Back to Journals » Pragmatic and Observational Research » Volume 13
Detection of Short-Term Side Effects of ChAdOx1 nCoV-19 Vaccine: A Cross-Sectional Study in a War-Torn Country
Authors Alshakka M, Hatem NAH , Badullah W , Alsakaf R, Rageh A, Yousef SA, Mohamed Ibrahim MI
Received 14 July 2022
Accepted for publication 22 August 2022
Published 25 August 2022 Volume 2022:13 Pages 85—91
DOI https://doi.org/10.2147/POR.S381836
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor David Price
Mohammed Alshakka1 ,† Najmaddin A H Hatem,2 Wafa Badullah,3 Rabab Alsakaf,4 Ali Rageh,1 Seena Abdulla Yousef,5 Mohamed Izham Mohamed Ibrahim6
1Section of Clinical Pharmacy, Faculty of Pharmacy, Aden University, Aden, Yemen; 2Department of Pharmacy Practice, College of Clinical Pharmacy, Hodeidah University, Alhodeidah, Yemen; 3Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Aden University, Aden, Yemen; 4Department of Post-Marketing Surveillance, National Pharmacovigilance Center, Supreme Board of Drugs and Medical Appliances, Aden, Yemen; 5Department of Community Medicine and Public Health, Faculty of Medicine and Health Sciences, Aden University, Aden, Yemen; 6Department of Clinical Pharmacy and Practice, College of Pharmacy, QU Health, Qatar University, Doha, Qatar
†Dr. Mohammed Alshakka passed away on February 11, 2022
Correspondence: Najmaddin A H Hatem, Department of Pharmacy Practice, College of Clinical Pharmacy, Hodeidah University, P.O. 3114, Alhodeidah, Yemen, Tel +967 775040472, Email [email protected]
Purpose: The chAdOx1 nCoV-19 vaccine is the first COVID-19 vaccine available in Yemen. Hence, this local-based study was used to identify the type and frequency of short-term side effects following 48 hours of the first shot of the vaccine.
Methods: A cross-section of vaccinated participants in Aden were surveyed by telephone. Descriptive statistics were used for statistical analysis.
Results: A total of 500 participants were included through convenient sampling. 27% of them were health care providers. Nearly 70% of the respondent experienced side effects. The top three side effects reported were fever (n=276, 55.2%), myalgia (n=270, 54%) and fatigue (n=247, 49.4%). Generally, most participants stated that they experienced the side effects after the first 24 hours of vaccination.
Conclusion: Side effects that participants experienced were not different from the literature, indicating a safe profile for the vaccine. Further studies are needed to identify the side effects after the second and third dose of the vaccine. In addition, more studies are required to assess the efficacy of the existing vaccines against new variants.
Keywords: COVID 19, vaccine hesitancy, pharmacovigilance, safety, side-effects
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by a novel coronavirus known as SARS-CoV-2.1,2 Wuhan, China, was the site of the virus’s initial detection in December 2019. The World Health Organization declared a pandemic on March 11, 2020.3 In response to this disaster has created global health, socioeconomic, and humanitarian crises. Hundreds of potential vaccines have been tested; some passing acceptable effectiveness and safety criteria and being released for use.4,5 As of July 10, 2022, 217 vaccine candidates were tested in 750 distinct trials in 78 different countries. The World Health Organization (WHO) has authorized ten vaccinations among them.6 The recombinant chimpanzee adenovirus-vectored vaccine from Oxford/AstraZeneca is one of the authorized vaccines utilized globally (ChAdOx1 nCoV-19). They used the modified chimpanzee adenovirus ChAdOx1 as a vector.4,7 At 22 days after the first dosage, the vaccine’s effectiveness is predicted to be 76.0%, and at two doses, it is 81.3%.8,9
In the opinion of scientists, the FDA/WHO-approved or currently in-development COVID-19 vaccines are anticipated to induce a broad immune response, offering at least some protection against potential virus strains in the future. However, numerous new virus varieties are developing as the virus infects people and spreads throughout the population. For example, South Africa first reported the B.1.1.529 (Omicron) variant to WHO in late November 2021, from South Africa. Recent evidence suggests that this new strain increases the risk of reinfection, increases the spread of transmission, and reinfection, and decreases the effectiveness of available vaccines.10 However, Omicron appears to have a milder clinical presentation, with symptoms mainly in the upper respiratory tract.11
The WHO provides a safety surveillance manual for COVID-19 vaccines, specifying that many vaccine safety standards must be met.12 According to a study by Soldatos, pharmacovigilance plays a significant role in enhancing vaccine safety.13 The World Health Organization defined Pharmacovigilance as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or other potential drug-related problems.”14 It is an important component of vaccine safety monitoring. Scientists from all over the world collaborate to produce safe and efficient vaccines to get over the pandemic. However, worries about the safety of the vaccines have arisen as a result of their rapid development, contributing to the unwillingness of people to take vaccine.15
Yemen is a conflict torn-country. It is considered the world’s worst humanitarian crisis and has one of the most fragile healthcare systems. It’s experiencing war and famine, with only 50% of health facilities fully functioning.16 Yemen reported its first case of COVID 19 on April 10, 2020, Yemen has experienced three waves of COVID-19 infections.17 As of 8 July 2022, 11,832 confirmed cases of COVID-19 with 2149 deaths were reported to WHO. As of 27 June 2022, 864,544 vaccine doses have been administered.18 And only nine hundred thousand doses arrived in Yemen through the COVAX program.19
As a result, post-market unfavorable events should be investigated, and enough proof should be gathered. Nevertheless, to the best of our knowledge, this study investigation is the first of its kind in Yemen. This study aimed to see if there were any short-term side effects after the first dosage of ChAdOx1 nCoV-19 vaccine in Aden-Yemen, and post public confidence in getting the vaccine.
Methodology
Study Setting
This study was conducted during the first vaccination campaign in late of April 2021 that was firstly kicked off in the southern port city of Aden-Yemen after the first shipment of 360,000 doses of Oxford/AstraZeneca AZD1222 COVID-19 vaccine from global COVAX facilities.20
Sample Size
The Raosoft sample size calculator (http://www.raosoft.com/samplesize.html) was used to estimate the sample size with a margin of error of 5%, a confidence interval of 95%, and a 50% response rate, and a 30% non-response rate. The estimated sample size was 490.
Study Tool and Data Collection
A telephone-administered survey Supplementary File 1. consists of two parts. The first part involved socio-demographic characteristics of vaccinated participants, which were taken before their first shot. The second part focused on the type of side effects experienced by participants with “Yes” or “No” alongside the timing of appearing them with “Before 24 h of vaccination” or “After 24h of vaccination”. The second part was obtained after 48 hours of vaccination by four final-year pharmacy students at Aden University using interview calls. Those side effects used in the second part of our survey were taken from previously reported clinical studies on the ChAdOx1 nCoV-19 vaccine.7–9 The study included participants who were willing to participate in the study, gave verbal and written agreement, and received the first shot of the vaccine. The interview calls last four weeks during the campaign to get 500 participants. Information concerning the vaccinated people and their telephone numbers were obtained from the Ministry of Health lists, which the vaccine health committee provided in Aden.
Data Analysis
The collected data was analyzed and evaluated to determine the types, frequency, and timing of side effects using IBM SPSS software V.19.
Results
The demographic characteristics of the survey on the side-effects of COVID-19 vaccine based on symptoms experienced among people in Aden are represented in Table 1. Seventy-five percent (n=375) of vaccinated people were male. Most of the vaccinated people were between 26–35 years (40.4%, n=202), and (27%, n=135) were health care providers, and (97.1%, n=95) of them were physicians.
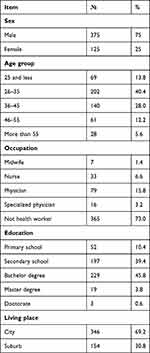 |
Table 1 Demographic Characteristics of Study Participants in Aden (n=500) |
The most-reported side effects of the vaccine among people in Aden city are illustrated in Table 2. More than half of the vaccinated people experienced fever (55.2%, n=276), and myalgia (54%, n=270), followed by fatigue (49.4%, n=247), headache (46.8%, n=234) and joint pain (45.8%, n=229).
 |
Table 2 Frequency of Reported Side Effects of ChAdOx1 nCoV-19 Vaccine and the Appearance of Symptoms Timing Among Peoples in Aden |
Moreover, Table 2. shows the vaccine’s side effects based on the appearance of symptoms time. The most-reported side effect before 24 hours was fever (77.2%, n=152) followed by headache (74.1%, n=194), fatigue (65.5%, n=129), joint pain (62.9%, n=124). Moreover, a slightly higher percentage of the same side effects were reported after 24 hours.
Discussion
Vaccines are the most successful public-health intervention because they prevent and control the spread of infectious illnesses, reducing mortality and morbidity. However, reactions to vaccination are possible, just as with other medications.21 Because of the low frequency of side effects, the small number of participants, and other study constraints, some of them are unlikely to show in pre-approval clinical investigations. As a result, post-marketing surveillance of side effects following vaccination is critical.22 Individuals’ confidence in vaccines varies widely and is influenced by various factors, such as vaccine awareness, political or religious beliefs, potential hazards, and economic and social situations.13,22
Despite that Yemen has allocated 4,774,000 doses of COVID 19 vaccine,19 as of Jun 19, 2022, only 1.5% of Yemeni people are fully vaccinated.23 Whereas the vast majority of those who claim to take the vaccine were working in Saudi Arabia as it was mandatory. A possible reason for this low vaccination rate among Yemeni people is vaccine availability since the vaccination campaigns were held only in the country’s southern governorates. At the same time, the vast majority of Yemenis live in the northern part, and the de facto Houthis authority in the north refuses the distribution of the vaccine in their controlled northern part of Yemen. As a conflict-country, women and children face many hinders in accessing vaccination facilities.19 An effort must be made by NGOs to coordinate with Yemeni authorities to ensure that the vaccination campaigns are conducted in safe and convenient access for all. Thus, we are confident that the most vital determinant of vaccine acceptance in this country is access to the vaccine. In other words, “supply could lead to an increase in demand”.24 Vaccine hesitancy plays a key role in vaccination acceptance. In early 2021 a survey conducted among northern residents in Yemen showed they considered the vaccine a deliberate “conspiracy” posing a threat to their health. Other believe that the vaccination campaign is a project to cause infertility among Muslims, and only 17% were willing to receive the vaccine.25 Furthermore, a study conducted among 36,000 participants from different Arabic countries stated that the most cited reason to reject the COVID-19 vaccines was concern about the side effects,26 also stated in a study held in Yemen in which 66.4% were fear of side effects.24
Hence, the current study was conducted in Aden-city in Yemen. We looked into the short-term side effects of the ChAdOx1 nCoV-19 vaccine after it was given. In this study, nearly 70% of the respondent experienced side effects. They reported various systemic side effects, including fever, myalgia, fatigue, headache, and joint pain. However, only a few people reported having syncope or other symptoms. The most common symptom was found to be fever. These findings were similar to other recently published studies.27–29 Most were mild to moderate, and all went away within a few days of vaccination.30 Huh et al reported that the incidences of anaphylactic shock did occur after vaccination in Korea.31 During our study, there was no incidence of such cases of anaphylactic shock, and only 21 participants reported mild and self-limiting hypersensitivity reactions. This is in line with a study conducted in Afghanistan.32 A cohort study conducted among 46 million adults in England reported an increased incidence of thrombotic events after ChAdOx1 vaccination.33 In contrast, other studies did not find any statistically significant increase in the risk of thrombotic events.34 This issue is still controversial in the literature; in the current study, no thrombotic events were reported, and no significant conditions necessitated hospitalization. Furthermore, most participants reported having side effects after the first 24hrs. This finding was in line with a study in Nepal that stated that fever and headache were reported 24hrs after getting the vaccine.35 The current study results show changes in the timing and the local and systemic adverse effects following immunization compared to prior clinical study data. These disparities could be attributable to racial and ethnic differences, those who have never been infected with COVID-19, and those who are older.36–38
More research into the side effects of COVID-19 vaccines could boost public confidence in vaccine safety, allowing the COVID-19 vaccination process to move more quickly. In addition, these data will dispel misunderstandings15 and conspiracy theories concerning the COVID-19 vaccine’s post-vaccination effects, which contribute to vaccine apprehension.
Limitations
The results were based on self-reported information by those who received the vaccine rather than clinically confirmed by physicians. Furthermore, the data was gathered through a phone-based survey, leading to report bias. Moreover, the study was conducted in Aden city, and the results cannot be generalized to the whole country. Besides, the study could not assess long-term side effects. Therefore, future studies need to assess side effects after the second dose and similar studies are needed to assess side effects for other types of approved vaccines in this country.
Conclusion
The side effects participants experienced were not different from the literature, indicating a safe profile for the vaccine. Most side effects were experienced after 24 hours of vaccination, and no serious symptoms were reported or hospitalization was needed. Health experts and physicians will be interested in the findings of this study. The findings raise awareness of the vaccination’s safety, effectiveness, and benefits, as no serious health issues were reported.
Ethics Approval
The study procedure was accepted by the ethics research committee of the faculty of medicine and health sciences at Aden University, the research Code (REC-109-2021), and the study protocol complied with the Declaration of Helsinki. After the trained data collectors explained the research’s goals, importance, and benefits, all participants gave verbal and written agreement.
Acknowledgments
We would like to acknowledge 5th year pharmacy students 2020/2021 at Aden University for helping in the data collection.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhu N, Zhang D, Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi:10.1056/NEJMoa2001017
2. Dhama K, Patel SK, Sharun K, et al. SARS-CoV-2 jumping the species barrier: zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Travel Med Infect Dis. 2020;37:101830. doi:10.1016/j.tmaid.2020.101830
3. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi:10.23750/abm.v91i1.9397
4. Onyeaka H, Al-Sharify ZT, Ghadhban MY, Al-Najjar SZ. A review on the advancements in the development of vaccines to combat coronavirus disease 2019. Clin Exp Vaccine Res. 2021;10(1):6–12. doi:10.7774/cevr.2021.10.1.6
5. Kirby T. Development of potential COVID-19 vaccines continues to accelerate. Lancet Microbe. 2020;1(3):e109. doi:10.1016/s2666-5247(20)30070-7
6. McGill COVID19 Vaccine Tracker Team. Vaccines candidates by trial phase; 2021. Available from: https://covid19.trackvaccines.org/vaccines/?fbclid=IwAR0rJdUx1T1j9TalYCJzwvnqm6MWluN2wwWtnuDP0GW6YvKLh3nW-Ex0ZKU.
7. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a Phase 1/2, single-blind, randomized controlled trial. Lancet. 2020;396(10249):467–478. doi:10.1016/S0140-6736(20)31604-4
8. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1
9. Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomized trials. Lancet. 2021;397(10277):881–891. doi:10.1016/S0140-6736(21)00432-3
10. World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. Available from: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern.
11. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant—United States, 1–8 December 2021. Morb Mortal Wkly Rep. 2021;70(50):1731. doi:10.15585/mmwr.mm7050e1
12. World Health Organization. Covid-19 vaccines: safety surveillance manual; 2020. Available from: https://apps.who.int/iris/handle/10665/338400.
13. Soldatos TG, Taglang G, Jackson DB. In silico profiling of clinical phenotypes for human targets using adverse event data. HighThroughput. 2018;7(4):37.
14. World Health Organization. The importance of pharmacovigilance – safety monitoring of medicinal products. Geneva, Switzerland: WHO Collaborating Centre for International Drug Monitoring; 2002.
15. Bitar AN, Zawiah M, Al-Ashwal FY, et al. Misinformation, perceptions towards COVID-19 and willingness to be vaccinated: a population-based survey in Yemen. PLoS One. 2021;16(10):e0248325. doi:10.1371/journal.pone.0248325
16. Global humanitarian overview 2021. Geneva: United Nations Office for the Coordination of Humanitarian Affairs; 2020. Available from: https://www.unocha.org/globalhumanitarian-overview-2021.
17. Reuters. War-ravaged Yemen confirms first coronavirus case, braces for more; April 10, 2020. Available from: https://www.reuters.com/article/us-healthcoronavirus-yemen-case/war-ravaged-yemenconfirms-first-coronavirus-case-braces-formore-idUSKCN21S0EI.
18. Yemen: WHO coronavirus disease (COVID-19) dashboard with vaccination data [Internet]. World Health Organization. Available from: https://covid19.who.int/region/emro/country/ye.
19. ACAPS. Yemen COVID-19: current situation and reasons for vaccine hesitancy. Available from: https://www.acaps.org/sites/acaps/files/products/files/20220110_acaps_yemen_analysis_hub_thematic_report_covid-19_and_vaccine_hesitancy_0.pdf.
20. Reuters. Yemen starts COVID-19 vaccination campaign. Available from: https://www.reuters.com/business/healthcare-pharmaceuticals/yemen-starts-covid-19-vaccination-campaign-2021-04-20/.
21. Larson H, Cooper L, Eskola J, Katz S, Ratzan S. Addressing the vaccine confidence gap. Lancet. 2011;378(9790):526–535. doi:10.1016/S0140-6736(11)60678-8
22. Pfizer COVID-19 vaccine EUA fact sheet for healthcare providers administering vaccine (vaccination providers). 2021:1–30. Available from: https://www.fda.gov/media/144413/download.
23. Our World in Data. Coronavirus (COVID-19) vaccinations statistics and research. Available from: https://ourworldindata.org/covid-vaccinations?country=YEM.
24. Noushad M, Al-Awar MS, Al-Saqqaf IS, Nassani MZ, Alrubaiee GG, Rastam S. Lack of access to COVID-19 vaccines could be a greater threat than vaccines hesitancy in low-income and conflict nations: the case of Yemen. Clin Infect Dis. 2022;ciac088. doi:10.1093/cid/ciac088
25. DARAJ. Yemen: We do not want vaccine. There is no corona here. We are fine. Available from: https://daraj.com/en/66891/.
26. qunaibi EA, Helmy M, Basheti I, Sultan IA. A high rate of COVID-19 vaccine hesitancy in a large-scale survey on Arabs. Elife. 2021;10:e68038. doi:10.7554/eLife.68038
27. World health organization. Coronavirus disease (COVID-19): vaccines safety; [updated September 20, 2021]. Available from: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines-safety.
28. Adam M, Gameraddin M, Alelyani M, et al. Evaluation of post-vaccination symptoms of two common COVID-19 vaccines used in Abha, Aseer Region, Kingdom of Saudi Arabia. Patient Prefer Adherence. 2021;15:1963–1970. doi:10.2147/PPA.S330689
29. Kadali RAK, Janagama R, Peruru S, et al. Adverse effects of COVID-19 mRNA-1273 vaccine: a randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. 2021;93(7):4420–4429. doi:10.1002/jmv.26996
30. Alhazmi A, Alamer E, Daws D, et al. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines. 2021;9(6):674. doi:10.3390/vaccines9060674
31. Huh K, Kim Y-E, Radnaabaatar M, et al. Estimating baseline incidence of condition potentially associated with vaccine adverse events: a call for surveillance system using the Korean National Health Insurance Claims Data. J Korean Med Sci. 2021;36(9):9. doi:10.3346/jkms.2021.36.e67
32. Azimi M, Dehzad WM, Atiq NA, Bahain B, Asady A. Adverse effects of the COVID-19 vaccine reported by lecturers and staff of Kabul University of Medical Sciences, Kabul, Afghanistan. Infect Drug Resist. 2021;14:4077–4083. doi:10.2147/IDR.S332354
33. Whiteley WN, Ip S, Cooper JA, et al. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: a population-based cohort study of 46 million adults in England. PLoS Med. 2022;19(2):e1003926. doi:10.1371/journal.pmed.103926
34. Wohlfahrt J. Association of AZD1222 and BNT162b2 COVID-19 vaccination with thromboembolic and thrombocytopenic events in frontline personnel: a retrospective cohort study. Ann Intern Med. 2022;175(4):541–546. doi:10.7326/M21-2452
35. Jha N, Palain S, Shankar PR, Dangal G. Pharmacovigilance of COVID-19 vaccines in the context of Nepal: an assessment based on early adverse drug reaction reports. J Pharm Health Serv Res. 2021;12(4):591–593. doi:10.1093/jphsr/rmab016
36. Meo S, Bukhari I, Akram J, Meo A, Klonoff D. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and moderna vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–1669. doi:10.26355/eurrev_202102_24877
37. Mascellino MT, Di Timoteo F, De Angelis M, Oliva A. Overview of the main anti-SARS-CoV-2 vaccines: mechanism of action, efficacy and safety. Infect Drug Resist. 2021;14:3459. doi:10.2147/IDR.S315727
38. Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of Phase 3 candidates. Npj Vaccines. 2021;6(1):1–17. doi:10.1038/s41541-021-00292-w
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
