Back to Journals » Infection and Drug Resistance » Volume 17
Detection of HIV-1 DNA/RNA in Peripheral Blood, Bone Marrow and Femoral Head of Patients with Osteonecrosis of the Femoral Head
Authors Li K, Liu B , Ma R, Zhang Q
Received 27 November 2023
Accepted for publication 23 January 2024
Published 12 February 2024 Volume 2024:17 Pages 551—559
DOI https://doi.org/10.2147/IDR.S449615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Kangpeng Li,* Bo Liu,* Rui Ma, Qiang Zhang
Department of Orthopedics, Beijing Ditan Hospital Affiliated to Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiang Zhang, Email [email protected]
Background: With the increasing life expectancy of people living with HIV (PLWH) following antiretroviral therapy (ART), there is a growing prevalence of chronic diseases such as osteonecrosis of the femoral head (ONFH). Compared with the more accessible blood, the viral infection profile in bone marrow and necrotic femoral heads in PLWH remains inadequately characterized.
Methods: Femoral head and bone marrow were collected from 15 PLWH undergoing total hip arthroplasty. For each femoral head, samples were obtained from the subchondral, necrotic, sclerotic, and normal areas. HIV DNA and HIV RNA assays were employed to evaluate disparities in viral load and reservoir between bone marrow and blood, as well as to quantify viral infection in distinct regions of the necrotic femoral head.
Results: Blood HIV RNA dropped below detectable levels in 8 patients (below 20 copies/mL). The median of bone marrow HIV RNA was 255.89 copies/mL. HIV DNA in blood and bone marrow was 296.35 and 454.31 copies/106 cells. HIV DNA in necrotic area was about half that in sclerotic area, HIV RNA was about twice that in normal area, the difference was statistically significant.
Conclusion: Despite using ART, there is still substantial active HIV and a potential reservoir in the bone marrow. Viral transcription was most active in the necrotic area of the femoral head, which may indicate that HIV itself is directly involved in ONFH.
Keywords: HIV, AIDS, bone marrow, femoral head, reservoir
A Letter to the Editor has been published for this article.
Introduction
According to UNAIDS, there are currently 39 million people living with HIV (PLWH) worldwide. By the end of 2022, China had reported 1.223 million PLWH cases and 418,000 deaths.1 Despite the application of antiretroviral therapy (ART) for nearly three decades, achieving complete success in conquering acquired immunodeficiency syndrome (AIDS) remains challenging due to the persistence of latent virus in various organs throughout the body.2 Our study focused on the bone marrow (BM), which serves as the primary site for hematopoiesis, encompassing the development, differentiation, and maturation of most immune cells.3 The interference of HIV infection on BM microenvironment leads to the imbalance of hematopoietic cell production, which participates in the depletion of CD4+ T cells.4 Additionally, BM serves as the site for CCR5Δ32/Δ32 hematopoietic stem cell transplantation, which has recently instilled hope for AIDS cure in patients, thereby highlighting the immense potential of BM research.5 Nevertheless, due to its limited accessibility compared to blood, BM remains inadequately explored.
With the increasing average life expectancy of PLWH, it is crucial to address the growing prevalence of chronic diseases. Osteonecrosis of the femoral head (ONFH) is among these conditions,6 and research has indicated that PLWH have a 100-fold higher incidence of ONFH compared to the general population in the absence of hip trauma.7 However, the underlying mechanisms remain unclear. It is reasonable to speculate that, in addition to the conventional factors contributing to ONFH, HIV itself may also play a role in the pathogenesis of femoral head destruction. Further investigation is warranted regarding the presence and extent of HIV infection within four key areas of necrotic femoral heads: subchondral, necrotic, sclerotic, and normal regions, as well as whether bone can serve as a reservoir for HIV persistence.
The quantification of plasma HIV RNA, namely viral load (VL), serves as a common indicator for assessing the rate of viral inhibition, evaluating disease progression in patients and monitoring the efficacy of ART.8 HIV DNA detection represents the most widely employed technology for studying HIV reservoirs, enabling not only assessment of disease progress but also comprehensive understanding of HIV distribution within tissues and organs throughout the body.9 Currently, various clinical techniques have been utilized to detect both HIV RNA and HIV DNA in peripheral blood (PB) with high concordance.10,11 However, expression patterns of HIV RNA and HIV DNA in BM and necrotic femoral head remain poorly characterized.
In this study, we collected samples of BM, PB, and femoral head to measure HIV DNA and RNA levels in order to evaluate the differences in viral load and reservoir size between BM and PB, as well as to assess the possibility and rationality of BM serving as a reservoir. Additionally, we quantified HIV infection in different areas of necrotic femoral head to speculate on the reasons for the high incidence of ONFH in PLWH.
Methods
Patients
All patients were from PLWH who needed total hip arthroplasty (THA) due to ONFH in the Department of Orthopedics, Beijing Ditan Hospital, Capital Medical University from March 2023 to September 2023. A total of 15 patients met the inclusion and exclusion criteria and obtained informed consent. The basic information of the patients was determined by inquiry, and a series of preoperative examinations were performed to determine the functional status of the patients’ heart, liver, kidney and other organs. Bone mineral density of patients was obtained by dual-energy X-ray absorptiometry (DXA).
Inclusion Criteria
(1) Informed consent must be signed voluntarily;
(2) PLWH confirmed by ELISA and Western blot
(3) Orthopedic disease requiring THA;
(4) Aged from 18 to 60 years old;
(5) The results of blood routine, urine routine, liver function and renal function tests were normal.
(6) No osteomyelitis (bone infection caused by bacteria or fungi), penetrating wound or contaminated wound;
(7) No opportunistic infections or cancer in the past 12 months.
Exclusion Criteria
(1) Female patients who are pregnant or lactating;
(2) Patients with a fever of more than 39 °C or requiring mechanical ventilation;
(3) Patients with autoimmune diseases, uncontrolled bleeding diseases, or severe past medical history;
(4) Patients with liver and kidney dysfunction or emergency surgery.
Collection of Samples
The anterior superior iliac spine was selected as the puncture point, and about 5 mL of bone marrow fluid was aspirated. Blood samples of 5 mL were drawn from the median cubital vein in the morning of the day of surgery. After centrifugation of bone marrow and blood, the supernatant was extracted for HIV RNA detection. The precipitated cells were resuspended and peripheral blood mononuclear cell (PBMC)/bone marrow mononuclear cell (BMMC) were extracted with lymphocyte separation medium for HIV DNA detection. The femoral head samples taken during THA were divided into two halves along the coronal plane, and 3.0mm*3.0mm*3.0mm bone blocks were removed from the subchondral area, necrotic area (where the collapse or cavity is most obvious), sclerotic area (White banded area with significantly increased density at the junction of necrotic and normal areas) and normal area by cylindrical steel sampler. The bone fragment was divided into two parts in half, extracted total DNA and RNA. RNA extraction steps: The bone blocks were put into a mortar and quickly ground with a little liquid nitrogen. Trizol was added at 100mg/mL and thoroughly homogenized. Chloroform, isopropanol and ethanol were then added and centrifuged. Finally, the precipitated RNA was dissolved in DEPC water and stored at −80°C. DNA extraction steps: the bone blocks were ground in the same way, the cells were broken by adding proteinase K and SDS, the proteins were digested, then extracted with phenol and phenol-chloride, and the supernatant was extracted after high-speed centrifugation. Ethanol was added for precipitation and washing, and finally dissolved in TE buffer and stored at −80°C.
HIV DNA/RNA Detection
HIV DNA was extracted from PBMC and BMMC using a nucleic acid extraction kit provided by Guangzhou SUPBIO company, followed by detection of HIV DNA using an HIV-1 DNA test kit (HIV-1 DNA Detection Kit by PCR-Fluorescent Probing, Guangzhou Supbio Company) using a Bio-Rad CFX96 (Bio-Rad Laboratories, Inc.). The same sample was tested twice, and the average of the two results was selected as the final quantitative result. The results were converted into “HIV DNA quantification value/cell quantification value ×106”, and the unit of HIV DNA quantification was “copies/106 PBMCs”. The quantification range of this assay was 20–5×106 copies/106 PBMCs. HIV RNA in the samples was detected using HIV-1 nucleic acid Detection Kit (PCR-Fluorescent Probing) (Guangzhou Supbio Company).
Statistical Analysis
Kolmogorov–Smirnov test was used to evaluate the normal distribution of measurement data, and Levene test was used to test the homogeneity of variance. Normal distribution variables were described by mean ± standard deviation, and non-normal distribution variables were described by median and interquartile ranges (IQR). Two groups of variables with normal distribution were analyzed by two independent sample t-test, and two groups of variables with non-normal distribution were analyzed by Mann–Whitney U-test. Three or more groups of variables were analyzed by one-way ANOVA. We do not log transform any of the data. Statistical analysis was performed using SPSS 22.0 (IBM Corporation, Armonk, New York, USA) and GraphPad Prism 6.0 (GraphPad Software, Inc. La Jolla, CA, USA). p <0.05 was considered statistically significant. In the statistical plots, p < 0.05 was marked as one *p <0.01 as **.
Results
Demographic and Clinical Characteristics of the Patients
A total of 15 patients who met the inclusion and exclusion criteria were included in the study, including 13 males and 2 females, with an average age of 41.8±6.69 years. In fact, obtaining BM was the greatest difficulty in this study because of the decrease in patients after the pandemic and the difficulty in obtaining informed consent. The actual number of recruiters was higher, but part of the iliac bone was too hard to be extracted by BM, so it was eventually abandoned. Except for one case of drug use, one case of blood transfusion and one case of heterosexual sex, the remaining patients were infected with homosexual sex. The mean duration of infection was 5.03±2.99 years. Tenofovir disoproxil/Lamivudine/Efavirenz (TDF+3TC+EFV) was the most commonly used regimen (7 people), followed by Bictegravir sodium/Emtricitabine/Tenofovir Alafenamide Fumarate (5 people). No drug resistance was detected in all patients. All patients came from 8 different provinces in China, with the largest number of patients in Beijing and Hebei Province. The mean BMD was 0.956 kg/m2 at the L1-L4 lumbar spine, 0.872 kg/m2 at the left hip, and 0.876 kg/m2 at the right hip. BMD was about 8% lower at the hip and 35% lower at the ward spot than at the lumbar spine. The basic information of the patients is provided in Table 1. The clinical characteristics are shown in Table 2.
 |
Table 1 Demographic Characteristics of the Patients |
 |
Table 2 Clinical Characteristics of the Patients |
Comparison of HIV Nucleic Acid Between BM and PB
About HIV RNA, 8 patients had blood VL below the detectable level (<20 copies/mL). Fourteen patients had detectable VL in BM, with a median of 255.89 copies/mL. The difference of HIV RNA between blood and bone marrow was statistically significant (P < 0.05) (Figure 1).
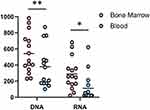 |
Figure 1 Comparison of HIV DNA and HIV RNA in the blood and bone marrow. p < 0.05 was marked as *, p< 0.01 as **. |
Regarding HIV DNA, all samples were detectable. The median of blood HIV DNA was 296.35 copies/106 PBMCs. Bone marrow HIV DNA was 454.31 copies/106 PBMCs. The difference was statistically significant (P < 0.01) (Figure 1).
Comparison of HIV Nucleic Acid in Different Regions of Necrotic Femoral Head
All 15 patients underwent THA. The femoral heads removed during the operation were tested for HIV DNA and RNA. The difference of HIV DNA between necrotic and sclerotic areas was statistically significant (P < 0.05). The difference of HIV RNA between necrotic and normal areas was statistically significant (P < 0.05). There was no difference between any other two groups (Figure 2).
 |
Figure 2 Comparison of HIV DNA and HIV RNA in different regions of necrotic femoral head. p < 0.05 was marked as *. |
In addition, HIV DNA and RNA were measured in synovial tissue taken during the THA. The results were 209.82 and 103.45. Synovial HIV DNA was statistically different from blood, but HIV RNA was not (Figure 3).
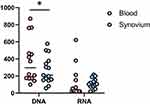 |
Figure 3 Comparison of HIV DNA and HIV RNA in blood and synovium. p < 0.05 was marked as *. |
Discussion
To the best of our knowledge, this is the first study to compare HIV DNA and RNA expression in human BM and PB. In the present study, blood HIV RNA fell below detectable levels in eight individuals and only one in BM. HIV RNA in bone marrow was more than twice that in blood, and HIV DNA was detected in all samples, indicating that although ART can effectively reduce the VL in blood, there is still a large amount of active HIV in BM and constitute a potential HIV reservoir. We speculate that the reasons for this difference between BM and PB are: (1) Difference in drug concentration. ART drug concentrations are tissue-specific, not only the same drug in different tissues, but also different drugs in the same tissue.12–15 The fact that the HIV is active even though the BM does not have obvious tissue barriers such as blood–brain barrier and blood-testis barrier suggests that BM may also be a site that performs specialized functions. (2) Differences in tissue microenvironment. The BM niche is a unique and complex environment that is not yet fully understood. It is composed of bone matrix and various non-hematopoietic cells, including endothelial cells, stromal cells, neuronal cells, and adipocytes.5 The susceptibility of these BM cells in the microenvironment to HIV is still unclear, and the susceptibility of hematopoietic stem and progenitor cells (HSPCs) at the apex of the hematopoietic process to HIV is still controversial.16 (3) Cell migration. HIV-infected CD4+T cells are rapidly depleted in the blood, and the homing theory finds that this population of cells migrates to the iliac and vertebral bone marrow twice as fast as in the regular state. Infected CD16+ monocytes have an increased ability to extravasate from the blood into tissues such as lymph nodes and BM and constitute a potential mechanism for transporting virus between tissue depots.17 (4) Active cell proliferation. BM is the only hematopoietic site in adults, which is rich in cells of different developmental stages of various blood cell lineages.3 Intramedullary cells proliferate rapidly and actively, which is conducive to the transmission of HIV between cells and the formation of a reservoir.
We further examined HIV DNA/RNA in four regions of the necrotic femoral head to provide evidence for revealing the reason for the high incidence of ONFH in PLWH. Numerically, the mean value of HIV nucleic acid in the four regions was significantly lower than that in BM and PB, which is understandable because bone does not have a suitable liquid environment and suitable cell population for HIV to proliferate. In the comparison between the areas, we found that the HIV RNA in the necrotic area was the highest in all regions, which partly explains why the structure of the necrotic area was the most damaged in the four regions. HIV may infect osteoblasts and osteoclasts, which are involved in bone formation and resorption, thus affecting the microstructure of bone trabeculae, leading to collapse of the femoral head. The bone mineral density of the hip is about 8% lower than that of the normal lumbar spine, and the ward point is 35% lower, which also fully supports the degree of collapse of the internal femoral head. Previous studies have also shown that HIV infection and ART drugs also affect bone quality and increase fracture risk in PLWH.18,19 On HIV DNA, the level of necrotic area was the lowest, which was about 45% lower than that of the highest sclerotic area. The necrotic area had the smallest reservoir but the highest HIV RNA, indicating that the necrotic area was the one with the most complete viral expression among the four regions of the femoral head. The sclerosis area is generally considered to be the coexistence of bone repair and osteonecrosis, and the repair rate is higher than that of necrosis. The active proliferation of cells in this area may be the reason for the rich content of HIV DNA. However, may be due to more pronounced immune function in this area, the expression of HIV RNA is not high, and they all present the state of latent virus reservoir. In addition to that, HIV RNA was detected in more than 90% of synovial and HIV DNA was detected in all the specimens. HIV RNA were higher than in all regions of the blood and femoral head, suggesting active HIV status. To the best of our knowledge, this is the first study to target HIV RNA and DNA in different areas of necrotic femoral head and in the synovium. It is worth mentioning that osteonecrosis is very common in PLWH. Many patients not only have ONFH with the highest incidence but also have different degrees of multiple necrosis in the shoulder, elbow, knee and ankle at the same time, which is an increasingly common phenomenon worthy of attention in the clinic.
This study also has some limitations: ① It is a single-center study with a small sample size. Due to our strict inclusion and exclusion criteria and the scarcity of bone marrow samples, we hope to continue to expand our sample pool and longitudinal data of patients at different time points in the future. ② Different ART regimens may have an impact on HIV detection. ③ The four areas of necrotic femoral head were selected according to the general observation, which may cause deviation. ④ The DNA/RNA of femoral head was partially lost in the process of extraction, grinding and lysis, resulting in the low value measured. ⑤ The gender difference in the study population was large, most of them were male, whether the conclusions can be extended to women needs to be further studied.
Conclusion
Despite using ART, there is still substantial active HIV and a potential reservoir in the bone marrow. Viral transcription was most active in the necrotic area of the femoral head, which may indicate that HIV itself is directly involved in ONFH. We recommend that special attention should be paid to the prevention of occupational exposure when bone marrow and femoral head are involved in orthopedic surgery for PLWH, and the virus contained in them is also one of the sources of infection.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was reviewed and approved by the Ethics Committee of Beijing Ditan Hospital, Capital Medical University. The approval number is NO.DTEC-KY2023-022-01. Written informed consent has been obtained from the patients and their anonymous information will be published in this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received for this article.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Du M, Liu M, Liu J. Global, regional, and national disease burden and attributable risk factors of HIV/AIDS in older adults aged 70 years and above: a trend analysis based on the Global Burden of Disease study 2019. Epidemiol Infect. 2023;152:e2. doi:10.1017/S0950268823001954
2. Li K, Liu B, Ma R, et al. HIV Tissue Reservoirs: current Advances in Research. AIDS Patient Care STDS. 2023;37(6):284–296. doi:10.1089/apc.2023.0028
3. Lucas D. Structural organization of the bone marrow and its role in hematopoiesis. Curr Opin Hematol. 2021;28(1):36–42. doi:10.1097/MOH.0000000000000621
4. Comazzetto S, Shen B, Morrison SJ. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev Cell. 2021;56(13):1848–1860. doi:10.1016/j.devcel.2021.05.018
5. Herd CL, Mellet J, Mashingaidze T, et al. Consequences of HIV infection in the bone marrow niche. Front Immunol. 2023;14:1163012. doi:10.3389/fimmu.2023.1163012
6. Zhao R, Ma R, Zhao C, et al. Risk Factors for Osteonecrosis of the Femoral Head in Human Immunodeficiency Virus-Positive Patients: a Retrospective Case-Control Study. AIDS Res Hum Retroviruses. 2022;38(11):869–874. doi:10.1089/AID.2021.0224
7. Morse CG, Mican JM, Jones EC, et al. The incidence and natural history of osteonecrosis in HIV-infected adults. Clin Infect Dis. 2007;44(5):739–748. doi:10.1086/511683
8. Anonymous. Single-Cell Multiomics Reveals Persistence of HIV-1 in Expanded Cytotoxic T Cell Clones - PubMed. n.d. Available from: https://pubmed.ncbi.nlm.nih.gov/35320704/.
9. Avettand-Fènoël V, Hocqueloux L, Ghosn J, et al. Total HIV-1 DNA, a Marker of Viral Reservoir Dynamics with Clinical Implications. Clin Microbiol Rev. 2016;29(4):859–880. doi:10.1128/CMR.00015-16
10. Hong F, Aga E, Cillo AR, et al. Novel Assays for Measurement of Total Cell-Associated HIV-1 DNA and RNA. J Clin Microbiol. 2016;54(4):902–911. doi:10.1128/JCM.02904-15
11. Meini G, Rossetti B, Bianco C, et al. Longitudinal analysis of HIV-1 coreceptor tropism by single and triplicate HIV-1 RNA and DNA sequencing in patients undergoing successful first-line antiretroviral therapy. J Antimicrob Chemother. 2014;69(3):735–741. doi:10.1093/jac/dkt426
12. Labarthe L, Gelé T, Gouget H, et al. Pharmacokinetics and tissue distribution of tenofovir, emtricitabine and dolutegravir in mice. J Antimicrob Chemother. 2022;77(4):1094–1101. doi:10.1093/jac/dkab501
13. Asmuth DM, Thompson CG, Chun T-W, et al. Tissue Pharmacologic and Virologic Determinants of Duodenal and Rectal Gastrointestinal-Associated Lymphoid Tissue Immune Reconstitution in HIV-Infected Patients Initiating Antiretroviral Therapy. J Infect Dis. 2017;216(7):813–818. doi:10.1093/infdis/jix418
14. Aquaro S, Borrajo A, Pellegrino M, et al. Mechanisms underlying of antiretroviral drugs in different cellular reservoirs with a focus on macrophages. Virulence. 2020;11(1):400–413. doi:10.1080/21505594.2020.1760443
15. Rosen EP, Deleage C, White N, et al. Antiretroviral drug exposure in lymph nodes is heterogeneous and drug dependent. J Int AIDS Soc. 2022;25(4):e25895. doi:10.1002/jia2.25895
16. Tsukamoto T. Hematopoietic Stem/Progenitor Cells and the Pathogenesis of HIV/AIDS. Front Cell Infect Microbiol. 2020;10:60. doi:10.3389/fcimb.2020.00060
17. Chen JJ-Y, Huang JC, Shirtliff M, et al. CD4 lymphocytes in the blood of HIV(+) individuals migrate rapidly to lymph nodes and bone marrow: support for homing theory of CD4 cell depletion. J Leukoc Biol. 2002;72(2):271–278. doi:10.1189/jlb.72.2.271
18. Chang C-J, Chan Y-L, Pramukti I, et al. People with HIV infection had lower bone mineral density and increased fracture risk: a meta-analysis. Arch Osteoporos. 2021;16(1):47. doi:10.1007/s11657-021-00903-y
19. McGee DM, Cotter AG. HIV and fracture: risk, assessment and intervention. HIV Med. 2023. doi:10.1111/hiv.13596
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
