460, Agilent, Santa Clara, CA, USA) with an Acquity UPLC Beh Shield RP18 (2.1×100 mm, 1.7 μm) column. The mobile phase was methanol (phase A): 0.05% formic acid in water with 5mM ammonium formate (phase B) = 85%:15%. Other parameters were a flow rate of 0.2mL/min for isometric elution and an injection volume of 3 μL. MS was operated in positive electrospray ionization mode. The scanning range was 40–500 m/z, the capillary voltage was 7 kV, the ventilation temperature was 350°C, the flow rate was 13 L/min and the atomizer was kept at 60 psi.
Results
MIC Changed with Incubation Time
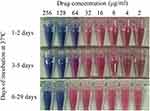
For strain M. hassiacum 95085, the time for cycloserine DST was about 4 days, from addition of the bacteria suspension to MIC reading. Adding the previous incubation time of cycloserine, the total incubation time was 4–32 days. From Figure 1, it is clear that with increased incubation time of cycloserine solution, the MIC value of strain M. hassiacum 95085 changed significantly from 64 μg/mL to 128 μg/mL. The color difference started as early as day 3, although it was not great enough to increase the MIC to the next dilution concentration. From day 6, the color of the 64 μg/mL tube was completely pink, and the MIC value had increased to 128 μg/mL. The results showed that the degradation and inactivation of cycloserine itself was indeed a confounding factor in the cycloserine DST of Mycobacterium, which may affect the final MIC value and the determination of drug resistance of a strain.
Quantitative and Qualitative Analysis of Cycloserine
Cycloserine solutions with the longest and shortest incubation times, ie, 1 day and 29 days, were analyzed by LC-MS (Figure 2). There were obvious changes in both the height and area of the cycloserine peak in the chromatograms, as well as the mass spectrums, which indicated that besides hydrolysis into hydroxylamine and serine, there may also be some structural changes in cycloserine that affect activity.
 |
Figure 2 The chromatograms (A) and mass spectrums from LC-MS (B) of two cycloserine solutions that had been incubated at 37 °C for 1 and 29 days, respectively. |
According to the color change of Alamar Blue in the DST of strain M. hassiacum 95085, we selected the first nine consecutive samples and three samples from the days 15, 20 and 29 of the cycloserine incubation for quantitative analysis by UPLC. The degradation of cycloserine was linear relative to time (Figure 3). With 256 μg/mL as the initial concentration, the degradation proportion of cycloserine was 3.35% at 6 days (8 μg/mL), and 8.5% at 2 weeks (21 μg/mL) (Table 1).
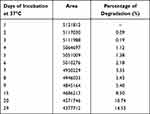 |
Table 1 The Peak Area and Percentage of Degradation of Cycloserine in HPLC |
 |
Figure 3 The degradation ratio map of cycloserine in MH medium. |
Discussion
Cycloserine, (R)-4-amino-1,2-oxazolidin-3-one, is a broad-spectrum antibiotic. It is produced by Streptomyces orchidaceus or synthesized by chemical methods. During the development of the new drug formulation, the stability of cycloserine has been comprehensively evaluated over pH range of 2.0–11.5 and an ionic concentration of 10–20 mM at 37°C.11–13 However, for DST, the drug stability is not usually tested under so many different conditions. The main consideration is the stability of the drugs under the storage conditions, ie, the standard storage solution or working solution at ˗20°C and the lyophilized plates, such as the Sensititre MYCOTB MIC plate, at room temperature. For most bacteria, the period of DST is 18–24 hours, the stability of the drugs at working concentrations in the culture medium is seldom considered. However, for Mycobacteria, even rapid-growth Mycobacteria, DST requires at least 3 days and M. tuberculosis DSTs requires 2–6 weeks. From the results of this study, it is clear that the long incubation time is a key problem in Mycobacterium cycloserine DST. Although the stock solution of cycloserine is stable for 1 month at ˗20°C and lyophilized cycloserine and cycloserine in capsules are stable for at least one year at room temperature,11 from the beginning of DST, cycloserine begin to degrade and becomes inactive gradually. Thus, phenomenon was evident after only 3 days.
Cycloserine was stable under alkaline conditions and showed the maximum rate of degradation at pH 4.7. The pH values of the MH and 7H9 media used in Mycobacterium DST were 7.3 ± 0.1 and 6.6 ± 0.2, respectively. At these neutral pH, degradation was not as rapid as under acidic pH. In phosphate buffer with the ionic strength adjusted with sodium chloride, the degradation rate of cycloserine was about 10% of the initial concentration within a week, 20% in 15 days and 30% in 22 days.11 During DST, cycloserine is added once, and the hydrolyzed and inactivated cycloserine cannot be supplemented to maintain an effective concentration, unlike the successive administration in patients through which an effective blood concentration can be maintained. Unlike the drugs that do not undergo hydrolysis and the drugs biodegraded by the test strains, the effect of the reduced proportion of cycloserine on drug sensitivity cannot be ignored. Therefore, it is inevitable that the DST outcomes of cycloserine are inconsistent with the clinical results and have poor clinical predictive values.
As for the inconsistency between the results of different cycloserine DST methods, this may be attributed to the different hydrolyzed proportions of cycloserine caused by the different test times. For standard LJ APM, the results are read from day 28 to 42, depending on the status of the growth control. For REMA, the incubation time is fixed at 7 days, then 30 μL of resazurin are added for color development and the MIC judgement. For MYCOTB DST, the results are read at two time points for each test, one when the drug-free control has visible cell growth, and the other after a longer incubation time of about 21 days. For MABA, as for APM, the end point depends on the growth control and the time ranges from 14 to 30 days for M. tuberculosis. The threshold/critical concentrations for cycloserine in DST were set by consideration of the peak drug level in serum (7.1 to 43.4 μg/mL). For APM, 25 μg/mL and 40 μg/mL have been used,8,14 for MYCOTB DST, >32 μg/mL has been used and for other liquid methods, 30 μg/mL15 has ever been selected. Our results showed that the effect of cycloserine inactivation on the MIC was equivalent to one dilution. This may explain why the agreement between exactly the same MIC values was only 50%, and the MIC ±1 doubling dilution agreement increased to more than 90% in previously reported studies.
To increase the clinical predictive efficacy of second-line anti-TB drugs, it has been suggested that both an in vitro test and blood serum should be included in the drug concentration monitoring and the ratio of the blood serum concentration to MIC could be used as a better indicator of clinical outcomes.9 For cycloserine, our results suggested that this would not be sufficient. Cycloserine is a bacteriostatic agent, not a bactericidal agente, and in DST medium, mycobacteria do not die. The final concentration that is effective at inhibiting bacterial growth (ie, the MIC) is the residual cycloserine that remains after degradation. We proposed that the amount of degraded and inactivated drugs might be deducted from the crude MIC to obtain a more accurate adjusted MIC, according to the degradation ratio of cycloserine proportional to the duration of DST for each Mycobacterium strain. According to the chromograph in our experiment, the degradation amount of cycloserine was 10.74% at 20 days, which was slower than the 30% degradation rate in phosphoric acid buffer.11 This showed that cycloserine in MH culture medium was more stable than in phosphoric acid buffer, and also revealed the difference degree of cycloserine degradation in the different solutions. More accurate values of cycloserine degradation in MH and 7H9 medium need to be measured for MIC adjustment in Mycobacteria DST.
Conclusion
The long incubation time and the deterioration of cycloserine reduced the potency of cycloserine in DST and led to poor agreements between the different testing methods and the lack of reliability of this in vitro test. The MIC value may be adjusted according to the time based degradation ratio of cycloserine to achieve better coincidence between in vitro experiments and clinical data. This, in turn, may provide more effective and reliable information on which clinicians can base their decision making regarding anti-TB therapy.
Funding
This work was supported by the National Mega-project for Innovative Drugs [grant number 2019ZX09721001-007-001].
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019.
2. World Health Organization. WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update. Geneva: World Health Organization; 2016.
3. Khosravi AD, Mirsaeidi M, Farahani A, et al. Prevalence of nontuberculous mycobacteria and high efficacy of D-cycloserine and its synergistic effect with clarithromycin against Mycobacterium fortuitum and Mycobacterium abscessus. Infect Drug Resist. 2018;11:2521–2532. doi:10.2147/IDR.S187554
4. Woods GL, Brown-Elliott BA, Conville PS, et al. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic, Actinomycetes; Approved Standard- Second Edition. CLSI Document M24-A2. Vol. M24-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2011.
5. Pasipanodya JG, Srivastava S, Gumbo T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis. 2012;55(2):169–177. doi:10.1093/cid/cis353
6. Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis. 2013;208(9):1464–1473. doi:10.1093/infdis/jit352
7. World Health Organization. Implementing Tuberculosis Diagnostics. Policy Framework. Geneva: World Health Organization; 2015.
8. Lee J, Armstrong DT, Ssengooba W, et al. Sensititre MYCOTB MIC plate for testing Mycobacterium tuberculosis susceptibility to first- and second-line drugs. Antimicrob Agents Chemother. 2014;58(1):11–18. doi:10.1128/AAC.01209-13
9. Yu X, Ma YF, Jiang GL, Chen ST, Wang GR, Huang HR. Sensititre(R) MYCOTB MIC plate for drug susceptibility testing of Mycobacterium tuberculosis complex isolates. Int J Tuberc Lung Dis. 2016;20(3):329–334. doi:10.5588/ijtld.15.0573
10. Schroder KH, Naumann L, Kroppenstedt RM, Reischl U. Mycobacterium hassiacum sp. nov., a new rapidly growing thermophilic mycobacterium. Int J Syst Bacteriol. 1997;47(1):86–91. doi:10.1099/00207713-47-1-86
11. Kaushal G, Ramirez R, Alambo D, Taupradist W, Choksi K, Sirbu C. Initial characterization of D-cycloserine for future formulation development for anxiety disorders. Drug Discov Ther. 2011;5(5):253–260. doi:10.5582/ddt.2011.v5.5.253
12. Iakhontova LF, Bruns BP, Kartseva VD, Kobzieva SN, Perevozskaia NA. Physico-chemical and sorption properties of d-cycloserine. Antibiotiki. 1969;14(3):205–210.
13. Kartseva VD, Iakhontova LF, Isaeva NL, Bruns BP. On the stability of crystalline D-cycloserine. Antibiotiki. 1967;12(9):772–775.
14. Falzon D, Jaramillo E, Schunemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38(3):516–528. doi:10.1183/09031936.00073611
15. Banu S, Rahman SM, Khan MS, et al. Discordance across several methods for drug susceptibility testing of drug-resistant Mycobacterium tuberculosis isolates in a single laboratory. J Clin Microbiol. 2014;52(1):156–163. doi:10.1128/JCM.02378-13
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.