Back to Journals » Eye and Brain » Volume 16
Detecting Abnormal Eye Movements in Patients with Neurodegenerative Diseases – Current Insights
Authors Sekar A, Panouillères MT, Kaski D
Received 18 October 2023
Accepted for publication 23 March 2024
Published 9 April 2024 Volume 2024:16 Pages 3—16
DOI https://doi.org/10.2147/EB.S384769
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 7
Editor who approved publication: Dr Rustum Karanjia
Akila Sekar,1 Muriel TN Panouillères,2,3 Diego Kaski1
1SENSE Research Unit, Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, London, UK; 2NeuroClues, Ottignies-Louvain-la-Neuve, Belgium; 3CIAMS, Université Paris-Saclay, Orsay, France
Correspondence: Diego Kaski, SENSE Research Unit, Department of Clinical and Movement Neurosciences, UCL Queen Square Institute of Neurology, London, UK, Email [email protected]
Abstract: This review delineates the ocular motor disturbances across a spectrum of neurodegenerative disorders, including Alzheimer’s Disease (AD) and related disorders (ADRD), Parkinson’s Disease (PD), atypical parkinsonism, and others, leveraging advancements in eye-tracking technology for enhanced diagnostic precision. We delve into the different classes of eye movements, their clinical assessment, and specific abnormalities manifesting in these diseases, highlighting the nuanced differences and shared patterns. For instance, AD and ADRD are characterized by increased saccadic latencies and instability in fixation, while PD features saccadic hypometria and mild smooth pursuit impairments. Atypical parkinsonism, notably Progressive Supranuclear Palsy (PSP) and Corticobasal Syndrome (CBS), presents with distinct ocular motor signatures such as vertical supranuclear gaze palsy and saccadic apraxia, respectively. Our review underscores the diagnostic value of eye movement analysis in differentiating between these disorders and also posits the existence of underlying common pathological mechanisms. We discuss how eye movements have potential as biomarkers for neurodegenerative diseases but also some of the existing limitations.
Keywords: Parkinson disease, eye-tracking technology, eye movements, parkinsonian disorders, neurodegenerative diseases
Introduction
Given that “the eyes are a window to the brain” it is perhaps unsurprising that the ocular motor assessment is a core part of the neurological examination. Disturbances in eye movements are indicative of pathological changes in the brain associated with neurodegeneration. The control of eye movements is governed by several brain regions spanning the brainstem, basal ganglia, cerebellum and the cerebral cortices. Our growing understanding of the neural circuits underpinning ocular motor function means that eye movement abnormalities can be, to some extent, localised to specific neurological lesions. Moreover, given a widespread distributed network for eye movement control, neurodegenerative disorders that result in early focal, and later diffuse pathology, commonly manifest eye movement abnormalities, creating a window not only to the brain, but also to pathological processes that affect it.
Eye movements are a useful tool in neurological practice. Firstly, an ocular motor examination is a non-invasive bedside test that is comparatively quicker to perform than other standard assessments (eg cognitive tests). Secondly, with advances in eye tracking technology, it is now possible to acquire precise quantitative metrics of ocular motor disturbance increasing the diagnostic value of eye movements. Finally, the degrees of freedom associated with eye movements are less compared to the assessment of symptoms such as tremor, therefore there is limited scope for variability and subjectivity.
Whilst several studies and reviews1 have provided an overview of ocular motor disturbance in neurodegenerative conditions, a discussion of the practical application of these findings in the differential diagnosis of neurodegenerative disorders is limited. Whether ocular motor findings can be used to feed predictive models2,3 for differential diagnosis has not yet been established. Furthermore, ocular motor tests or paradigms can be clinical - those that can be performed at the bedside - or experimental, requiring assessments to be performed using an eye tracker and screen. There has been perhaps less focus on bridging the gap between clinical and experimental ocular motor assessments that could be of diagnostic utility to patients or indeed be part of biomarkers for disease or treatment monitoring. Synthesis of these findings from both modes of acquisition and exploration of subtypes within class of eye movements such as saccades and pursuit and methods to quantify eye movements at the bedside is necessary to establish eye movements as a tool for differential diagnosis.
Here, we provide a brief overview of the different classes of eye movements, how these are assessed clinically and how they are recorded experimentally, and how these become abnormal across a range of neurodegenerative disorders, focusing on Alzheimer’s disease (AD), and related disorder (ADRD), Parkinson’s disease (PD) and atypical parkinsonism. The review aims to provide a practical approach to eye tracking that can be employed to acquire high-quality clinical data.
Classes of Eye Movements
Eye movements broadly fall under two groups, ones that stabilise the image on the retina and those that shift the field of vision to bring the area of interest into focus.1,4 Within these two categories, there are several functional classes of eye movements, each intended to serve a specific purpose. Here, we summarise these and provide a list of the key terms used in ocular motor research and their definitions (Table 1).
 |
Table 1 Key Terms in Ocular Motor Research, Examinations and Analysis and Their Definitions |
Saccades (Figure 1) are rapid eye movements that shift the field of vision from one point of fixation to another. There is a wide spectrum of saccades ranging from reflexive prosaccades driving gaze towards a novel stimulus, to more complex volitional saccades elicited voluntarily towards a stable target in the environment. Among volitional saccades, memory guided saccades involve fixating towards a previously remembered target and anti-saccades, move the field of vision away from a visual target. The saccade parameters (speed, accuracy, duration, latency) and saccadic intrusions such as square-wave jerks provide useful information both to assess functioning of the saccadic system and to provide insight into the localisation of brain damage.5–7
Vergence eye movements can both stabilise the image on the retina and shift the field of view. Vergence eye movements allow us to track the depth of a moving object ie, either moving towards us or away from us. Divergence occurs when an object of interest is moving from near to far and convergence where an object being tracked is being displaced from far to near.8 In both cases, the eyes are moving in the opposite direction. Abnormalities of convergence are assessed using the near point of convergence test (the closest point to one’s face) at which a single image can be perceived or positive fusional vergence test.9 Note that accommodation is tested by shifting the visual focus from distant objects to near objects.
We distinguish several types of movements that stabilise the image on the retina:
- To achieve maximum visual acuity, the displacement of the image from the centre of the fovea must be limited which involves making small eye movements called fixational eye movements. There are three categories of fixational eye movements, tremor, drifts and microsaccades with tremor being the smallest and microsaccades being the largest. Abnormalities in fixational eye movements present in the form of nystagmus, saccadic intrusions – particularly square wave jerks - and gaze distractibility.10–12 Abnormal eye movements can be detected during the attempted fixation period. Nystagmus describes an involuntary oscillatory eye movement either in the horizontal, vertical, or torsional (rotatory) direction. Nystagmus has two phases, the slow phase which shifts the eye away from the target and the fast phase which bring back the eyes to the target and is traditionally used to classify the direction of the nystagmus.13 The presence of nystagmus is classified as abnormal and can be associated with a range of neurological disorders (eg, cerebellar, cortical, or vestibular lesions)14 or may occur as part of congenital nystagmus (not covered in this review).15
- Smooth pursuit eye movements allow us to follow a target as it moves within the visual field therefore cannot be elicited in the absence of a visual target. If a target is moving at a faster speed than the visual system can process, approximately around 30 degrees/second, catch-up saccades are elicited to keep the target in the line of sight.16 Abnormal pursuit occurs when the ocular velocity is unable to match that of the target and catch-up saccades are performed in speeds slower than 30 degrees/second. Such saccades, or saccadic intrusions give rise to the “broken” appearance of pursuit movements, sometimes referred to as “saccadic pursuit”.17
- Two classes of eye movements stabilise the image on the retina during head movements, vestibular eye movements hold the image during brief head rotations and translations18 whereas optokinetic eye movements hold the image steady during sustained visual motion. Impaired vestibular and optokinetic reflexes lead to the loss of visual acuity during head movements or visual motion.19,20
Methods of Recording Eye Movements
Eye movement recording methods play a pivotal role in understanding visual perception, cognitive processes, and diagnosing eye movement disorders. Several techniques are employed for this purpose, including video oculography (VOG) which can use either infrared or non-infrared oculography, electro-oculography (EOG) and magnetic search coils.
VOG, also known as video-based eye tracking, employs high-speed cameras to capture images or videos of the eye, enabling researchers to analyse eye movement patterns. This method is highly accurate and provides precise spatial and temporal information regarding eye movements, including saccades, smooth pursuit, and fixations.21 Infrared oculography is a technique that uses infrared light sources and detectors to track eye movements. These systems typically illuminate the eye with infrared light and capture the reflection off the cornea and/or pupil. Infrared oculography is non-invasive and works well in low-light conditions.22 Non-infrared oculography, in contrast to its infrared counterpart, uses visible light sources and cameras to record eye movements. Non-infrared oculography systems may use visible light reflections from the cornea or pupil to monitor eye movements.23 It is particularly useful for studying eye movements in well-lit environments and can provide high-resolution tracking of gaze points.24 VOG is widely used in both research and clinical settings for diagnosing and monitoring eye conditions like strabismus and nystagmus. However, the use of VOG can be challenging in cases where detecting the pupil is difficult because of severe disease progression resulting in droopy eyelids, excessive blinking and inability to keep the eye open,25 features that can be seen across a range of neurodegenerative conditions.
EOG is an electrophysiological technique that measures the electrical potential difference between different points on the skin around the eye. This method relies on the fact that the cornea and retina have distinct electrical potentials, and as the eye moves, the potential difference changes.26 Electrodes are typically placed near the corners of the eyes to record these changes. EOG is especially useful for detecting slow eye movements, such as those involved in reading or tracking slowly moving objects. Compared to VOG, EOG is more cost-effective but is associated with noise in horizontal and vertical signals and has a longer setup time.27
Magnetic search coils represent a sophisticated method for recording eye movements, being more reliable than EOG and VOG with high spatial and temporal resolution. The coils utilize the principles of electromagnetic induction, allowing for precise tracking of the eye’s movements by measuring changes in magnetic fields generated by miniature coils placed near the eye.28 There are two key drawbacks with the use of magnetic search coils: firstly, it is invasive and requires the administration of anaesthetic drops29 and secondly, there are artifacts from eyelid motion during vertical eye movement recordings.22
Whilst clinical assessment can be informative, training in the execution and interpretation of these eye movements is often lacking in most medical (including neurology) training programmes. Recording of eye movements thus provides quantitative data of eye movements (Table 2) that standardises the assessment and allows more detailed (offline) interpretation and review.
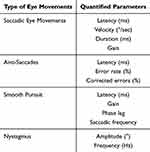 |
Table 2 Quantitative Metrics Acquired from Classes of Eye Movements |
VOG allows objective recording of the presence of specific deficits in one or another of the eye movement parameters. Indeed, in certain cases, the saccadic eye movements of a patient may be slow, but the clinician may struggle in identifying whether it is due to a prolonged latency or a decreased velocity. An eye tracking examination will be able to decipher between these two potential alterations, and thus lead to very different interpretation (eg: a delayed latency can be found in Posterior Cortical Atrophy due to occipital/parietal dysfunction, while reduced velocity is present in Progressive Supranuclear Palsy). One of the drawbacks of using an eye tracker is that, in order to limit the variability and to make sure to focus only on eye movements, the patient is often in a very constrained environment (eg: head stabilised with a chin-rest or bite bar). This ensures that only eye movements are used by the patient, but it does not allow evaluation of whether the patient uses the head or the entire trunk to compensate for some deficits. Despite a huge amount of research having been done on eye movements in the past 120 years, little of this has reached clinical practice. This is probably due to the fact that indeed little training is provided in most medical training programmes and that most of the available tools are fit for research purpose but not clinical practice.
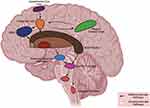
Ocular Motor Abnormalities in Neurodegeneration
Figure 2 summarises the main ocular motor abnormalities seen in patient with neurodegenerative diseases, based on key research publications.
Alzheimer’s Disease
The use of eye movements in the clinical diagnosis of AD is not widespread as there remains a debate as to whether there are specific ocular motor abnormalities.1 This may reflect differences in experimental paradigms and patient heterogeneity (related to disease onset and duration). Several studies have found correlations between neuropsychological scores and eye movements suggesting that cognitive impairment and loss of executive function from atrophy in the cortices may be driving the ocular motor abnormalities.30–32 In experimental settings, studies have revealed abnormalities such as instability in fixation,33 saccadic abnormalities primarily increased latency and increased errors during the anti-saccade task associated with a reduced number of self-corrections.30,31,34
Saccadic latencies in prosaccades are found to be correlated with dementia and executive functioning in AD with anti-saccade latencies increasing from mild cognitive impairment (MCI), a percussor to AD, to patients with a confirmed diagnosis of AD.35 Additionally, MCI and AD also produce greater numbers of pro-saccades and have shorter fixation periods as the complexity of the visual stimuli increases compared to healthy controls, suggestive of a dysfunction in both the occipito‐temporal network and the occipitoparietal network.36,37 Increased saccadic latencies and increased anti-saccades error rates coupled with little self-corrections and shorter fixation durations are distinct features of AD with papers consistently reporting findings within these eye movement classes. While abnormal fixational eye movement can be clinically seen, widespread use of saccades requires the quantification of saccadic metrics at the bedside potentially using a portable eye tracker. Furthermore, eye movements with voluntary and memory components have been under explored, perhaps providing better insights into the cognitive decline of AD. Lastly, eye movement abnormalities (increased error rate) have been noted in the prodromal stages of AD in anti-saccade tasks.38
Posterior Cortical Atrophy
Posterior cortical atrophy (PCA) is regarded as the visual variant of AD. Indeed, it has a similar pathology with defective beta-amyloid and tau protein but associated with atrophy in the parietal, occipital and temporal cortices resulting in a decline in visual perception.39 There have been to date very few studies exploring ocular motor findings in patients with PCA. Overall, PCA presents with deficits in saccades, fixation and smooth pursuit although the data in a clinical setting are limited and as for AD, appears to be non-specific, reflecting widespread cortical and sub-cortical involvement at the time of testing. Abnormal fixation in PCA was found to be associated with large saccadic intrusions at a higher frequency, while smaller square wave jerks were more prevalent during prolonged fixation periods in AD.40–43
PCA patients have difficulty initiating visually guided saccades, visible through an increase of saccade latency.40 A single-case study evaluated both visually guided saccades and memory guided saccades in a patient with PCA; for the visually guided saccades, they corroborated previous findings of impaired saccades arising from weak inputs from the occipital-parietal cortex.44 They also revealed that both visually guided saccades and memory guided saccades were consistently hypometric.44 Smooth pursuit profiles in PCA closely resembled those in AD with decreased pursuit gain compared to controls and the presence of saccadic instructions.40 In one case study, the increased saccade latency in a patient was the first argument for further investigation that was able to lead the clinician to provide the diagnosis of PCA.
Overall, fixational (square wave jerks) and saccadic (increased pro-saccadic latency, saccadic hypometria) are common but non-specific findings of PCA.
Frontotemporal Dementia
Frontotemporal dementia (FTD) has three subtypes: behavioural variant, progressive non-fluent aphasia and semantic dementia.45 Patients with progressive supranuclear palsy (PSP) or corticobasal syndrome (CBS) can also present with FTD pathology. The experimental data in FTD is conflicting therefore eye movement abnormalities are not considered a clinically useful measure to identify fronto-temporal degeneration. Some studies have found impaired pursuit while others have reported normal smooth pursuit movements.46,47 In saccades, while some researchers have shown increased saccadic latency, decreased velocity and lower accuracy associated with atrophy in the frontal eye fields and cingulate eye fields48–50 others have reported normal velocity and no significant difference in accuracy compared to controls.46,49 Consensus, however, has been found in anti-saccade tasks where patients with FTD had increased error rates compared to controls51 in keeping with the role of the frontal lobes in inhibiting pro-saccades. Compared to AD, PSP and CBS, patients with FTD were able to spontaneously self-correct anti-saccadic errors,47 which is intriguing and suggests that at least in some patients with FTD, attentional mechanisms may be able to override more reflexive frontal lobe dysfunction.
Patients with FTD are unable to fixate on points for prolonged durations with frequent saccadic intrusions pointing towards malfunctioning in the saccadic inhibition pathway and atrophy in the orbitofrontal cortex.52,53 One group assessed spatial anticipation in FTD using an ocular motor adaptation of the Brixton spatial anticipation test, where patients predict where a target would appear and execute the eye movement based on a set rule. After applying machine learning techniques, the authors were able to differentiate between controls and FTD in all trials,54 suggesting the combination of machine learning and ocular motor metrics has the potential to provide diagnostic data. However, the study compared FTD with controls where there are likely to be significant differences; comparison between other forms of neurodegeneration is essential to establish proof of concept.
Key ocular motor features of FTD are increased anti-saccadic errors and increased fixational eye movements (“sticky eyes”), both of which can be clinically assessed without an eye tracker. However, eye movements alone are of insufficient diagnostic value for FTD.
Parkinson’s Disease
In PD, eye movement abnormalities tend to be present in later stages of the disease and are not as prominent as those observed in atypical Parkinsonism. Saccadic hypometria and the presence of square-wave jerks are two fairly consistent ocular motor features of PD, together with impaired smooth pursuit. Saccades in PD are primarily characterised by hypometria due to the affected outputs from the substantia nigra pars reticulata projections to the superior colliculus.55 Some studies have also found increased latency in voluntary saccades with reflexive saccades being more preserved in PD compared to healthy controls56 and also found an association with saccadic latency and disease duration.57 The associated hypometria and latency has also been seen for memory guided saccades in PD, with voluntary saccades being most significantly affected compared to reflexive saccades and memory guided saccades.55 Saccades in the vertical direction are slightly more affected in PD compared to the horizontal direction.1 PD patients exhibit impaired ability to suppress reflexive behaviours, as in the case of anti-saccade tasks. However, research findings on anti-saccades in PD are conflicting. Some studies report higher anti-saccade error rates, even in early PD stages, while others find no substantial differences in error rates or latency across PD progression.58–61
A recent study was able to demonstrate that the use of eye-tracking could help capture saccadic bradykinesia in PD.62 Such a measure could contribute to objectively monitoring disease progression and further research is needed to see the potential correlations with bradykinesia assessed with the Unified Parkinson’s Disease Rating Scale (UPDRS), a rating scale assessing the motor and non-motor symptoms of PD.63 Another study was also able to show that the increase in anti-saccades latency could be a predictive marker to develop gait freezing in patients with PD.64
PD patients commonly display abnormalities in smooth pursuit eye movements, with reduced smooth pursuit gain. These abnormalities are often mixed with saccadic pursuit, particularly in advanced PD patients, possibly due to a failure to control extraneous saccades or executive and attentional dysfunction.65–67 Similar to saccades, smooth pursuit movements in the vertical direction were more abnormal than in the horizontal direction possibly related to task difficulty in overcoming gravity.68
From a practical perspective, patients with idiopathic PD tend to have normal eye movements but may manifest hypometric voluntary (more than reflexive) saccades. Repetitive saccades may decrement in velocity also over time (saccadic bradykinesia) but this can only be reliably identified on oculography.
Progressive Supranuclear Palsy
PSP is driven by the accumulation of tau protein in the brain69 and the most prominent clinical signs are postural instability, akinesia accompanied with gait freezing, cognitive dysfunction particularly in language and speech components, and ocular motor disturbances including vertical supranuclear gaze palsy, slowed vertical saccades and increased square wave jerks.70 Disproportional impairment of vertical saccadic velocity compared to horizontal saccades, known as vertical supranuclear gaze palsy, is regarded as one of the first core clinical features of PSP.70 The decreased velocity in the vertical plane leads to more continuous oblique like saccades, called “round the houses” trajectory.71 This points towards the involvement of brainstem and is supported by the presence of other indicators of brainstem dysfunction such as increased square wave jerks and gaze instability as seen in PSP.72 High frequency and high amplitude square wave jerks are most prominent in PSP compared to other Parkinsonian syndromes and are mediated by the supratentorial cortical structures in the temporal lobe.73 In a comparative study between amyotrophic sclerosis, PSP and controls, it was found that the occurrence of square wave jerks is a function of the amplitude of the saccadic intrusions rather than the group.74 However, this was not considered for other Parkinsonian syndromes such as MSA and CBS where square wave jerks are distinctly present. Later stages of PSP is identified with a marked decrease in voluntary vertical gaze which eventually progresses into the horizontal plane.75
Saccades in PSP are markedly hypometric which can also be attributed to the decreased firing of excitatory burst neurones in the brainstem.76,77 Some reports have presented a “zig-zag” or “saw tooth” pattern where hypometric vertical saccades had a horizontal component, alike round the houses.78,79 This pattern could be the combination of square wave jerks and hypometric vertical saccades pointing towards increased disease as a major compensatory mechanism employing more horizontal saccades and impairment of inhibitory burst neurones.80 Volitional and memory guided saccade function are less reported in PSP. A study found that volitional saccades are less accurate and have lower velocity compared to reflexive saccades in both healthy controls and PSP suggesting a selective impairment of networks in the frontal eye fields.81,82 A more clinically relevant finding here was that the velocity and accuracy of saccades did not decrease over one-minute time scales contrary to what was observed in PD.62,81 In PSP, smooth pursuit movements have small saccades leading to a saccadic pursuit or cogwheel pursuit.83 Optokinetic nystagmus in PSP is also impaired by the eye drifting in the direction of the slow phase during the fast phase13 and this can be a useful clinical sign. Loss of quick phases in torsional vestibulo-ocular reflexes has also been found.84 PSP is also associated with a strong disinhibition characterised by a large increase of error rates in anti-saccade tasks.
The core ocular motor features of PSP are thus slowed and eventually limited vertical eye movements, frequent square wave jerks, broken smooth pursuit, and large error rates in anti-saccades, the combination of which is likely of diagnostic value.
Multiple System Atrophy
MSA is an α-synucleinopathy divided into two distinct subtypes, MSA-P, parkinsonian subtype and MSA-C, cerebellar subtype. Each subtype presents with different clinical features and progression patterns, with MSA-C exhibiting cerebellar signs such as ataxia and MSA-P primarily showing bradykinesia and autonomic dysfunction. There are however features of MSA-C type seen also in MSA-P, making it difficult to differentiate between these subtypes85 and most studies exploring eye movement function in MSA have not made clear distinction between the two. Clinically, while assessing eye movements, prominent signs are excessive square-wave jerks, mild supranuclear gaze palsy, gaze-evoked nystagmus, positional down-beat nystagmus, saccadic hypometria, impaired smooth pursuit, and reduced vestibulo-ocular reflex (VOR) suppression, reflective of the underlying pathology in the striato-nigral and olivopontocerebellar systems.1
Smooth pursuit gain in MSA is lower than healthy controls86 and a study by Zhou et al reported that patients with MSA-C had lower smooth pursuit gain compared to patients with MSA-P.87 Although smooth pursuit deficits in MSA and PSP are similar, PSP was not associated with volume loss in the pontocerebellar region,88 a core neural substate for smooth pursuit movements.89 This may be because smooth pursuit involves several other neural pathways or that disease-specific compensatory mechanisms are different. Saccadic intrusions are present in smooth pursuit eye movements in MSA.90 While MSA-C patients present with “broken-up” pursuit movements due to decreased smooth pursuit gain, MSA-P can also have anticipatory saccades wherein the eyes anticipate the moving target.66 Saccadic velocities are regarded to be normal in MSA compared to other Parkinsonian syndromes with mild-moderate saccadic hypometria91 and some patients also having mild vertical supranuclear gaze palsy.86 In anti-saccade tasks, MSA patients (sub-type not specified) have a higher error rate than PD and controls and anti-saccade latencies did not improve over time as seen in controls and PD suggesting a possible progression marker.92
Compared to PD and CBS, patients with MSA have a high frequency of square wave jerks, although less than that seen in patients with PSP.93,94 Additionally, square wave jerks in MSA are occasionally larger in amplitude resulting in a feature known as macro-square wave jerks.95 Cerebellar signs in MSA-C are associated with gaze-evoked, downbeat, and rebound nystagmus.96 The VOR suppression in MSA is abnormal clinically, a differentiating feature from other Parkinsonian sndromes.97 MSA-P patients with no clinical cerebellar signs can also exhibit cerebellar ocular motor abnormalities making the differential diagnosis difficult.98
Therefore, the combination of increased square wave jerks, saccadic hypometria, saccadic smooth pursuit and VOR suppression failure could strongly point towards MSA-P rather over PD, where cerebellar features are not to be expected. Lastly, convergence eye movements are predominantly preserved in MSA whereas abnormalities are likely to be noted in Dementia with Lewy Bodies (DLB) and PD.68
Corticobasal Syndrome
CBS is a four-repeat tauopathy, defined by the accumulation of the specific isoform of tau proteins with four microtubule domains.99 The motor symptoms of CBS include limb rigidity, myoclonus, limb dystonia and “alien limb syndrome” which manifests as involuntary limb movements and apraxia characterised by the inability to carry out limb movements.100,101 Apraxia also manifests in ocular motor dysfunction with CBS patients having saccadic apraxia or increased saccadic latency, which is the time elapsed between the appearance of a target and the initiation of a saccade.93 As degeneration in CBS is often lateralised, the saccadic latency is also likely to be ipsilateral to the side experiencing apraxia.86,102 Although saccadic latency is affected in CBS, saccadic metrics and velocity are normal compared to PD and PSP, providing a key clinical differentiator.103
CBS patients have a high error rate and increased latencies in anti-saccade tasks compared to controls and PD patients and are also unable to self-correct errors in anti-saccade tasks spontaneously.47,104 Atrophy in the parietal cortex supports the increased latency in prosaccades and a dysfunction in the frontal eye field could suggest a common mechanism for the latencies in both prosaccades and anti-saccades. Interestingly, when single tasks with either prosaccades or anti-saccades were performed in an experimental setting in PD, PSP, and CBS, it was found that anti-saccade error rates were similar in CBS and PD with PSP having much higher error rates. However when mixed tasks were performed, CBS patients showed a significant increased prosaccade and anti-saccade error rate suggesting anti-saccades have differential effects in single and mixed tasks.104
Other eye movement abnormalities can occur in people with CBS; however, they are often milder than those in people with other Parkinsonian disorders. Two further characteristics of CBS include impaired OKN and increased square wave jerks.13 Studies have also revealed reduced pursuit gain, restricted movement, and abnormal smooth pursuit with abrupt “jolting” eye movements.93,105 Vertical supranuclear gaze palsy is observed in varying degrees in CBS with corticobasal degeneration pathology and is less prevalent in the early stages. Vertical supranuclear gaze palsy can be present in up to 20% of patients early in the disease and up to 50% of patients overall in cases with CBS with PSP pathology.93,106 Variations have been noted in atypical CBS instances, such as impaired vertical saccades and an early lowering of horizontal saccade velocity.105 These changes, however, rarely entail early square-wave jerks or increased saccadic delay, which are more typical of PSP.
Overall, CBS is defined by ocular motor apraxia, with increased latency of voluntary saccades, that may be lateralised to the most affected apraxic (limb) side.
Dementia with Lewy Bodies
Dementia with Lewy bodies is pathologically associated with the accumulation of α-synuclein protein into Lewy bodies and Lewy neurites.107 Although patients with DLB show signs of cognitive impairment similar to AD, the clinical manifestations of DLB are more closely related to PD dementia (PDD).103 Notable symptoms include abnormal sleep patterns, visual hallucinations, fluctuations in attention and impaired visual perception.108 Systematic reporting of ocular motor disturbance in DLB is limited both clinically and experimentally.
A study involving 20 DLB patients compared their saccadic eye movements with those of individuals with PDD. The results revealed that DLB patients exhibited impairments in both reflexive and voluntary saccadic execution, along with difficulties in performing more complex saccadic eye movement tasks (saccadic prediction, decision, and anti-saccades).103 Compared to AD and PD, DLB showed more prominent signs of saccadic deficits possibly because of the neurodegeneration seen in cortical and subcortical regions.109 Other studies have revealed prolonged latency in various types of horizontal saccades, impaired predictive saccades, and difficulties in suppressing saccades.103,110 Another detailed examination of horizontal and vertical saccades in DLB patients demonstrated a reduced tendency to produce saccades with a shorter latency also known “express saccades” in visual tasks where there is a gap between the fixation target and the saccade target.103 Additionally, these DLB patients exhibited reduced saccade velocity and accuracy and increased saccadic latency in reflexive and voluntary saccades which increased with disease severity.102
Case reports have described a subset of DLB patients who present with vertical gaze palsies, often accompanied by horizontal gaze abnormalities.111–113 These cases could potentially be mistaken for PSP, underscoring the need for caution when interpreting vertical gaze palsy deficits and distinguishing between various Parkinsonian syndromes. Convergence insufficiency has been observed in DLB (and may eventually be followed by akinesia and rigidity),114 but these are not disease-specific.
In most cases DLB is seen with increased saccadic latency, reduced gain and increased number of errors in complex saccadic tasks.
Future Directions
In this review, we summarised the ocular motor impairments seen in Parkinsonian syndromes as well as in Alzheimer’s disease and related disorders. Eye tracking has been used in experimental setting for more than 120 years but has only recently entered routine clinical practice in movement disorders and cognitive neurology. The ocular motor assessment aids clinicians to provide quantitative and unbiased markers for diagnosis, disease progression and monitoring as well being of possible prognostic value. However, given the overlap of ocular motor disturbances across neurodegenerative disorders and the expertise required to operate certain eye-trackers, the translation of oculography from “bench to bedside” is still evolving. Experimental setups can provide intricate and detailed data about ocular motor abnormalities, but their use is restricted due to their desktop-mounted setup for eye recordings in a clinical setting. New technologies for clinical practice need to emerge through the development of mobile eye tracking devices, either using a portable eye tracker or developing smartphone-based approaches.115 These new technologies should allow for an easy ocular motor assessment that could be performed both by specialists and non-specialists. However, they face the challenge of providing a lower sampling rate than experimental set-up and this will need to be controlled to ensure still a high quality of data so that markers extracted from such device are reliable and informative. With recent progress in machine learning could emerge some powerful algorithms that would help in classifying patients in this continuum of neurodegenerative diseases. Machine learning techniques may also allow identification of more complex patterns of eye movements that will both contribute to diagnosis and to predict disease trajectory.116 This contribution to diagnosis could be a major step to help triage of patients in pharmaceutical trials.
It is well known that in neurodegenerative diseases such as PD and AD, the neural degeneration starts fifteen to twenty years prior the onset of symptoms.117,118 In the coming years, longitudinal studies of large cohorts using eye-tracking and machine learning could allow us to identify the ocular motor markers that are modified at a pre-symptomatic step of the disease. Screening within the general population could be of particular interest given new developments in disease-modifying treatments aimed at slowing down neuronal death.
Conclusions
The study of eye movements offers a unique window into the brain’s health and dysfunction. As our understanding of the neural circuits governing eye movements and their alterations in neurodegenerative diseases continues to grow, so does the potential for improving early diagnosis and patient care in these challenging conditions. However, challenges persist in the clinical application of these assessments, including the need for differentiation among various neurodegenerative disorders with overlapping eye movement abnormalities. To address this, future research should focus on developing mobile eye tracking solutions that are adaptable to clinical settings, harnessing the power of machine learning to refine diagnostic models, and ensuring the reliability of eye movement measurements.
This review of ocular motor disturbances in neurodegenerative diseases elucidates significant disparities and commonalities among disorders like AD, PD, and various forms of atypical parkinsonism, providing a nuanced perspective on their diagnostic and pathological implications. Notably, the distinct ocular motor features identified in each condition—ranging from saccadic abnormalities in AD to the pronounced vertical gaze palsy in PSP—underscore the potential of ocular motor assessments as tools for early diagnosis and monitoring disease progression. Furthermore, the shared patterns of eye movement disturbances across different diseases hint at possible common underlying neurodegenerative processes, offering intriguing avenues for future research. To harness the full diagnostic potential of eye movement analysis, advancements in technology and machine learning are imperative for developing accessible, accurate, and clinician-friendly eye-tracking systems. Ultimately, this review highlights the role of eye movement studies in unravelling the complex interplay of neurodegenerative diseases, paving the way for novel diagnostic strategies and a deeper understanding of their shared pathological foundations.
Abbreviations
AD, Alzheimer’s Disease; PD, Parkinson’s Disease; MCI, Mild Cognitive Impairment; FTD, Frontotemporal dementia; PCA, Posterior cortical atrophy; PSP, Progressive Supranuclear Palsy; CBS, Corticobasal Syndrome; DLB, Dementia with Lewy Bodies; VOG, Video Oculography; EOG, Electro-Oculography.
Disclosure
Dr Muriel Panouillères is an employee of P3Lab company that develops a medical eyetracking device. The authors report no other conflicts of interest in this work.
References
1. Anderson TJ, MacAskill MR. Eye movements in patients with neurodegenerative disorders. Nat Rev Neurol. 2013;9(2):74–85. doi:10.1038/nrneurol.2012.273
2. Przybyszewski AW, Śledzianowski A, Chudzik A, Szlufik S, Koziorowski D. Machine learning and eye movements give insights into neurodegenerative disease mechanisms. Sensors. 2023;23(4):2145. doi:10.3390/s23042145
3. Birawo B, Kasprowski P. Review and evaluation of eye movement event detection algorithms. Sensors. 2022;22(22):8810. doi:10.3390/s22228810
4. Leigh RJ, Zee DS. The Neurology of Eye Movements.
5. Terao Y, Fukuda H, Hikosaka O. What do eye movements tell us about patients with neurological disorders? An introduction to saccade recording in the clinical setting. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(10):772–801. doi:10.2183/pjab.93.049
6. Calikusu FZ, Akkus S, Kochan Kizilkilic E, et al. Atypical findings: atypical parkinsonian syndromes or Atypical parkinsonian syndromes look-alikes. Clin Neurol Neurosurg. 2023;233:107975. doi:10.1016/j.clineuro.2023.107975
7. Irving EL, Steinbach MJ, Lillakas L, Babu RJ, Hutchings N. Horizontal saccade dynamics across the human life span. Invest Ophthalmol Vis Sci. 2006;47(6):2478–2484. doi:10.1167/iovs.05-1311
8. Mays LE. Neural control of vergence eye movements: convergence and divergence neurons in midbrain. J Neurophysiol. 1984;51(5):1091–1108. doi:10.1152/jn.1984.51.5.1091
9. Lavrich JB. Convergence insufficiency and its current treatment. Curr Opin Ophthalmol. 2010;21(5):356–360. doi:10.1097/ICU.0b013e32833cf03a
10. Abadi RV, Gowen E. Characteristics of saccadic intrusions. Vision Res. 2004;44(23):2675–2690. doi:10.1016/j.visres.2004.05.009
11. Lemos J, Eggenberger E. Saccadic intrusions: review and update. Curr Opin Neurol. 2013;26(1):59–66. doi:10.1097/WCO.0b013e32835c5e1d
12. Herishanu YO, Sharpe JA. Normal square wave jerks. Invest Ophthalmol Vis Sci. 1981;20(2):268–272.
13. Kassavetis P, Kaski D, Anderson T, Hallett M. Eye movement disorders in movement disorders. Mov Disord Clin Pract. 2022;9(3):284–295. doi:10.1002/mdc3.13413
14. Shemesh AA, Zee DS. Eye movement disorders and the cerebellum. J Clin Neurophysiol. 2019;36(6):405–414. doi:10.1097/WNP.0000000000000579
15. Zahidi AA, Woodhouse JM, Erichsen JT, Dunn MJ. Infantile nystagmus: an optometrist’s perspective. Clin Optom. 2017;9:123–131. doi:10.2147/OPTO.S126214
16. Schraa-Tam C. Functional MRI Studies into the Neuroanatomical Basis of the Eye Movements [Ph.D. thesis]. Erasmus MC: University Medical Center Rotterdam; 2009.
17. Sharpe J, Wong AM. Anatomy and physiology of ocular motor systems. Walsh Hoyt’s Clin Neuro Ophthalmol. 2005;16:809–885.
18. Szmulewicz DJ, Waterston JA, MacDougall HG, et al. Cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS): a review of the clinical features and video-oculographic diagnosis. Ann N Y Acad Sci. 2011;1233(1):139–147. doi:10.1111/j.1749-6632.2011.06158.x
19. Wang L, Söderberg PG, Tengroth B. Influence of target direction, luminance and velocity on monocular horizontal optokinetic nystagmus. Acta Ophthalmol. 1993;71(5):578–585. doi:10.1111/j.1755-3768.1993.tb04645.x
20. Cheng M, Outerbridge JS. Optokinetic nystagmus during selective retinal stimulation. Exp Brain Res. 1975;23(2):129–139. doi:10.1007/BF00235455
21. Wade NJ, Tatler BW, Heller D. Dodge-ing the issue: dodge, Javal, Hering, and the measurement of saccades in eye-movement research. Perception. 2003;32(7):793–804. doi:10.1068/p3470
22. Leigh RJ, Zee DS. The Neurology of Eye Movements. Oxford University Press; 2015.
23. Friedrich MU, Schneider E, Buerklein M, et al. Smartphone video nystagmography using convolutional neural networks: conVNG. J Neurol. 2023;270(5):2518–2530. doi:10.1007/s00415-022-11493-1
24. Turuwhenua J, Yu TY, Mazharullah Z, Thompson B. A method for detecting optokinetic nystagmus based on the optic flow of the limbus. Vision Res. 2014;103:75–82. doi:10.1016/j.visres.2014.07.016
25. Pleshkov M, Zaitsev V, Starkov D, Demkin V, Kingma H, van de Berg R. Comparison of EOG and VOG obtained eye movements during horizontal head impulse testing. Front Neurol. 2022;13:917413. doi:10.3389/fneur.2022.917413
26. Creel DJ. The electrooculogram. Handb Clin Neurol. 2019;160:495–499.
27. Eggert T. Eye movement recordings: methods. Dev Ophthalmol. 2007;40:15–34.
28. Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145.
29. Newman JL, Phillips JS, Cox SJ. Reconstructing animated eye movements from electrooculography data to aid the diagnosis of vestibular disorders. Int J Audiol. 2022;61(1):78–83. doi:10.1080/14992027.2021.1883196
30. Currie J, Ramsden B, McArthur C, Maruff P. Validation of a clinical antisaccadic eye movement test in the assessment of dementia. Arch Neurol. 1991;48(6):644–648. doi:10.1001/archneur.1991.00530180102024
31. Crawford TJ, Higham S, Renvoize T, et al. Inhibitory control of saccadic eye movements and cognitive impairment in Alzheimer’s disease. Biol Psychiatry. 2005;57(9):1052–1060. doi:10.1016/j.biopsych.2005.01.017
32. Heuer HW, Mirsky JB, Kong EL, et al. Antisaccade task reflects cortical involvement in mild cognitive impairment. Neurology. 2013;81(14):1235–1243. doi:10.1212/WNL.0b013e3182a6cbfe
33. Molitor RJ, Ko PC, Ally BA. Eye movements in Alzheimer’s disease. J Alzheimers Dis. 2015;44(1):1–12. doi:10.3233/JAD-141173
34. Fletcher WA, Sharpe JA. Saccadic eye movement dysfunction in Alzheimer’s disease. Ann Neurol. 1986;20(4):464–471. doi:10.1002/ana.410200405
35. Costanzo E, Lengyel I, Parravano M, et al. Ocular biomarkers for Alzheimer disease dementia: an umbrella review of systematic reviews and meta-analyses. JAMA Ophthalmol. 2023;141(1):84–91. doi:10.1001/jamaophthalmol.2022.4845
36. Mosimann UP, Felblinger J, Ballinari P, Hess CW, Müri RM. Visual exploration behaviour during clock reading in Alzheimer’s disease. Brain. 2004;127(Pt 2):431–438. doi:10.1093/brain/awh051
37. Parra MA, Della Sala S, Logie RH, Morcom AM. Neural correlates of shape-color binding in visual working memory. Neuropsychologia. 2014;52:27–36. doi:10.1016/j.neuropsychologia.2013.09.036
38. Holden JG, Cosnard A, Laurens B, et al. Prodromal Alzheimer’s disease demonstrates increased errors at a simple and automated anti-saccade task. J Alzheimers Dis. 2018;65(4):1209–1223. doi:10.3233/JAD-180082
39. Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol. 2012;11(2):170–178. doi:10.1016/S1474-4422(11)70289-7
40. Shakespeare TJ, Kaski D, Yong KX, et al. Abnormalities of fixation, saccade and pursuit in posterior cortical atrophy. Brain. 2015;138(Pt 7):1976–1991. doi:10.1093/brain/awv103
41. Jones A, Friedland RP, Koss B, Stark L, Thompkins-Ober BA. Saccadic intrusions in Alzheimer-type dementia. J Neurol. 1983;229(3):189–194. doi:10.1007/BF00313742
42. Schewe HJ, Uebelhack R, Vohs K. Abnormality in saccadic eye movement in dementia. Eur Psychiatry. 1999;14(1):52–53. doi:10.1016/S0924-9338(99)80716-0
43. Pin G, Trompette C, Ceccaldi M, Felician O, Koric L. Interest of eye movement study in early diagnosis of posterior cortical atrophy: a case-report. Rev Neurol. 2023;179(3):246–248. doi:10.1016/j.neurol.2022.10.007
44. Terao Y, Fukuda H, Tokushige SI, Inomata-Terada S, Hamada M, Ugawa Y. Saccades abnormalities in posterior cortical atrophy - A case report. Clin Neurophysiol. 2017;128(2):349–350. doi:10.1016/j.clinph.2016.12.005
45. Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 2008;64(1):4–14. doi:10.1002/ana.21426
46. Boxer AL, Garbutt S, Rankin KP, et al. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26(23):6354–6363. doi:10.1523/JNEUROSCI.0549-06.2006
47. Garbutt S, Matlin A, Hellmuth J, et al. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer’s disease. Brain. 2008;131(Pt 5):1268–1281. doi:10.1093/brain/awn047
48. Meyniel C, Rivaud-Péchoux S, Damier P, Gaymard B. Saccade impairments in patients with fronto-temporal dementia. J Neurol Neurosurg Psychiatry. 2005;76(11):1581–1584. doi:10.1136/jnnp.2004.060392
49. Burrell JR, Hornberger M, Carpenter RH, Kiernan MC, Hodges JR. Saccadic abnormalities in frontotemporal dementia. Neurology. 2012;78(23):1816–1823. doi:10.1212/WNL.0b013e318258f75c
50. Douglass A, Walterfang M, Velakoulis D, Abel L, Savage S. Behavioral variant frontotemporal dementia performance on a range of saccadic tasks. J Alzheimers Dis. 2018;65(1):231–242. doi:10.3233/JAD-170797
51. Russell LL, Greaves CV, Convery RS, et al. Eye movements in frontotemporal dementia: abnormalities of fixation, saccades and anti-saccades. Alzheimers Dement. 2021;7(1):e12218.
52. Peters F, Perani D, Herholz K, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cognit Disord. 2006;21(5–6):373–379. doi:10.1159/000091898
53. Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134(Pt 9):2502–2512. doi:10.1093/brain/awr173
54. Primativo S, Clark C, Yong KXX, et al. Eyetracking metrics reveal impaired spatial anticipation in behavioural variant frontotemporal dementia. Neuropsychologia. 2017;106:328–340. doi:10.1016/j.neuropsychologia.2017.10.014
55. Terao Y, Fukuda H, Yugeta A, et al. Initiation and inhibitory control of saccades with the progression of Parkinson’s disease - changes in three major drives converging on the superior colliculus. Neuropsychologia. 2011;49(7):1794–1806. doi:10.1016/j.neuropsychologia.2011.03.002
56. Chambers JM, Prescott TJ. Response times for visually guided saccades in persons with Parkinson’s disease: a meta-analytic review. Neuropsychologia. 2010;48(4):887–899. doi:10.1016/j.neuropsychologia.2009.11.006
57. Zhou MX, Wang Q, Lin Y, et al. Oculomotor impairments in de novo Parkinson’s disease. Front Aging Neurosci. 2022;14:985679. doi:10.3389/fnagi.2022.985679
58. Antoniades CA, Demeyere N, Kennard C, Humphreys GW, Hu MT. Antisaccades and executive dysfunction in early drug-naive Parkinson’s disease: the discovery study. Mov Disord. 2015;30(6):843–847. doi:10.1002/mds.26134
59. Hanuška J, Rusz J, Bezdicek O, et al. Eye movements in idiopathic rapid eye movement sleep behaviour disorder: high antisaccade error rate reflects prefrontal cortex dysfunction. J Sleep Res. 2019;28(5):e12742. doi:10.1111/jsr.12742
60. Nagai K, Kaneko Y, Suzuki M, et al. Multimodal visual exploration disturbances in Parkinson’s disease detected with an infrared eye-movement assessment system. Neurosci Res. 2020;160:50–56. doi:10.1016/j.neures.2019.11.003
61. Ranchet M, Orlosky J, Morgan J, Qadir S, Akinwuntan AE, Devos H. Pupillary response to cognitive workload during saccadic tasks in Parkinson’s disease. Behav Brain Res. 2017;327:162–166. doi:10.1016/j.bbr.2017.03.043
62. Koohi N, Bancroft MJ, Patel J, et al. Saccadic Bradykinesia in Parkinson’s disease: preliminary observations. Mov Disord. 2021;36(7):1729–1731. doi:10.1002/mds.28609
63. Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738–750. doi:10.1002/mds.10473
64. Gallea C, Wicki B, Ewenczyk C, et al. Antisaccade, a predictive marker for freezing of gait in Parkinson’s disease and gait/gaze network connectivity. Brain. 2021;144(2):504–514. doi:10.1093/brain/awaa407
65. Zhang Y, Yan A, Liu B, et al. Oculomotor performances are associated with motor and non-motor symptoms in Parkinson’s disease. Front Neurol. 2018;9:960. doi:10.3389/fneur.2018.00960
66. Pinkhardt EH, Kassubek J, Süssmuth S, Ludolph AC, Becker W, Jürgens R. Comparison of smooth pursuit eye movement deficits in multiple system atrophy and Parkinson’s disease. J Neurol. 2009;256(9):1438–1446. doi:10.1007/s00415-009-5131-5
67. Wu CC, Cao B, Dali V, et al. Eye movement control during visual pursuit in Parkinson’s disease. PeerJ. 2018;6:e5442.
68. Corin MS, Elizan TS, Bender MB. Oculomotor function in patients with Parkinson’s disease. J Neurol Sci. 1972;15(3):251–265. doi:10.1016/0022-510X(72)90068-8
69. Kovacs GG. Invited review: neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol. 2015;41(1):3–23. doi:10.1111/nan.12208
70. Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32(6):853–864. doi:10.1002/mds.26987
71. Quinn N. The ”round the houses” sign in progressive supranuclear palsy. Ann Neurol. 1996;40(6):951. doi:10.1002/ana.410400630
72. Fearon C, Field R, Donlon E, et al. The ”round the houses” sign and ”zig-zag” sign in progressive supranuclear palsy and other conditions. Parkinsonism Relat Disord. 2020;81:94–95. doi:10.1016/j.parkreldis.2020.10.030
73. Anagnostou E, Karavasilis E, Potiri I, et al. A cortical substrate for Square-Wave Jerks in progressive supranuclear palsy. J Clin Neurol. 2020;16(1):37–45. doi:10.3988/jcn.2020.16.1.37
74. Becker W, Behler A, Vintonyak O, Kassubek J. Patterns of small involuntary fixation saccades (SIFSs) in different neurodegenerative diseases: the role of noise. Exp Brain Res. 2023;241(7):1821–1833. doi:10.1007/s00221-023-06633-6
75. Troost BT, Daroff RB. The ocular motor defects in progressive supranuclear palsy. Ann Neurol. 1977;2(5):397–403. doi:10.1002/ana.410020509
76. Terao Y, Tokushige SI, Inomata-Terada S, Fukuda H, Yugeta A, Ugawa Y. Deciphering the saccade velocity profile of progressive supranuclear palsy: a sign of latent cerebellar/brainstem dysfunction? Clin Neurophysiol. 2022;141:147–159. doi:10.1016/j.clinph.2020.12.023
77. Lemos J, Pereira D, Almendra L, et al. Cortical control of vertical and horizontal saccades in progressive supranuclear palsy: an exploratory fMRI study. J Neurol Sci. 2017;373:157–166. doi:10.1016/j.jns.2016.12.049
78. Kim MK, Lee D, Yang X, Kim HJ, Choi JY, Kim JS. Saw-tooth vertical saccades in progressive supranuclear palsy. J Neurol. 2023;270(7):3644–3646. doi:10.1007/s00415-023-11696-0
79. Abate F, Picillo M, Della Rocca G, Barone P, Erro R. The ”zig-zag” sign in Progressive Supranuclear Palsy. Parkinsonism Relat Disord. 2020;79:86–87. doi:10.1016/j.parkreldis.2020.08.014
80. Shaikh AG, Factor SA, Juncos J. Saccades in progressive supranuclear palsy - maladapted, irregular, curved, and slow. Mov Disord Clin Pract. 2017;4(5):671–681. doi:10.1002/mdc3.12491
81. Wright IH, Sekar A, Jensen MT, et al. Reflexive and volitional saccadic eye movements and their changes in age and progressive supranuclear palsy. J Neurol Sci. 2022;443:120482. doi:10.1016/j.jns.2022.120482
82. Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53(3):603–635. doi:10.1152/jn.1985.53.3.603
83. Karatas M. Internuclear and supranuclear disorders of eye movements: clinical features and causes. Eur J Neurol. 2009;16(12):1265–1277. doi:10.1111/j.1468-1331.2009.02779.x
84. Ling X, Kim HJ, Lee JH, et al. Loss of torsional quick eye movements during head roll in progressive supranuclear palsy: a new diagnostic marker. J Neurol. 2023;270(4):2230–2236. doi:10.1007/s00415-023-11578-5
85. Armstrong RA. Visual signs and symptoms of multiple system atrophy. Clin Exp Optom. 2014;97(6):483–491. doi:10.1111/cxo.12206
86. Anderson T, Luxon L, Quinn N, Daniel S, David Marsden C, Bronstein A. Oculomotor function in multiple system atrophy: clinical and laboratory features in 30 patients. Mov Disord. 2008;23(7):977–984. doi:10.1002/mds.21999
87. Zhou H, Sun Y, Wei L, et al. Quantitative assessment of oculomotor function by videonystagmography in multiple system atrophy. Clin Neurophysiol. 2022;141:15–23. doi:10.1016/j.clinph.2022.05.019
88. Vintonyak O, Gorges M, Müller HP, et al. Patterns of eye movement impairment correlate with regional brain atrophy in neurodegenerative parkinsonism. Neurodegener Dis. 2017;17(4–5):117–126. doi:10.1159/000454880
89. Lanfranchi S, Jerman O, Vianello R. Working memory and cognitive skills in individuals with Down syndrome. Child Neuropsychol. 2009;15(4):397–416. doi:10.1080/09297040902740652
90. Lal V, Truong D. Eye movement abnormalities in movement disorders. Clin Park Relat Disord. 2019;1:54–63. doi:10.1016/j.prdoa.2019.08.004
91. Linder J, Wenngren BI, Stenlund H, Forsgren L. Impaired oculomotor function in a community-based patient population with newly diagnosed idiopathic parkinsonism. J Neurol. 2012;259(6):1206–1214. doi:10.1007/s00415-011-6338-9
92. Brooks SH, Klier EM, Red SD, et al. Slowed prosaccades and increased antisaccade errors as a potential behavioral biomarker of multiple system atrophy. Front Neurol. 2017;8:261. doi:10.3389/fneur.2017.00261
93. Vidailhet M, Rivaud S, Gouider-Khouja N, et al. Eye movements in parkinsonian syndromes. Ann Neurol. 1994;35(4):420–426. doi:10.1002/ana.410350408
94. Otero-Millan J, Serra A, Leigh RJ, Troncoso XG, Macknik SL, Martinez-Conde S. Distinctive features of saccadic intrusions and microsaccades in progressive supranuclear palsy. J Neurosci. 2011;31(12):4379–4387. doi:10.1523/JNEUROSCI.2600-10.2011
95. Klotz L, Klockgether T. Multiple system atrophy with macro square-wave jerks. Mov Disord. 2005;20(2):253–254. doi:10.1002/mds.20298
96. Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi:10.1212/01.wnl.0000324625.00404.15
97. Rascol O, Sabatini U, Fabre N, et al. Abnormal vestibuloocular reflex cancellation in multiple system atrophy and progressive supranuclear palsy but not in Parkinson’s disease. Mov Disord. 1995;10(2):163–170. doi:10.1002/mds.870100206
98. Lee JY, Lee WW, Kim JS, Kim HJ, Kim JK, Jeon BS. Perverted head-shaking and positional downbeat nystagmus in patients with multiple system atrophy. Mov Disord. 2009;24(9):1290–1295.
99. VandeVrede L, Ljubenkov PA, Rojas JC, Welch AE, Boxer AL. Four-repeat tauopathies: current management and future treatments. Neurotherapeutics. 2020;17(4):1563–1581. doi:10.1007/s13311-020-00888-5
100. Belfor N, Amici S, Boxer AL, et al. Clinical and neuropsychological features of corticobasal degeneration. Mech Ageing Dev. 2006;127(2):203–207. doi:10.1016/j.mad.2005.09.013
101. Parmera JB, Rodriguez RD, Studart Neto A, Nitrini R, Brucki SMD. Corticobasal syndrome: a diagnostic conundrum. Dement Neuropsychol. 2016;10(4):267–275. doi:10.1590/s1980-5764-2016dn1004003
102. Rivaud-Péchoux S, Vidailhet M, Gallouedec G, Litvan I, Gaymard B, Pierrot-Deseilligny C. Longitudinal ocular motor study in corticobasal degeneration and progressive supranuclear palsy. Neurology. 2000;54(5):1029–1032. doi:10.1212/WNL.54.5.1029
103. Mosimann UP, Müri RM, Burn DJ, Felblinger J, O’Brien JT, McKeith IG. Saccadic eye movement changes in Parkinson’s disease dementia and dementia with Lewy bodies. Brain. 2005;128(Pt 6):1267–1276. doi:10.1093/brain/awh484
104. Rivaud-Péchoux S, Vidailhet M, Brandel JP, Gaymard B. Mixing pro- and antisaccades in patients with parkinsonian syndromes. Brain. 2007;130(Pt 1):256–264. doi:10.1093/brain/awl315
105. Rinne JO, Lee MS, Thompson PD, Marsden CD. Corticobasal degeneration. A clinical study of 36 cases. Brain. 1994;117(Pt 5):1183–1196. doi:10.1093/brain/117.5.1183
106. Ling H, de Silva R, Massey LA, et al. Characteristics of progressive supranuclear palsy presenting with corticobasal syndrome: a cortical variant. Neuropathol Appl Neurobiol. 2014;40(2):149–163. doi:10.1111/nan.12037
107. Outeiro TF, Koss DJ, Erskine D, et al. Dementia with Lewy bodies: an update and outlook. Mol Neurodegener. 2019;14(1):5. doi:10.1186/s13024-019-0306-8
108. Fernández-Arcos A, Morenas-Rodríguez E, Santamaria J, et al. Clinical and video-polysomnographic analysis of rapid eye movement sleep behavior disorder and other sleep disturbances in dementia with Lewy bodies. Sleep. 2019;42(7). doi:10.1093/sleep/zsz086
109. McKeith I, Mintzer J, Aarsland D, et al. Dementia with Lewy bodies. Lancet Neurol. 2004;3(1):19–28. doi:10.1016/S1474-4422(03)00619-7
110. Kapoula Z, Yang Q, Vernet M, Dieudonné B, Greffard S, Verny M. Spread deficits in initiation, speed and accuracy of horizontal and vertical automatic saccades in dementia with Lewy bodies. Front Neurol. 2010;1:138. doi:10.3389/fneur.2010.00138
111. Brett FM, Henson C, Staunton H. Familial diffuse Lewy body disease, eye movement abnormalities, and distribution of pathology. Arch Neurol. 2002;59(3):464–467. doi:10.1001/archneur.59.3.464
112. Nakashima H, Terada S, Ishizu H, et al. An autopsied case of dementia with Lewy bodies with supranuclear gaze palsy. Neurol Res. 2003;25(5):533–537. doi:10.1179/016164103101201788
113. de Bruin VM, Lees AJ, Daniel SE. Diffuse Lewy body disease presenting with supranuclear gaze palsy, parkinsonism, and dementia: a case report. Mov Disord. 1992;7(4):355–358. doi:10.1002/mds.870070410
114. Armstrong RA. A comparison of visual problems in the parkinsonian syndromes. Intern Med Rev. 2018;4(3):32.
115. de Villers-Sidani É, Voss P, Guitton D, Cisneros-Franco JM, Koch NA, Ducharme S. A novel tablet-based software for the acquisition and analysis of gaze and eye movement parameters: a preliminary validation study in Parkinson’s disease. Front Neurol. 2023;14:1204733. doi:10.3389/fneur.2023.1204733
116. Bredemeyer O, Patel S, FitzGerald JJ, Antoniades CA. Oculomotor deficits in Parkinson’s disease: increasing sensitivity using multivariate approaches. Front Digit Health. 2022;4:939677. doi:10.3389/fdgth.2022.939677
117. Meissner WG. When does Parkinson’s disease begin? From prodromal disease to motor signs. Rev Neurol. 2012;168(11):809–814. doi:10.1016/j.neurol.2012.07.004
118. Raskin J, Cummings J, Hardy J, Schuh K, Dean RA. Neurobiology of Alzheimer’s disease: integrated molecular, physiological, anatomical, biomarker, and cognitive dimensions. Curr Alzheimer Res. 2015;12(8):712–722. doi:10.2174/1567205012666150701103107
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.