Back to Journals » Drug Design, Development and Therapy » Volume 8
Design, synthesis, and anticancer activity of novel berberine derivatives prepared via CuAAC “click” chemistry as potential anticancer agents
Authors Jin X, Yan T, Yan L, Li Q, Wang R, Hu Z, Jiang Y, Sun Q, Cao Y
Received 27 February 2014
Accepted for publication 23 April 2014
Published 6 August 2014 Volume 2014:8 Pages 1047—1059
DOI https://doi.org/10.2147/DDDT.S63228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Xin Jin1,2,* Tian-Hua Yan,3,* Lan Yan,1 Qian Li,4 Rui-Lian Wang,1 Zhen-Lin Hu,1 Yuan-Ying Jiang,1 Qing-Yan Sun,1 Yong-Bing Cao1
1School of Pharmacy, Second Military Medical University, Shanghai, People's Republic of China; 2School of Pharmacy, FuJian University of Traditional Chinese Medicine, Fuzhou, People's Republic of China; 3Department of Pharmacology, School of Pharmacy, China Pharmaceutical University, Nanjing, People's Republic of China; 4Diakite Biological Technology Co., Ltd, Shanghai, People's Republic of China
*These authors contributed equally to this work
Abstract: A series of novel derivatives of phenyl-substituted berberine triazolyls has been designed and synthesized via copper-catalyzed azide-alkyne cycloaddition click chemistry in an attempt to develop antitumor agents. All of the compounds were evaluated for anticancer activity against a panel of three human cancer cell lines, including MCF-7 (breast), SW-1990 (pancreatic), and SMMC-7721 (liver) and the noncancerous human umbilical vein endothelial cell (HUVEC) cell lines. The results indicated that most of the compounds displayed notable anticancer activities against the MCF-7 cells compared with berberine. Among these derivatives, compound 16 showed the most potent inhibitory activity against the SW-1990 and SMMC-7721 cell lines, with half-maximal inhibitory concentration (IC50) values of 8.54±1.97 µM and 11.87±1.83 µM, respectively. Compound 36 exhibited the most potent inhibitory activity against the MCF-7 cell line, with an IC50 value of 12.57±1.96 µM. Compound 16 and compound 36 exhibited low cytotoxicity in the HUVEC cell line, with IC50 values of 25.49±3.24 µM and 30.47±3.47 µM. Furthermore, compounds 14, 15, 16, 17, 18, 32, and 36 exhibited much better selectivity than berberine toward the normal cell line HUVEC.
Keywords: berberine, anticancer, click chemistry, structure–activity relationship
Introduction
In the past several decades, researchers have struggled to find effective clinical approaches for the treatment of cancer and have searched for novel anticancer agents.1–3 Berberine, an isoquinoline alkaloid isolated from the roots and stem bark of the Berberis species, is widely used as a traditional medicine for treating diarrhea and gastrointestinal disorders.4 In the past several years, berberine has shown a wide range of biochemical and pharmacological activities.5–11 Research on the anticancer activity of berberine in particular has received widespread attention and has achieved fairly good results.12–19 However, berberine was poorly absorbed in the intestines and thus showed a low inhibitory effect on cancer cell growth, which seriously affected the application prospects of berberine as an anticancer drug.20–21
The concept of “click” chemistry was introduced in 2001. The catchy term “click” refers to the facile, efficient, selective, and versatile chemical transformations that lead to a single reaction product.22,23 Although different chemical reactions (eg, cycloadditions, nucleophilic substitutions, additions to carbon–carbon double bonds) can be considered to be of the “click” type, copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) is regarded as a prime example of “click” chemistry.24,25 This reaction is regioselective, chemoselective, and moreover can be performed in aqueous medium at room or physiological temperature. Several recent reports have confirmed that CuAAC is a very useful method in bioapplications as diverse as drug discovery, drug delivery, and gene therapy.26–32 Thus, CuAAC has become a very popular tool in drug research.
To enhance the inhibitory function of berberine, the functionalized benzyl-modified berberine derivative compounds 4–36 were synthesized via CuAAC “click” chemistry from berberrubine and relevant azides and screened for cytotoxicity in vitro in human MCF-7 breast adenocarcinoma cells, human SW-1990 pancreatic carcinoma cells, and human SMMC-7721 liver carcinoma cells. This study is presented in two parts for clarity, the synthesis of new triazolyl berberine derivatives and the results of testing their anticancer properties. Several types of functional groups, specifically triazolyl berberine derivatives, were investigated to determine the structure–activity relationship.
Materials and methods
Synthesis and purification of triazolyl berberine derivatives
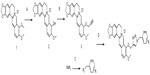
In this study, 33 triazolyl berberine derivatives were synthesized. This is the first report of any of them. The initial synthesis of the triazolyl berberine derivatives is illustrated in Figure 1. First, structural modifications at berberine C-9 (compound 1) can be envisaged after converting the methoxy group to a hydroxyl to obtain berberrubine (compound 2). Stirring compound 1 with dimethylformamide (DMF) at 190°C yielded berberrubine.33 Second, compound 3 was synthesized with an overall yield of 60% from berberrubine and propargyl bromide by a nucleophilic substitution reaction. Azides were prepared from their corresponding benzyl halides by diazotization in acidic conditions followed by displacement with sodium azide, with good to excellent yield (approximately 85% yield). Reaction of compound 3 with aromatic azides in the presence of CuSO4 · 5H2O and sodium ascorbate in DMF resulted in the derivatives 4–36 in excellent yields. The structures of the synthesized target compounds were elucidated by 1H nuclear magnetic resonance (NMR), 13C NMR, and mass spectrometry (MS). By employing the above reaction conditions, a series of triazolyl berberine derivatives that have varied substitutions on the aromatic ring were synthesized from a range of aromatic azides. All spectral data were in accordance with the assumed structures.
Chemistry
1H NMR and 13C NMR spectra were recorded using tetramethylsilane (TMS) as the internal standard in dimethyl sulfoxide (DMSO)-d6 with a Bruker BioSpin GmbH (Billerica, MA, USA) spectrometer at 300 MHz and 75 MHz, respectively. The chemical shifts are reported in ppm relative to TMS as the internal standard, and coupling constants were measured in Hz. Electrospray ionization (ESI)-MS was recorded on an Agilent 1100 LC/MSD (70 eV) spectrometer (Agilent Technologies, Santa Clara, CA, USA). High-resoution mass spectrometry was recorded on a Waters Micromass® Q-Tof micro™ mass spectrometer (Milford, MA, USA). All other reagents and starting materials were purchased and used as received (Sigma-Aldrich Co, St Louis, MO, USA; Tokyo Chemical Industry Co, Ltd, Tokyo, Japan; Adamas Reagent, Ltd, Shanghai, China). Reactions were monitored by analytical thin-layer chromatography (TLC) using silica gel 60 F254 plates, and spots were visualized by ultraviolet light irradiation (254 nm). Flash column chromatography was performed with silica gel (200–300 mesh). All reagents were purchased from Sigma-Aldrich Co, (St Louis, MO, USA). All solvents and reagents were analytical pure, and no further purification was needed. All starting materials were commercially available.
To a solution of NaN3 (50 mg, 0.77 mmol) in DMF (10 mL), a benzyl compound (0.6 mmol) was added, and the reaction mixture was sonicated at 35°C until completion, monitored by TLC. Compound 3 (160 mg, 0.4 mmol), CuSO4·5H2O (30 mg, 0.12 mmol) and sodium ascorbate (50 mg, 0.25 mmol) were added to this mixture. The mixture was stirred at room temperature for 3 hours, filtered and then evaporated under vacuum, while being monitored by TLC. The crude mixture was extracted with dichloromethane (3×40 mL), and the combined organic layer was dried over sodium sulfate and purified through column chromatography to give pure compounds 4–36 at 50%–90% yields.
Synthesis of berberrubine (compound 2)
Commercially available berberine (compound 1, 1.1 g) chloride was heated at 190°C in a vacuum oven under reduced pressure (20–30 mmHg) for 1–2 hours to obtain berberrubine (compound 2, 2.885 mg) in 90% yield. Dark red solid; yield 88% 1H NMR (300 MHz, DMSO): δ 9.19 (s, 1H), 7.90 (s, 1H), 7.45 (s, 1H), 7.34 (s, 1H), 6.80 (s, 2H), 6.00 (s, 2H), 4.85 (s, 2H), 3.83 (s, 3H), 3.09 (s, 2H); 13C NMR (DMSO): δ 162.7, 149.6, 149.4, 148.0, 145.8, 134.0, 132.2, 129.2, 122.5, 121.4, 120.1, 118.2, 107.7, 106.9, 104.3, 101.8, 55.2, 54.1, 27.5; high-resolution (HR)-ESI-MS calculated for C19H16NO4+ [M+1-Cl]+ 323.1079, found 323.1078.
Synthesis of 9-O-(propyne) berberine chloride (compound 3)
To a solution of compound 2 (540 mg, 1 mmol) in acetonitrile, propargyl bromide (240 mg, 1.2 mmol) was added and the reaction mixture was stirred at 75°C for 4 hours. Reaction was monitored by TLC and the crude product was subjected to column chromatography to give pure compound 3 (350 mg, 65% yield). Yellow solid, 1H NMR (300 MHz, DMSO): δ 9.88 (s, 1H), 8.97 (d, J=7.8 Hz, 1H), 8.22 (t, J=8.8 Hz, 1H), 8.03 (dd, J=14.8, 9.1 Hz, 1H), 7.80 (s, 1H), 7.09 (s, 1H), 6.18 (s, 2H), 5.09 (d, J=2.4 Hz, 2H), 4.96 (t, J=6.1 Hz, 2H), 4.09 (d, J=5.6 Hz, 3H), 3.62 (t, J=2.4 Hz, 1H), 3.21 (t, J=6.1 Hz, 2H); 13C NMR (75 MHz, DMSO): δ 151.20, 150.38, 148.19, 145.82, 141.19, 138.12, 133.42, 131.22, 127.03, 124.76, 122.58, 120.90, 120.79, 108.92, 105.96, 102.60, 80.30, 79.26, 61.44, 57.65, 55.79, 26.82; HR-ESI-MS calculated for C22H18NO4+ [M+1-Cl]+ 361.1235, found 361.1239.
General procedure for the synthesis of compounds 4–36
To a solution of NaN3 (50 mg, 0.77 mmol) in DMF (10 mL), compound of benzyl (0.6 mmol) was added and the reaction mixture was sonicated at 35°C until completion, monitored by TLC. To this mixture, compound 3 (160 mg, 0.4 mmol), CuSO4 · 5H2O (30 mg, 0.12 mmol) and sodium ascorbate (50 mg, 0.25 mmol) were added. The mixture was stirred at room temperature for 3 hours, filtered, and then evaporated under vacuum, monitored by TLC. The crude mixture was extracted with dichloromethane (3×40 mL) and the combined organic layer was dried over sodium sulfate and purified through column chromatography to give pure compounds 4–36 in 50%–90% yield.
9-O-[4-ethyl-(1-benzyl)-1H-1,2,3-triazole] berberine chloride (compound 4)
Yellow solid, yield 74%; 1H NMR (300 MHz, DMSO): δ 9.64 (s, 1H), 8.94 (d, J=15.5 Hz, 1H), 8.38 (s, 1H), 8.21 (t, J=9.3 Hz, 1H), 8.03 (t, J=12.0 Hz, 1H), 7.79 (s, 1H), 7.19 (ddd, J=38.5, 14.7, 7.5 Hz, 6H), 6.18 (s, 2H), 5.57 (s, 2H), 5.46 (s, 2H), 4.80 (s, 2H), 4.06 (s, 3H), 3.11 (s, 2H); 13C NMR (75 MHz, DMSO): δ 151.49, 150.38, 148.22, 145.65, 143.07, 141.82, 137.89, 136.48, 133.36, 131.11, 129.17, 128.51, 128.07, 127.03, 126.45, 125.94, 124.52, 122.50, 120.87, 120.78, 115.67, 108.93, 105.92, 102.61, 66.82, 57.67, 55.78, 53.24, 26.81; HR-ESI-MS calculated for C29H25N4O4+ [M+1-Cl]+ 494.1903, found 494.1908.
9-O-[1-(2-fluorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 5)
Yellow solid, yield 70%; 1H NMR (300 MHz, DMSO): δ 9.66 (s, 1H), 8.92 (s, 1H), 8.33 (s, 1H), 8.20 (d, J=9.1 Hz, 1H), 8.00 (d, J=9.2 Hz, 1H), 7.80 (s, 1H), 7.33 (d, J=6.9 Hz, 1H), 7.16 (t, J=9.3 Hz, 3H), 7.09 (s, 1H), 6.18 (s, 2H), 5.63 (s, 2H), 5.46 (s, 2H), 4.83 (s, 2H), 4.06 (s, 3H), 3.14 (s, 2H); 13C NMR (75 MHz, DMSO): δ 158.36, 150.94, 149.86, 147.71, 145.26, 142.46, 141.50, 137.37, 133.01, 130.74, 130.59, 130.50, 126.50, 125.50, 124.84, 123.98, 121.95, 120.46, 120.25, 115.77, 115.47, 108.42, 105.39, 102.10, 66.31, 56.99, 55.42, 47.02, 26.38; HR-ESI-MS calculated for C29H24BrN4O4+ [M+1-Cl]+ 572.0980, found 572.0986.
9-O-[1-(3-fluorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 6)
Yellow solid, yield 63%; 1H NMR (300 MHz, DMSO): δ 9.69 (s, 1H), 8.93 (s, 1H), 8.47 (s, 1H), 8.20 (d, J=9.2 Hz, 1H), 8.00 (d, J=9.0 Hz, 1H), 7.78 (s, 1H), 7.34 (dd, J=14.2, 7.5 Hz, 1H), 7.03 (dt, J=23.2, 6.5 Hz, 4H), 6.18 (s, 2H), 5.62 (s, 2H), 5.47 (s, 2H), 4.85 (s, 2H), 4.07 (s, 3H), 3.13 (s, 2H); 13C NMR (75 MHz, DMSO): δ 150.96, 149.93, 147.78, 145.38, 142.65, 141.46, 137.47, 132.99, 130.90, 130.73, 130.58, 126.66, 125.63, 124.02, 123.78, 122.00, 120.34, 120.23, 115.13, 114.83, 114.49, 108.39, 105.52, 102.16, 66.59, 57.01, 55.51, 52.20, 26.39; HR-ESI-MS calculated for C29H24BrN4O4 + [M+1-Cl]
9-O-[1-(4-fluorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 7)
Yellow solid, yield 65%; 1H NMR (300 MHz, DMSO): δ 9.69 (s, 1H), 8.95 (s, 1H), 8.41 (s, 1H), 8.24 (d, J=9.2 Hz, 1H), 8.05 (d, J=9.1 Hz, 1H), 7.82 (s, 1H), 7.35–7.03 (m, 5H), 6.21 (s, 2H), 5.60 (s, 2H), 5.49 (s, 2H), 4.86 (s, 2H), 4.10 (s, 3H), 3.16 (s, 2H); 13C NMR (75 MHz, DMSO): δ 151.03, 149.83, 147.84, 145.22, 142.66, 141.58, 137.44, 132.75, 132.44, 130.93, 130.07, 129.96, 127.01, 125.42, 124.54, 124.28, 122.24, 120.44, 120.31, 115.73, 115.44, 108.49, 105.53, 102.46, 66.78, 57.08, 55.64, 52.51, 26.74; HR-ESI-MS calculated for C29H24BrN4O4+ [M+1-Cl]+ 572.0980, found 572.0985.
9-O-[1-(2-chlorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 8)
Yellow solid, yield 59%; 1H NMR (300 MHz, DMSO): δ 9.65 (s, 1H), 8.91 (s, 1H), 8.40 (s, 1H), 8.17–8.09 (m, 1H), 8.00 (d, J=9.4 Hz, 1H), 7.75 (s, 1H), 7.38 (d, J=7.5 Hz, 1H), 7.29–7.21 (m, 2H), 7.05 (s, 2H), 6.15 (s, 2H), 5.64 (s, 2H), 5.45 (s, 2H), 4.82 (s, 2H), 4.04 (s, 3H), 3.10 (s, 2H); 13C NMR (75 MHz, DMSO): δ 151.13, 149.98, 147.83, 145.29, 142.46, 141.28, 137.44, 133.22, 133.01, 132.72, 130.72, 130.58, 130.39, 129.70, 127.79, 126.54, 126.18, 124.29, 122.15, 120.48, 108.59, 105.60, 102.26, 66.25, 57.19, 55.50, 50.77, 34.21, 26.47; HR-ESI-MS calculated for C29H24ClN4O4+ [M+1-Cl]+ 528.1486, found 528.1490.
9-O-[1-(3-chlorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 9)
Yellow solid, yield 68%; 1H NMR (300 MHz, DMSO): δ 9.67 (s, 1H), 8.93 (s, 1H), 8.20 (dd, J=18.4, 12.4 Hz, 2H), 8.00 (d, J=6.1 Hz, 1H), 7.79 (s, 1H), 7.09 (dd, J=7.3, 3.1 Hz, 4H), 6.85 (s, 1H), 6.18 (s, 2H), 5.56 (s, 2H), 5.47 (s, 2H), 4.80 (s, 2H), 4.06 (d, J=2.9 Hz, 3H), 3.11 (s, 2H); 13C NMR (75 MHz, DMSO): δ 150.97, 149.87, 147.71, 145.18, 142.39, 141.22, 137.36, 132.83, 130.59, 130.46, 130.33, 126.12, 125.47, 124.07, 123.97, 122.01, 120.36, 120.31, 115.33, 114.63, 114.69, 108.42, 105.40, 102.10, 66.22, 57.00, 55.25, 52.94, 26.29; HR-ESI-MS calculated for C29H24ClN4O4+ [M+1-Cl]+ 528.1486, found 528.1492.
9-O-[1-(4-chlorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 10)
Yellow solid, yield 62%; 1H NMR (300 MHz, DMSO): δ 9.65 (s, 1H), 8.91 (s, 1H), 8.41 (s, 1H), 8.20 (d, J=9.2 Hz, 1H), 8.01 (d, J=9.1 Hz, 1H), 7.77 (s, 1H), 7.31 (t, J=14.0 Hz, 2H), 7.15 (d, J=8.4 Hz, 2H), 7.07 (s, 1H), 6.17 (s, 2H), 5.55 (d, J=19.2 Hz, 2H), 5.46 (s, 2H), 4.82 (s, 2H), 4.07 (s, 3H), 3.10 (d, J=6.1 Hz, 2H); 13C NMR (75 MHz, DMSO): δ 151.01, 149.93, 147.77, 145.18, 142.64, 141.33, 137.41, 134.99, 132.88, 130.72, 130.62, 129.55, 128.69, 126.55, 125.58, 124.07, 123.94, 122.01, 120.36, 120.27, 114.57, 108.47, 105.49, 102.13, 66.31, 57.08, 55.34, 51.95, 26.35; HR-ESI-MS calculated for C29H24ClN4O4+ [M+1-Cl]+ 528.1486, found 528.1489.
9-O-[1-(2-bromobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 11)
Yellow solid, yield 54%; 1H NMR (300 MHz, DMSO): δ 9.66 (s, 1H), 8.93 (s, 1H), 8.34 (s, 1H), 8.21 (d, J=9.2 Hz, 1H), 8.01 (d, J=9.1 Hz, 1H), 7.79 (s, 1H), 7.60 (d, J=2.1 Hz, 1H), 7.45–7.38 (m, 1H), 7.37 (d, J=2.1 Hz, 1H), 7.15–6.97 (m, 2H), 6.18 (s, 2H), 5.65 (s, 2H), 5.48 (s, 2H), 4.83 (s, 2H), 4.08 (s, 3H), 3.22–3.02 (m, 2H); 13C NMR (75 MHz, DMSO): δ 151.46, 150.40, 148.24, 146.09, 142.88, 142.01, 138.11, 134.49, 134.15, 133.37, 132.76, 132.23, 131.08, 129.57, 128.28, 127.11, 126.34, 124.60, 122.49, 120.82, 120.79, 108.95, 106.16, 102.78, 66.91, 57.54, 55.97, 50.56, 27.05; HR-ESI-MS calculated for C29H24BrN4O4+ [M+1-Cl]+ 573.0980, found 573.0986.
9-O-[1-(3-bromobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 12)
Yellow solid, yield 50%; 1H NMR (300 MHz, DMSO): δ 9.67 (s, 1H), 8.91 (d, J=7.6 Hz, 1H), 8.49 (s, 1H), 8.17 (d, J=9.2 Hz, 1H), 7.99 (d, J=9.1 Hz, 1H), 7.75 (s, 1H), 7.49–7.35 (m, 2H), 7.24 (t, J=7.7 Hz, 1H), 7.19–7.09 (m, 1H), 7.06 (s, 1H), 6.16 (s, 2H), 5.60 (s, 2H), 5.46 (s, 2H), 4.84 (t, J=5.8 Hz, 2H), 4.07 (d, J=8.0 Hz, 3H), 3.12 (t, J=5.8 Hz, 2H); 13C NMR (75 MHz, DMSO): δ 151.38, 150.33, 148.17, 145.63, 143.16, 141.82, 139.06, 137.80, 133.31, 131.43, 131.36, 131.05 (2C), 127.30, 126.92, 126.11, 124.51, 122.39, 122.27, 120.82, 120.77, 108.91, 105.90, 102.59, 66.83, 57.51, 55.80, 52.39, 26.83; HR-ESI-MS calculated for C29H24BrN4O4+ [M+1-Cl]+ 573.0980, found 573.0986.
9-O-[1-(4-bromobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 13)
Yellow solid, yield 58%; 1H NMR (300 MHz, DMSO) δ 9.66 (s, 1H), 8.93 (s, 1H), 8.55–8.29 (m, 1H), 8.22 (d, J=9.2 Hz, 1H), 8.01 (d, J=8.8 Hz, 1H), 7.79 (s, 1H), 7.49 (d, J=7.8 Hz, 2H), 7.08 (s, 3H), 6.18 (s, 2H), 5.52 (d, J=29.6 Hz, 2H), 5.47 (s, 2H), 4.82 (s, 2H), 4.07 (d, J=4.6 Hz, 3H), 3.12 (s, 2H); 13C NMR (75 MHz, DMSO): δ 151.73, 150.72, 148.55, 146.05, 143.40, 142.18, 138.18, 136.11, 133.63, 132.18, 131.56, 131.38(2C), 130.38, 126.86, 126.33, 124.96, 122.78, 122.26, 121.13, 120.96, 109.41, 106.16, 102.91, 67.13, 57.77, 56.30, 52.78, 27.06; HR-ESI-MS calculated for C29H24BrN4O4+ [M+1-Cl]+ 573.0980, found 573.0987.
9-O-[1-(2-methylbenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 14)
Yellow solid, yield 61%; 1H NMR (300 MHz, DMSO): δ 9.67 (s, 1H), 8.92 (s, 1H), 8.42 (s, 1H), 8.20 (d, J=9.2 Hz, 1H), 8.00 (d, J=9.1 Hz, 1H), 7.78 (s, 1H), 7.36–7.22 (m, 3H), 7.15–7.05 (m, 2H), 6.18 (s, 2H), 5.60 (s, 2H), 5.47 (s, 2H), 4.83 (d, J=6.0 Hz, 2H), 4.07 (s, 3H), 3.17–3.07 (m, 2H), 1.30–1.20 (m, 3H); 13C NMR (75 MHz, DMSO): δ 150.97, 149.94, 147.83, 145.38, 145.25, 142.68, 141.44, 138.43, 137.46, 133.35, 132.89, 130.60, 128.04, 127.65, 126.61, 126.39, 125.64, 124.09, 122.02, 120.43, 120.32, 108.51, 105.50, 102.22, 66.44, 57.02, 55.35, 52.06, 29.09, 26.39; HR-ESI-MS calculated for C30H27N4O4+ [M+1-Cl]+ 539.1726, found 539.1732.
9-O-[1-(4-methylbenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 15)
Yellow solid, yield 74%; 1H NMR (300 MHz, DMSO) δ 9.62 (s, 1H), 8.94 (d, J=7.7 Hz, 1H), 8.35 (s, 1H), 8.26–8.16 (m, 1H), 8.03 (t, J=11.6 Hz, 1H), 7.80 (s, 1H), 7.11–6.91 (m, 5H), 6.18 (s, 2H), 5.48 (d, J=9.8 Hz, 4H), 4.76 (t, J=6.0 Hz, 2H), 4.08 (s, 3H), 3.08 (d, J=5.9 Hz, 2H), 2.15 (s, 3H); 13C NMR (75 MHz, DMSO) δ 151.56, 150.37, 148.22, 145.62, 142.96, 141.70, 137.84, 133.50, 133.30, 131.09, 129.64, 127.92, 127.04, 125.86, 124.51, 122.56, 120.86, 120.77, 108.93, 105.94, 102.61,100.02, 66.69, 57.53, 55.71, 52.96, 26.76, 21.00; HR-ESI-MS calculated for C30H27N4O4+ [M+1-Cl]+ 539.1726, found 539.1731.
9-O-[1-(4-tert-butylbenzy1)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 16)
Yellow solid, yield 68%; 1H NMR (300 MHz, DMSO): δ 9.71 (s, 1H), 8.96 (s, 1H), 8.40 (s, 1H), 8.21 (d, J=9.1 Hz, 1H), 8.01 (d, J=9.1 Hz, 1H), 7.80 (s, 1H), 7.28 (d, J=8.2 Hz, 2H), 7.13–6.96 (m, 3H), 6.17 (s, 2H), 5.54 (s, 2H), 5.46 (s, 2H), 4.88 (s, 2H), 4.06 (s, 3H), 3.15 (s, 2H), 1.20 (s, 9H); 13C NMR (75 MHz, DMSO): δ 155.73, 151.21, 150.25, 145.67, 148.07, 142.78, 141.57, 138.13, 133.60, 132.96, 130.98, 127.28, 126.64, 125.38, 124.41, 122.13, 120.38, 120.30, 120.22, 119.12, 108.34, 105.65, 102.35, 100.43, 78.61, 57.21, 55.56, 52.50, 34.45, 30.95, 29.23; HR-ESI-MS calculated for C33H33N4O4+ [M+1-Cl]+ 550.2501, found 550.2507.
9-O-[1-(3-methoxybenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 17)
Yellow solid, yield 65%; 1H NMR (300 MHz, DMSO): δ 9.65 (s, 1H), 8.91 (s, 1H), 8.41 (s, 1H), 8.19 (d, J=9.2 Hz, 1H), 8.00 (d, J=9.1 Hz, 1H), 7.78 (s, 1H), 7.22–7.12 (m, 1H), 7.08 (s, 1H), 6.81–6.73 (m, 2H), 6.64 (d, J=7.5 Hz, 1H), 6.17 (s, 2H), 5.53 (s, 2H), 5.46 (s, 2H), 4.81 (t, J=5.3 Hz, 2H), 4.07 (s, 3H), 3.67 (s, 3H), 3.12 (d, J=5.3 Hz, 2H); 13C NMR (75 MHz, DMSO): δ 159.35, 150.99, 149.88, 147.72, 145.13, 142.58, 141.32, 137.43, 137.37, 132.86, 130.61, 129.84, 126.52, 125.49, 124.03, 122.00, 120.38, 120.29, 119.61, 113.63, 113.20, 108.43, 105.45, 102.12, 66.32, 57.04, 55.29, 55.10, 52.66, 26.33; HR-ESI-MS calculated for C30H27N4O5+ [M+1-Cl]+ 523.1981, found 523.1987.
9-O-[1-(4-methoxybenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 18)
Yellow solid, yield 78%; 1H NMR (300 MHz, DMSO): δ 9.65 (s, 1H), 8.94 (s, 1H), 8.48 (s, 1H), 8.17 (d, J=8.6 Hz, 1H), 8.01 (d, J=8.3 Hz, 1H), 7.76 (s, 1H), 7.09 (d, J=8.1 Hz, 3H), 6.79 (d, J=7.0 Hz, 2H), 6.16 (s, 2H), 5.47 (s, 2H), 5.44 (s, 2H), 4.83 (s, 2H), 4.05 (s, 3H), 3.64 (s, 3H), 3.09 (s, 2H); 13C NMR (75 MHz, DMSO): δ 159.57, 151.57, 150.39, 148.23, 145.68, 143.03, 141.83, 137.86, 137.34, 133.42, 131.12, 129.75, 128.45, 127.04, 125.92, 124.63, 122.56, 120.84, 120.56, 119.12, 114.53, 108.95, 106.04, 102.65, 66.86, 57.62, 55.84, 55.64, 52.80, 26.88; HR-ESI-MS calculated for C30H27N4O5+ [M+1-Cl]+ 523.1981, found 523.1989.
9-O-[4-(trifluoromethoxy)benzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 19)
Yellow solid, yield 85%; 1H NMR (300 MHz, DMSO): δ 9.67 (s, 1H), 8.91 (s, 1H), 8.54 (s, 1H), 8.16 (d, J=9.3 Hz, 1H), 8.02 (d, J=9.2 Hz, 1H), 7.75 (s, 1H), 7.33–7.21 (m, 4H), 7.05 (s, 1H), 6.14 (s, 2H), 5.62 (s, 2H), 5.45 (s, 2H), 4.84 (t, J=5.9 Hz, 2H), 4.04 (s, 3H), 3.16–3.06 (m, 2H); 13C NMR (75 MHz, DMSO): δ 151.15, 150.02, 148.15, 147.86, 145.31, 142.79, 141.41, 137.50, 135.58, 133.03, 130.76, 129.93, 126.62, 125.94, 124.31, 122.12, 121.38, 120.48, 120.42, 108.59, 105.62, 102.26, 66.37, 57.22, 55.51, 52.01, 26.50; HR-ESI-MS calculated for C30H24F3N4O5+ [M+1-Cl]+ 577.1698, found 577.1702.
9-O-[1-(3-(trifluoromethyl)benzyl-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 20)
Yellow solid, yield 83%; 1H NMR (300 MHz, DMSO): δ 9.73 (s, 1H), 8.95(s, 1H), 8.57(s, 1H), 8.21 (d, J=9.2 Hz, 1H), 8.01 (d, J=9.1 Hz, 1H), 7.80 (s, 1H), 7.74(d, J=8.1 Hz, 2H), 7.40 (d, J=8.0 Hz, 2H), 7.12 (s, 1H), 6.24(s, 2H), 5.77 (s, 2H), 5.52 (s, 2H), 4.93 (t, J=5.5 Hz, 2H), 4.14 (s, 3H), 3.16 (d, J=5.6 Hz, 2H); 13C NMR (75 MHz, DMSO): δ 150.80, 149.72, 147.56, 145.04, 142.62, 141.41, 137.22, 132.67, 131.79, 130.52, 129.76 (2C), 126.33, 125.36, 124.99, 124.88, 124.69, 124.59, 123.84, 121.80, 120.32, 120.23, 108.37, 105.38, 102.05, 65.78, 56.78, 54.83, 51.50, 25.54; HR-ESI-MS calculated for C30H24F3N4O4+ [M+1-Cl]+ 562.1749, found 562.1756.
9-O-[1-(4-(trifluoromethyl)benzyl-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 21)
Yellow solid, yield 88%; 1H NMR (300 MHz, DMSO): δ 9.75 (s, 1H), 8.96 (s, 1H), 8.56 (s, 1H), 8.23 (d, J=9.2 Hz, 1H), 8.04 (d, J=9.1 Hz, 1H), 7.80 (s, 1H), 7.71 (d, J=8.1 Hz, 2H), 7.39 (d, J=8.0 Hz, 2H), 7.10 (s, 1H), 6.20 (s, 2H), 5.77 (s, 2H), 5.51 (s, 2H), 4.91 (t, J=5.5 Hz, 2H), 4.11 (s, 3H), 3.18 (d, J=5.6 Hz, 2H); 13C NMR (75 MHz, DMSO): δ 150.86, 149.68, 148.06, 145.24, 142.66, 141.71, 137.32, 132.77, 132.09, 130.32, 129.72, 126.37, 125.34, 124.97, 124.78, 124.64, 124.53, 123.90, 121.83, 120.40, 120.33, 108.39, 105.41, 102.15, 65.88, 56.48, 54.63, 52.21, 25.04; HR-ESI-MS calculated for C30H24F3N4O4+ [M+1-Cl]+ 562.1749, found 562.1755.
9-O-[1-(2-benzonitrile)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 22)
Yellow solid, yield 79%; 1H NMR (300 MHz, DMSO): δ 9.67 (s, 1H), 8.92 (s, 1H), 8.39 (s, 1H), 8.20 (d, J=9.1 Hz, 1H), 8.00 (d, J=9.3 Hz, 1H), 7.90–7.75 (m, 2H), 7.65 (t, J=7.4 Hz, 1H), 7.49 (t, J=7.5 Hz, 1H), 7.23 (d, J=7.3 Hz, 1H), 7.08 (s, 1H), 6.18 (s, 2H), 5.78 (s, 2H), 5.49 (s, 2H), 4.84 (s, 2H), 4.07 (s, 3H), 3.14 (s, 2H); 13C NMR (75 MHz, DMSO): δ 151.14, 150.00, 147.63, 145.14, 142.65, 141.36, 141.29, 137.36, 132.88, 132.78, 130.65, 128.32, 126.48, 126.07, 124.14, 122.06, 120.32, 118.36, 110.78, 108.37, 105.38, 102.17, 66.20, 57.12, 55.29, 52.12, 26.31; HR-ESI-MS calculated for C30H24N5O4+ [M+1-Cl]+ 519.1828, found 519.1832.
9-O-[1-(4-benzonitrile)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 23)
Yellow solid, yield 83%; 1H NMR (300 MHz, DMSO): δ 9.68 (s, 1H), 8.92 (s, 1H), 8.52 (s, 1H), 8.20 (d, J=9.0 Hz, 1H), 8.01 (d, J=8.9 Hz, 1H), 7.82–7.66 (m, 3H), 7.23 (d, J=7.7 Hz, 2H), 7.05 (s, 1H), 6.16 (s, 2H), 5.71 (s, 2H), 5.48 (s, 2H), 4.82 (s, 2H), 4.08 (s, 3H), 3.07 (s, 2H); 13C NMR (75 MHz, DMSO): δ 151.04, 150.00, 147.83, 145.18, 142.68, 141.46, 141.24, 137.35, 132.85, 132.61, 130.56, 128.28, 126.52, 126.01, 124.11, 122.02, 120.30, 118.38, 110.85, 108.41, 105.41, 102.14, 66.23, 57.09, 55.31, 52.09, 26.33; HR-ESI-MS calculated for C30H24N5O4+ [M+1-Cl]+ 519.1828, found 519.1833.
9-O-[1-(4-nitrobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 24)
Yellow solid, yield 85%; 1H NMR (300 MHz, DMSO): δ 9.70 (s, 1H), 8.91 (s, 1H), 8.63 (s, 1H), 8.24–8.04 (m, 3H), 7.99 (d, J=9.1 Hz, 1H), 7.72 (s, 1H), 7.36 (d, J=8.7 Hz, 2H), 7.03 (s, 1H), 6.16 (s, 2H), 5.77 (d, J=9.0 Hz, 2H), 5.48 (s, 2H), 4.87 (t, J=5.6 Hz, 2H), 4.08 (d, J=2.9 Hz, 3H), 3.07 (d, J=5.4 Hz, 2H); 13C NMR (75 MHz, DMSO): δ 151.45, 150.34, 148.17, 147.51, 145.64, 143.89, 143.19, 141.73, 137.74, 133.29, 132.45, 130.95, 129.22, 126.95, 126.55, 124.53, 124.22, 122.42, 120.70, 118.58, 110.65, 108.82, 105.83, 102.58, 66.75, 57.55, 55.75, 52.27, 26.78; HR-ESI-MS calculated for C29H24N5O6+ [M+1-Cl]+ 539.1726, found 539.173.
9-O-[1-(4-methyl formate benzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 25)
Yellow solid, yield 69%; 1H NMR (300 MHz, DMSO): δ 9.61 (s, 1H), 8.87 (s, 1H), 8.41 (s, 1H), 8.21 (d, J=8.5 Hz, 1H), 8.01 (d, J=7.9 Hz, 1H), 7.84–7.68 (m, 3H), 7.11 (d, J=7.4 Hz, 2H), 7.03 (s, 1H), 6.17 (s, 2H), 5.65 (s, 2H), 5.49 (s, 2H), 4.75 (s, 2H), 4.08 (s, 3H), 3.81 (s, 3H); 13C NMR (75 MHz, DMSO): δ 165.74, 151.30, 150.06, 147.84, 145.20, 142.71, 141.24, 141.09, 137.38, 132.83, 130.54, 129.45, 129.20, 127.52, 126.65, 125.91, 124.16, 122.19, 120.25, 108.43, 105.48, 102.19, 66.14, 57.06, 55.35, 52.18, 26.36; HR-ESI-MS calculated for C31H27N4O6+ [M+1-Cl]+ 552.1930, found 552.1936.
9-O-[1-(4-(methylsulfonyl)benzyl-4-ethyl-1H-1, 2, 3-triazole) berberine chloride (compound 26)
Yellow solid, yield 73%; 1H NMR (300 MHz, DMSO): δ 9.73 (s, 1H), 8.93 (s, 1H), 8.53 (s, 1H), 8.19 (d, J=9.2 Hz, 1H), 8.01 (d, J=9.2 Hz, 1H), 7.89 (d, J=8.3 Hz, 2H), 7.77 (s, 1H), 7.45 (d, J=8.3 Hz, 2H), 7.07 (s, 1H), 6.17 (s, 2H), 5.77 (s, 2H), 5.47 (s, 2H), 4.90 (t, J=5.6 Hz, 2H), 4.06 (s, 3H), 3.19 (s, 3H), 3.17 (s, 2H); 13C NMR (75 MHz, DMSO): δ 150.86, 149.91, 147.75, 145.27, 142.83, 141.71, 141.57, 140.52, 137.47, 132.94, 130.65, 128.60, 127.45, 126.54, 125.84, 124.01, 121.87, 120.36, 120.27, 108.45, 105.47, 102.13, 66.54, 57.08, 55.40, 52.12, 43.44, 26.39; HR-ESI-MS calculated for C30H27N4O6S+ [M+1-Cl]+ 572.1651, found 572.1656.
9-O-[1-(2, 4-difluorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 27)
Yellow solid, yield 74%; 1H NMR (300 MHz, DMSO): δ 9.70 (s, 1H), 8.96 (d, J=8.6 Hz, 1H), 8.46 (s, 1H), 8.23 (d, J=9.2 Hz, 1H), 8.05 (t, J=7.6 Hz, 1H), 7.82 (d, J=6.8 Hz, 1H), 7.37 (tt, J=13.3, 5.2 Hz, 2H), 7.15–7.00 (m, 2H), 6.20 (s, 2H), 5.61 (s, 2H), 5.49 (s, 2H), 4.88 (t, J=5.8 Hz, 2H), 4.11 (d, J=6.2 Hz, 3H), 3.16 (t, J=5.8 Hz, 2H); 13C NMR (75 MHz, DMSO): δ 150.95, 149.91, 147.73, 145.20, 142.70, 141.45, 137.40, 132.88, 131.89, 130.60, 126.55, 125.52, 124.02, 121.95, 120.34, 120.29, 118.02, 117.80, 117.35, 117.06, 108.44, 105.44, 102.13, 100.24, 66.37, 57.06, 55.48, 51.58, 26.45; HR-ESI-MS calculated for C29H23F2N4O4+ [M+1-Cl]+ 530.1687, found 530.1692.
9-O-[1-(2, 6-difluorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 28)
Yellow solid, yield 63%; 1H NMR (300 MHz, DMSO): δ 9.66 (s, 1H), 8.93 (s, 1H), 8.34 (s, 1H), 8.19 (d, J=8.9 Hz, 1H), 8.00 (d, J=8.7 Hz, 1H), 7.79 (s, 1H), 7.43 (s, 1H), 7.09 (s, 3H), 6.18 (s, 2H), 5.63 (s, 2H), 5.44 (s, 2H), 4.85 (s, 2H), 4.06 (s, 3H), 3.15 (s, 2H); 13C NMR (75 MHz, DMSO): δ 159.26, 150.98, 150.15, 149.83, 147.96, 145.19, 142.77, 141.62, 137.76, 133.14, 131.69, 130.66, 126.66, 125.65, 124.09, 122.02, 120.49, 112.05, 111.93, 111.79, 108.53, 108.30, 105.51, 102.19, 99.15, 66.29, 57.08, 55.47, 26.46; HR-ESI-MS calculated for C29H23F2N4O4+ [M+1-Cl]+ 530.1687, found 530.1691.
9-O-[1-(3, 4-difluorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 29)
Yellow solid, yield 67%; 1H NMR (300 MHz, DMSO): δ 9.70 (s, 1H), 8.97 (d, J=16.3 Hz, 1H), 8.45 (s, 1H), 8.25 (t, J=8.7 Hz, 1H), 8.06 (dd, J=15.2, 9.2 Hz, 1H), 7.82 (d, J=6.8 Hz, 1H), 7.38 (dt, J=17.9, 9.0 Hz, 2H), 7.10 (d, J=10.8 Hz, 2H), 6.21 (s, 2H), 5.61 (s, 2H), 5.49 (s, 2H), 4.88 (s, 2H), 4.10 (s, 3H), 3.21–3.06 (m, 2H); 13C NMR (75 MHz, DMSO): δ 150.97, 149.92, 147.75, 145.62, 142.71, 141.39, 137.75, 137.42, 133.71, 132.89, 130.62, 126.56, 125.52, 124.05, 121.97, 120.36, 120.28, 117.98, 117.88, 117.30, 117.12, 108.46, 105.45, 102.14, 66.37, 57.07, 55.36, 51.60, 26.36; HR-ESI-MS calculated for C29H23F2N4O4+ [M+1-Cl]+ 530.1687, found 530.1690.
9-O-[1-(4-chloro-2-fluorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 30)
Yellow solid, yield 66%; 1H NMR (300 MHz, DMSO): δ 9.64 (s, 1H), 8.91 (s, 1H), 8.24 (d, J=37.4 Hz, 2H), 7.90 (d, J=64.3 Hz, 2H), 7.36 (d, J=23.5 Hz, 3H), 7.08 (s, 1H), 6.18 (s, 2H), 5.66 (s, 2H), 5.44 (s, 2H), 4.82 (s, 2H), 4.06 (s, 3H), 3.14 (s, 2H); 13C NMR (75 MHz, DMSO): δ 159.56, 151.01, 149.92, 147.77, 145.23, 142.20, 141.31, 137.39, 134.82, 132.89, 131.88, 131.75, 130.62, 126.51, 125.73, 124.08, 122.02, 120.98, 120.38, 115.06, 114.77, 108.48, 105.45, 102.15, 66.24, 57.03, 55.38, 44.47, 26.37; HR-ESI-MS calculated for C29H23ClFN4O4+ [M+1-Cl]+ 545.1391, found 545.1397.
9-O-[1-(2-bromo-4-fluorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 31)
Yellow solid, yield 62%;1H NMR (300 MHz, DMSO): δ 9.66 (s, 1H), 8.91 (s, 1H), 8.43 (s, 1H), 8.20 (d, J=9.0 Hz, 1H), 7.99 (d, J=9.1 Hz, 1H), 7.77 (s, 1H), 7.60 (d, J=8.1 Hz, 1H), 7.21 (d, J=9.5 Hz, 1H), 7.07 (s, 1H), 6.90 (d, J=8.4 Hz, 1H), 6.16 (s, 2H), 5.59 (s, 2H), 5.46 (s, 2H), 4.83 (s, 2H), 4.07 (s, 3H), 3.10 (s, 2H); 13C NMR (75 MHz, DMSO): δ 150.93, 149.91, 147.74, 145.19, 142.69, 141.35, 138.22, 138.18, 137.39, 133.82, 132.84, 130.57, 126.54, 125.64, 125.33, 123.99, 121.94, 120.32, 120.23, 116.25, 115.95, 108.42, 105.45, 102.10, 66.33, 57.04, 55.29, 51.54, 26.32; HR-ESI-MS calculated for C29H23BrFN4O4+ [M+1-Cl]+ 590.0886, found 590.0890.
9-O-[1-(2, 4-dichlorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 32)
Yellow solid, yield 73%; 1H NMR (300 MHz, DMSO): δ 9.67 (s, 1H), 8.92 (s, 1H), 8.31 (s, 1H), 8.19 (d, J=8.1 Hz, 1H), 8.01 (s, 1H), 7.79 (s, 1H), 7.60 (s, 1H), 7.22 (s, 1H), 7.03 (d, J=28.1 Hz, 2H), 6.18 (s, 2H), 5.64 (s, 2H), 5.49 (s, 2H), 4.83 (s, 2H), 4.07 (s, 3H), 3.13 (s, 2H); 13C NMR (75 MHz, DMSO): δ 151.62, 150.58, 145.84, 143.12, 139.79, 135.46, 133.39, 131.24, 130.96, 130.85, 128.76, 127.14, 126.45, 123.38, 122.65, 122.34, 120.93, 120.84, 109.05, 105.99, 102.84, 102.77, 98.27, 96.17, 67.35, 58.44, 56.21, 54.21, 27.63; HR-ESI-MS calculated for C29H23Cl2N4O4+ [M+1-Cl]+ 562.1096, found 562.1094.
9-O-[1-(2, 5-dichlorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 33)
Yellow solid, yield 53%; 1H NMR (300 MHz, DMSO): δ 9.64 (s, 1H), 8.91 (s, 1H), 8.26 (s, 1H), 8.18 (d, J=9.2 Hz, 1H), 7.99 (d, J=9.1 Hz, 1H), 7.78 (s, 1H), 7.48 (d, J=7.5 Hz, 2H), 7.37 (dd, J=9.0, 7.1 Hz, 1H), 7.08 (s, 1H), 6.18 (s, 2H), 5.74 (d, J=5.8 Hz, 2H), 5.44 (s, 2H), 4.81 (s, 2H), 4.06 (s, 3H), 3.13 (s, 2H); 13C NMR (75 MHz, DMSO): δ 151.03, 149.93, 147.78, 145.24, 142.06, 141.27, 137.38, 135.83, 132.90, 131.64, 130.60, 130.26, 128.93, 126.51, 125.69, 124.09, 122.05, 120.38, 120.33, 108.50, 105.46, 102.16, 66.19, 57.04, 55.39, 48.62, 30.73, 26.37; HR-ESI-MS calculated for C29H23Cl2N4O4+ [M+1-Cl]+ 562.1096, found 562.1095.
9-O-[1-(2, 6-dichlorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 34)
Yellow solid, yield 58%; BBR-57 1H NMR (300 MHz, DMSO): δ 9.65 (s, 1H), 8.92 (s, 1H), 8.32 (s, 1H), 8.18 (d, J=9.2 Hz, 1H), 7.99 (d, J=9.1 Hz, 1H), 7.78 (s, 1H), 7.41 (td, J=8.2, 6.2 Hz, 1H), 7.35–7.20 (m, 2H), 7.08 (s, 1H), 6.17 (s, 2H), 5.66 (s, 2H), 5.44 (s, 2H), 4.83 (t, J=5.8 Hz, 2H), 4.06 (s, 3H), 3.15 (d, J=5.9 Hz, 2H); 13C NMR (75 MHz, DMSO): δ 162.85, 159.53, 150.97, 149.87, 147.72, 145.20, 142.18, 141.30, 137.34, 134.79, 132.86, 131.85, 130.57, 126.47, 125.74, 124.04, 121.98, 120.95, 120.34, 115.03, 114.74, 108.44, 105.42, 102.12, 66.23, 57.00, 55.34, 44.42, 26.34; HR-ESI-MS calculated for C29H23Cl2N4O4+ [M+1-Cl]+ 562.1096, found 562.1092.
9-O-[1-(3, 4-dichlorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 35)
Yellow solid, yield 76%; 1H NMR (300 MHz, DMSO): δ 9.66 (s, 1H), 8.88 (s, 1H), 8.47 (s, 1H), 8.18 (d, J=9.2 Hz, 1H), 8.00 (d, J=9.1 Hz, 1H), 7.75 (s, 1H), 7.52 (dd, J=10.4, 4.9 Hz, 2H), 7.17–7.03 (m, 2H), 6.16 (s, 2H), 5.60 (s, 2H), 5.46 (s, 2H), 4.83 (d, J=5.3 Hz, 2H), 4.07 (s, 3H), 3.12 (d, J=5.5 Hz, 2H); 13C NMR (75 MHz, DMSO): δ 150.92, 149.89, 147.74, 145.15, 142.62, 141.30, 137.32, 136.81, 132.85, 131.23, 131.00, 130.93, 130.57, 129.88, 128.09, 126.45, 125.57,123.90, 121.92, 120.37, 120.28, 108.46, 105.22, 101.88, 66.19, 56.81, 55.30, 51.21, 26.16; HR-ESI-MS calculated for C29H23Cl2N4O4+ [M+1-Cl]+ 562.1096, found 562.1094.
9-O-[1-(2, 4, 6-trichlorobenzyl)-4-ethyl-1H-1, 2, 3-triazole] berberine chloride (compound 36)
Yellow solid, yield 63%; 1H NMR (300 MHz, DMSO): δ 9.57 (s, 1H), 8.90 (s, 1H), 8.17 (d, J=9.1 Hz, 1H), 8.07–7.92 (m, 2H), 7.81 (s, 1H), 6.75 (s, 2H), 6.18 (s, 2H), 5.44 (s, 4H), 4.65 (s, 2H), 4.05 (s, 3H), 3.10 (s, 2H); 13C NMR (75 MHz, DMSO): δ 151.15, 149.93, 147.80, 145.19, 141.93, 141.05, 137.72, 137.53, 137.32, 132.81, 131.02, 130.98, 130.59, 128.98, 128.62, 126.51, 124.99, 124.06, 122.19, 120.40, 120.25, 108.50, 105.50, 102.29, 66.02, 57.03, 55.17, 47.50, 19.22; HR-ESI-MS calculated for C29H22Cl3N4O4+ [M+1-Cl]+ 596.0706, found 596.0708.
Cell culture
Human umbilical vein endothelial cell (HUVEC), SW-1990 (human pancreatic carcinoma), and SMMC-7721 (human liver carcinoma) cell lines were provided by Research and Development Center for New Drugs, School of Pharmacy, Second Military Medical University, Shanghai, People’s Republic of China. MCF-7 (human breast adenocarcinoma) cells were provided by the Shanghai Institute of Materia Medica (Chinese Academy of Sciences). All adherent cell lines, including MCF-7, SW-1990, and SMMC-7721, were cultured in a humidified 5% CO2 atmosphere at 37°C. Cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum and 100 units/mL penicillin; 0.1 mg/mL of streptomycin and HUVEC were cultivated in Endothelial Cell Growth Medium. This medium was prepared with 485 mL of Endothelial Basal Medium (without serum) and 15 mL of growth supplement (3%) of fetal bovine serum. HUVEC cells were cultivated between passages 1 and 7.
Cytotoxic activity
The in vitro cytotoxicity of the newly synthesized target compounds 4–36 against MCF-7, SW-1990, SMMC-7721, and HUVEC cell lines was measured by an assay was based on the cleavage of the yellow tetrazolium salt MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich) to form purple formazan crystals in viable cells. The cells were plated in 96-well culture plates at a density of 10,000 (MCF-7), 5,000 (SW-1990), 6,000 (SMMC-7721), and 5,000 (HUVEC) cells per well and incubated for 24 hours at 37°C in a 5% CO2 incubator. The compounds were dissolved in DMSO and diluted with culture medium. The compounds were added to the wells at final concentrations of 1.95, 3.9, 7.8, 15.625, 31.25, 62.5, 125, and 250 μM before incubation at 37°C in a 5% CO2 incubator for 48 hours. After that, the cells were treated with 10% volume ratio (v/v) MTT dye solution (5 mg/mL) for 4 hours. The media with MTT solution was replaced with DMSO (150 μL). The 96-well culture plates were then gently shaken in the dark for 30 minutes, and absorbance at 570 nm and 630 nm (background) was measured with a microtiter plate reader. The half-maximal inhibitory concentration (IC50) value was determined from the chart of cell viability (%) against compound dose (μM).34
In vitro cytotoxic activities were evaluated for all synthesized compounds against the MCF-7, SW-1990, SMMC-7721, and HUVEC cell lines. Berberine was taken as the reference standard in this study. Berberine and compounds 4–36 were dissolved in DMSO and diluted with culture medium containing 0.1% DMSO. Cells were treated with culture medium containing 0.1% DMSO. The percent inhibition of viability at each concentration of the compounds was calculated with respect to the control, and IC50 values were estimated with the software SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Each experiment was repeated three times, and the results are summarized in Table 1. The response parameter calculated was IC50 values (Table 1), which corresponded to the compound concentration causing 50% mortality in net cells.
Statistical analysis
Each experiment was performed at least in triplicate. The data are presented as mean ± standard deviation. Using analysis of variance, statistical significance was determined. Mean values with probability values of P<0.05 were taken as statistically significant.
Results and discussion
In vivo anticancer activity
From the IC50 values (Table 1) and percentage inhibition data (Figures 2–4), it is obvious that most of the tested compounds displayed better anticancer activities than that of the reference drug berberine with an IC50 value of 121.91±11.26 μM (MCF-7) and 68.06±7.76 μM (SMMC-7721). However, the activity of berberine against SW-1990 cells was better than that of the derivatives, with an IC50 value of 27.64±3.04 μM. Berberine only exhibited greater than 50% inhibition against SW-1990 cell growth at 62.5 μM. In addition, the tested compounds generally showed a lower effect on noncancer cells (HUVECs) than the reference drug berberine, which had an IC50 value of 18.33±2.31 μM. For example, compounds 4, 14, 15, 17, 18, 26 and 32 against HUVEC cell line had IC50 values of 71.92±9.04, 67.69±8.95, 145.71±19.01, 61.66±6.78, 71.18±9.03, 74.62±9.21 and 65.96±7.26, respectively.
Compound 16 has a tert–butyl group substituted phenyl, which inhibited the human pancreatic carcinoma SW-1990 cell line and human liver carcinoma SMMC-7721 cell line, with IC50 values of 8.54±1.97 μM and 11.87±1.83 μM, while it had a lower effect than berberine on noncancerous HUVEC cells, with an IC50 value of 25.49±3.24 μM. In comparison, the IC50 values of compound 16 against MCF-7, SW-1990, and SMMC-7721 were 8, 3, and 6 times lower than that of berberine. However, it was less potent than compound 36 against the human breast adenocarcinoma MCF-7 cell line, with an IC50 value of 15.80±2.14 μM. Figures 2–4 show that compound 16 exhibited greater than 50% inhibition of MCF-7, SW-1990, and SMMC-7721 cell growth at 7.8 μM.
Compound 36 has 1, 3, 5-trichloro substituents instead of an R position. It showed the best activity of all of the derivatives against the MCF-7 cell line, with an IC50 value of 12.57±1.96 μM. It was less potent than compound 16 against the SW-1990 cell line (IC50 value of 13.32±2.36 μM) and the SMMC-7721 cell line (IC50 value of 18.68±2.69 μM), and displayed lower cytotoxicity than berberine to HUVEC cells, with an IC50 value of 30.47±3.47 μM. Furthermore, compound 36 exhibited greater than 45% inhibition of MCF-7 cell growth at 7.8 μM (Figure 2), which is much better than the reference drug berberine and other target compounds.
Compounds 4 (R=−H), 5–7 (R=−2-F, –3-F, −4-F), 8–10 (R=−2-Cl, –3-Cl, −4-Cl), 11–13 (R=−2-Br, –3-Br, −4-Br), 14-15 (R=−2-CH3, −4-CH3), 17–18 (R=−3-OCH3, −4-OCH3), and 24 (R=−4-NO2) were single substitution at the R position. Among them, compound 14 inhibited the SMMC-7721 cell line, with an IC50 value of 19.95±2.33 μM. Compound 15 inhibited the MCF-7, SW-1990, and SMMC-7721 cell lines, with IC50 values of 37.83±4.66 μM, 32.03±4.23 μM, and 39.37±4.98 μM, respectively. However, compound 24 exhibited weaker activities than compound 15 against the three cancer cell lines (MCF-7, SW-1990, SMMC-7721), with IC50 values of 206.47±23.59 μM, 54.23±6.55 μM, and 63.81±8.90 μM, respectively. Compound 4 contained no group on the substituted phenyl ring and exhibited weaker anticancer activities against the three cancer cell lines than compound 15 but better activities than compound 13. Compounds 5, 6, and 7 have a single fluorine instead of an aromatic ring at the ortho, meta, and para positions. However, these compounds showed weaker anticancer activities than any other compounds with electron-withdrawing substituents on the phenyl group (compounds 8–13).
Among the derivatives tested, compounds 25 and 26 had 4-methyl formate and 4-methylsulfonyl at the R position, respectively, which inhibited against four cancer cells lines with IC50 values of 248.55±20.13 μM (MCF-7), 165.27±18.94 μM (SW-1990), and 53.49±5.84 μM (SMMC-7721), and 206.84±19.65 μM (MCF-7), 100.76±9.01 μM (SW-1990), and 69.68±8.93 μM (SMMC-7721), respectively.
From the data, we can indicate that adding disubstituted groups on the phenyl ring of compounds 27–35 showed moderate effect against three human cancer cell lines. However, compounds 32–35 had a dichlorine group at the R position and had enhanced activity compared to that of other electron-withdrawing group derivatives (compounds 27–31). Compounds 32 and 35 had 2, 4-dichloro and 3, 4-dichloro substituents on the phenyl group, respectively. Compounds 32 and 35 inhibited against the MCF-7, SW-1990, and SMMC-7721 cell lines, with IC50 values of 28.20±3.46 μM (compound 32, MCF-7), 30.78±4.32 μM (compound 35, MCF-7), 28.09±2.68 μM (compound 32, SW-1990), 20.35±2.35 μM (compound 35, SW-1990), 27.94±2.53 μM (compound 32, SMMC-7721), and 16.15±2.92 μM (compound 35, SMMC-7721). However, the IC50 value of compound 35 against HUVECs was four times lower than that of 32. It is obvious that compound 32 showed weaker cytotoxicity than compound 35 against the noncancer HUVEC cell line. Besides, among these derivatives, compound 33 showed the most potent cytotoxicity against HUVECs, with an IC50 value of 7.88±1.12 μM.
Compared to compound 32 (R=−2, 4-2Cl) the activity of compound 30 (R=−2-F, 4-Cl) was weaker. Similarly, compound 27 had 2-4-difluorine substituents on the phenyl group, but the cytotoxicity of compound 27 against the three cancer cell lines was lower than that of compound 31 (R=−2-Br, 4-F).
Conclusions
This study described the construction of a new series of triazole-containing berberine derivatives using CuAAC “click” chemistry. All of the compounds synthesized were screened for anticancer activity against a panel of three human cancer cell lines, MCF-7 (breast), SW-1990 (pancreatic), and SMMC-7721 (liver), and the noncancer HUVEC (human umbilical vein endothelial cell) cell line. From the data, it was evident that most of the target compounds exhibited better anticancer activity against all the tested cancer cell lines than the reference drug berberine used in this study. Compounds 16 and 36, which had tert–butyl and 1,3,5-trichlorosubstituents on the phenyl group, were the most promising target compounds. It was noted that highly active anticancer compounds showed lower cytotoxicity to the noncancerous HUVEC cells compared with berberine. Detailed mechanistic studies are currently underway in our laboratory.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (General Program No 81273556/H3106) and by Shanghai Key Projects of Basic Research (Number 11JC1415400).
Disclosure
The authors report no conflicts of interest in this work.
References
Ciardiello F, Tortora G. A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res. 2001:7(10):2958–2970. | |
Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56(11):1649–1659. | |
Aird RE, Cummings J, Ritchie AA, et al. In vitro and in vivo activity and cross resistance profiles of novel ruthenium (II) organometallic arene complexes in human ovarian cancer. Br J Cancer. 2002;86(10):1652–1657. | |
Grycová L, Dostál J, Marek R. Quaternary protoberberine alkaloids. Phytochemistry. 2007;68(2):150–175. | |
Iwasa K, Kamigauchi M, Ueki M, Taniguchi M. Antibacterial activity and structure-activity relationships of berberine analogs. Eur J Med Chem. 1996;31(6):469–478. | |
Iwasa K, Kim HS, Wataya Y, Lee DU. Antimalarial activity and structure-activity relationships of protoberberine alkaloids. Eur J Med Chem. 1998;33(1):65–69. | |
Vennerstrom JL, Lovelace JK, Waits VB, Hanson WL, Klayman DL. Berberine derivatives as antileishmanial drugs. Antimicrob Agents Chemother. 1990;34(5):918–921. | |
Yang P, Song DQ, Li YH, et al. Synthesis and structure-activity relationships of berberine analogues as a novel class of low-density- lipoprotein receptor up-regulators. Bioorg Med Chem Lett. 2008; 18(16):4675–4677. | |
Samosorn S, Tanwirat B, Muhamad N, et al. Antibacterial activity of berberine-NorA pump inhibitor hybrids with a methylene ether linking group. Bioorg Med Chem. 2009;17(11):3866–3872. | |
Park KD, Lee JH, Kim SH, Kang TH, Moon JS, Kim SU. Synthesis of 13-(substituted benzyl) berberine and berberrubine derivatives as antifungal agents. Bioorg Med Chem Lett. 2006;16(15):3913–3916. | |
Chen WH, Pang JY, Qin Y, Peng Q, Cai Z, Jiang ZH. Synthesis of linked berberine dimers and their remarkably enhanced DNA-binding affinities. Bioorg Med Chem Lett. 2005;15(10):2689–2692. | |
Hoshi A, Ikekawa T, Ikeda Y, Shirakawa S, Iigo M. Antitumor activity of berberrubine derivatives. Gann. 1976;67(2):321–325. | |
Lo CY, Hsu LC, Chen MS, et al. Synthesis and anticancer activity of a novel series of 9-O-substituted berberine derivatives: a lipophilic substitute role. Bioorg Med Chem Lett. 2013;23(1):305–309. | |
Pang JY, Qin Y, Chen WH, Luo GA, Jiang ZH. Synthesis and DNA-binding affinities of monomodified berberines. Bioorg Med Chem. 2005;13(20):5835–5840. | |
Zhang WJ, Ou TM, Lu YJ, et al. 9-Substituted berberine derivatives as G-quadruplex stabilizing ligands in telomeric DNA. Bioorg Med Chem. 2007;15(16):5493–5501. | |
Ma Y, Ou TM, Hou JQ, et al. 9-N-Substituted berberine derivatives: stabilization of G-quadruplex DNA and down-regulation of oncogene c-myc. Bioorg Med Chem. 2008;16(16):7582–7591. | |
Li QY, Zu YG, Liu TY, et al. Generation of reactive oxygen species by a novel berberine-bile acid analog mediates apoptosis in hepatocarcinoma SMMC-7721 cells. Biochem Biophys Res Commun. 2013;433(4):432–437. | |
Franceschin M, Rossetti L, D’Ambrosio A, et al. Natural and synthetic G-quadruplex interactive berberine derivatives. Bioorg Med Chem Lett. 2006;16(6):1707–1711. | |
Beausoleil E, Chauvignac C, Taverne T, et al. Structure-activity relationship of isoform selective inhibitors of Rac1/1b GTPase nucleotide binding. Bioorg Med Chem Lett. 2009;19(19):5594–5598. | |
Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol. 2002;91(4):193–197. | |
Chen CM, Chang HC. Determination of berberine in plasma, urine and bile by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1995;665(1):117–123. | |
Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001;40(11):2004–2021. | |
Lutz JF; Nanotechnology for Life Science Research Group. 1,3-dipolar cycloadditions of azides and alkynes: a universal ligation tool in polymer and materials science. Angew Chem Int Ed Engl. 2007;46(7):1018–1025. | |
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41(14):2596–2599. | |
Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67(9):3057–3064. | |
Binder WH, Sachsenhofer R. “Click” chemistry in polymer and materials science. Macromol Rapid Commun. 2007;28(1):15–54. | |
Bock VD, Hiemstra H, van Maarseveen JH. CuI-catalyzed alkyne-azide “click” cycloadditions from a mechanistic and synthetic perspective. Eur J Org Chem. 2006;2006(1):51–68. | |
Fournier D, Hoogenboom R, Schubert US. Clicking polymers: a straightforward approach to novel macromolecular architectures. Chem Soc Rev. 2007;36(8):1369–1380. | |
Angell YL, Burgess K. Peptidomimetics via copper-catalyzed azide-alkyne cycloadditions. Chem Soc Rev. 2007;36(10):1674–1689. | |
Wang Q, Chittaboina S, Barnhill HN. Highlights in organic chemistry advances in 1,3-dipolar cycloaddition reaction of azides and alkynes – a prototype of “click” chemistry. Lett Org Chem. 2005;2(4):293–301. | |
Hua Y, Flood AH. Click chemistry generates privileged CH hydrogen-bonding triazoles: the latest addition to anion supramolecular chemistry. Chem Soc Rev. 2010;39(4):1262–1271. | |
Nandivada H, Jiang X, Lahann J. Click chemistry: versatility and control in the hands of materials scientists. Adv Mat. 2007;19(17):2197–2208. | |
Bodiwala HS, Sabde S, Mitra D, Bhutani KK, Singh IP. Synthesis of 9-substituted derivatives of berberine as anti-HIV agents. Eur J Med Chem. 2011;46(4):1045–1049. | |
Sieuwerts AM, Klijn JG, Peters HA, Foekens JA. The MTT tetrazolium salt assay scrutinized: how to use this assay reliably to measure metabolic activity of cell cultures in vitro for the assessment of growth characteristics, IC50-values and cell survival. Eur J Clin Chem Clin Biochem. 1995;33(11):813–823. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.