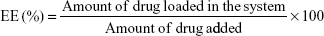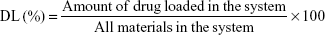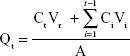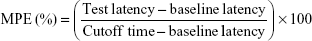Back to Journals » Drug Design, Development and Therapy » Volume 11
Design and evaluation of lidocaine- and prilocaine-coloaded nanoparticulate drug delivery systems for topical anesthetic analgesic therapy: a comparison between solid lipid nanoparticles and nanostructured lipid carriers
Received 3 May 2017
Accepted for publication 22 August 2017
Published 18 September 2017 Volume 2017:11 Pages 2743—2752
DOI https://doi.org/10.2147/DDDT.S141031
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Peijun You,1 Ran Yuan,2 Chuanyu Chen1
1Department of Anesthesiology, Shandong Jining No 1 People’s Hospital, Shandong, People’s Republic of China; 2Department of Anesthesiology, Affiliated Hospital of Jining Medical College, Jining, Shandong, People’s Republic of China
Purpose: Topical anesthesia analgesic therapy has diverse applicability in solving the barrier properties of skin and unfavorable physicochemical properties of drugs. Lidocaine (LID) combined with prilocaine (PRI) has been used as a topical preparation for dermal anesthesia for treatment of conditions such as paresthesia.
Materials and methods: In this study, for combination anesthesia and overcoming the drawbacks of LID and PRI, respectively, LID- and PRI-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) were prepared and characterized by determination of their particle size, drug loading capacity, stability, in vitro drug release behavior and in vitro cellular viability. Ex vivo skin permeation and in vivo anesthesia analgesic efficiency of these two systems were also evaluated and compared.
Results: Results revealed that combination delivery of the dual drugs exhibited more remarkable efficiency than signal drug-loaded systems. SLN systems have better ex vivo skin permeation ability than NLCs. NLC systems revealed a stronger in vivo anesthesia analgesic effect than SLN systems.
Conclusion: It can be concluded that SLNs and NLCs have different advantages, and that both carriers are promising dual drug delivery systems for topical anesthetic analgesic therapy.
Keywords: topical anesthesia, prilocaine, lidocaine, solid lipid nanoparticles, nanostructured lipid carriers
Introduction
Topical anesthesia has diverse applicability in alleviating pain, anxiety and discomfort caused by needle insertion and local anesthetic injection prior to anesthesia during surface skin surgical procedures.1–3 Drug delivery of local anesthetics (LAs) via the skin brings a lot of advantages such as easier application, longer sustained drug release, less systemic adverse reactions and higher patient compliance.4 Among the commercially available topical anesthetic products, the main dosage forms include gels, ointments, creams and so on, which noninvasively deliver LA agents such as prilocaine (PRI), lidocaine (LID) and benzocaine in locally required areas.5,6 Eutectic mixture of local anesthetic (EMLA) cream (LID 2.5% and PRI 2.5%) developed by Astra USA, Inc., which was approved by the US Food and Drug Administration in 1992, has been widely used on normal intact skin for local analgesia, and in genital mucus membranes for superficial minor surgery and as pretreatment for infiltration anesthesia. The eutectic mixture of LID and PRI has a melting point below room temperature, and therefore, both LA agents exist as liquid oil rather than as crystals. However, EMLA induces short duration of action and several clinical side effects such as edema and erythema, mostly because of its emulsion structure and formulation.7–9
Currently available literature indicates exploration of nanosystems for topical delivery of both LA agents (LID and PRI), such as phospholipid microemulsions, nanoemulsion and nanostructured lipid carriers (NLCs).4,10,11 Negi et al developed phospholipid microemulsion-based gel formulation and nanoemulsion to modify the in vivo efficacy of the eutectic mixture of LID and PRI.4,10 Results revealed that this system could enhance skin permeability and compliance, and therefore has been proposed as a more useful alternative for the topical delivery of LID and PRI. Ribeiro et al used factorial design to optimize a process for the preparation of NLC for delivery of LID and PRI.11 These results focused on in vitro research, and there was lack of in vivo evaluation. In conclusion, researchers have focused on ideal formulations for local anesthesia that promote the permeation of the drug at the site of application and a rapid and sustained onset of action, thus enhancing the efficacy and reducing toxicity.
Lipid-based delivery systems are composed of biocompatible and biodegradable lipids that can be utilized for controlled release, skin delivery and drug protection in the field of topical anesthesia.12–15 Among various lipid nanoparticles, solid lipid nanoparticles (SLNs) and NLCs are considered to be the most suitable ones due to the following advantages: high adhesion to the skin, thus forming a uniform film on the stratum corneum and enhancing skin permeation; increased chemical stability, thus preventing leaching during storage; opaque nature and the absence of any thickeners, thus bringing a pleasant esthetic character and skin feel; and controlled release, thus keeping persistent anesthetic effect.12,16–18 SLNs are composed of solid lipid or a blend of solid lipids, which are in the solid state at room and body temperature, and NLCs have been developed using the blend of both solid lipids and liquid lipids, which show a melting point lower and a drug loading higher than those of SLNs, respectively.19,20
In this study, LID and PRI were co-encapsulated in SLNs as well as NLCs. The formulations were evaluated for various physicochemical parameters including particle size, zeta potential, drug loading capacity (DL), in vitro drug release, in vitro cytotoxicity, in vitro skin permeation, in vivo anesthetic and analgesic activity in animal models.
Materials and methods
Materials
LID, PRI, DMEM, MTT and dimethyldioctadecylammonium bromide (DDAB; purity ≥98.0%), were purchased from Sigma-Aldrich (St Louis, MO, USA). Injectable soya lecithin (ISL) was obtained from Lipoid GmbH (Ludwigshafen, Germany). Compritol® 888 ATO, Precirol® ATO 5 and glycerol monostearate (GMS) were obtained as gifts from Gattefossé (Lyon, France). All other chemicals and reagents were of analytical grade or high-performance liquid chromatography (HPLC) grade and were used without further purification.
Animals
Mouse embryonic fibroblasts (BALB/c-3T3 cells) were obtained from the American Type Culture Collection (Manassas, VA, USA). BALB/c-3T3 cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA). Cells were grown as suspension cultures and maintained in a humidified atmosphere at 37°C±2°C and 5% CO2.
Wistar rats (9–11 weeks old, 301–350 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Rats were housed in the following conditions: 25°C±2°C, 30%–70% humidity and 12/12 h light/dark cycle. All animal experiments complied with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No 8023, revised 1978). Ethics approval was received from the Medical Ethics Committee of Shandong Jining No 1 People’s Hospital (Reference number: SDJNPH20170306001).
Preparation of SLNs
LID and PRI co-encapsulated SLNs (LID/PRI SLNs) were prepared using nanoprecipitation and solvent evaporation method.21,22 Lipid phase: LID (50 mg), PRI (50 mg), ISL (200 mg) and GMS (250 mg) were dissolved in 10 mL of chloroform. Aqueous phase: 100 mg DDAB was dissolved in 40 mL of Milli-Q water. Lipid phase was added to aqueous phase drop by drop, and stirred at 600 rpm and at room temperature for 12 h to get LID/PRI SLNs. LID-encapsulated SLNs (LID SLNs), PRI-encapsulated SLNs (PRI SLNs) and blank SLNs (SLNs) were prepared following the same procedures.
Preparation of NLCs
LID and PRI co-encapsulated NLCs (LID/PRI NLCs) were prepared by solvent diffusion method.23,24 Lipid phase: Compritol 888 ATO (200 mg), Precirol ATO 5 (100 mg) and GMS (200 mg) were mixed together and heated to 80°C±2°C to get a lipid dispersion; LID (50 mg), PRI (50 mg) and ISL (200 mg) were dissolved in 2 mL of dimethyl formamide and added to the lipid dispersion. Aqueous phase: 100 mg DDAB was dissolved in 45 mL of Milli-Q water, stirred at 600 rpm and heated to 30°C. The lipid phase was rapidly injected into the stirred aqueous phase and the resulting suspension was then dispersed by dialysis against pH 7.4 PBS for 4 h to get LID/PRI NLCs. LID-encapsulated NLCs (LID NLCs), PRI-encapsulated NLCs (PRI NLCs) and blank NLCs (NLCs) were prepared following the same procedures.
Preparation of SLNs and NLCs freeze-dried powder
All kinds of SLN and NLC suspensions prepared above were centrifuged at 10,000 rpm for 10 min and the supernatant was discarded.14,25,26 The pellet was washed with Milli-Q water for three times. Mannitol (5.0%, w/v) was added as a cryoprotectant for the freeze-drying process. The suspensions were frozen at −40°C, and then prefreezing was continued at −80°C for 24 h. Lyophilization was carried out at a pressure of 0.1 mbar and a temperature of −60°C for 48 h. The obtained SLN and NLC powders were collected and kept for further analysis.
Particle size, size distribution and zeta potential
The particle size (presented as volume-mean particle diameter), size distribution (presented as polydispersion indices [PDIs]) and zeta potential of SLNs and NLCs were measured by dynamic light scattering with Zetasizer Nano-ZS (Malvern Instruments, Malvern, UK).27
Drug encapsulation efficiency and loading capacity
The drug encapsulation efficiency (EE) and the DL were evaluated by quantifying the amount of LID or PRI encapsulated in SLNs or NLCs by gel filtration method using Sephadex column (Sephadex G-50 Column).28 Sephadex G-50 mini column centrifugation technique is a chromatographic method in which molecules in solution are separated by their size and, in some cases, molecular weight. It is generally applied to large molecules or macromolecular complexes such as proteins and polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. The LID or PRI was separated from the SLNs or NLCs, and HPLC was used to measure the amount of drugs. A liquid chromatographic pump with autosampler connected to a UV–vis detector was used for the quantitative determination of LID and PRI. A Purospher C18 (5 mm, 250×4.6 mm) column was used with 77:23 methanol–NaH2PO4 (0.02 M; pH 5) buffer as the mobile phase and a flow rate of 1 mL/min. Volumes of 20 mL were injected in the column and the LID and PRI were analyzed at a wavelength of 210 nm. The column temperature was maintained at 30°C.4 The EE and DL of drug-encapsulated SLNs or NLCs were calculated as follows:
|
|
The SLN and NLC freeze-dried powders were suspended with Milli-Q water at 25°C±2°C before the test. Stability of SLNs and NLCs was evaluated by measuring the mean diameter, PDI and zeta potential for 120 days.29 The results were calculated at predetermined time intervals (0, 15, 30, 60, 90 and 120 days).
In vitro drug release
In vitro release behaviors of the drugs from SLNs and NLCs were determined using Franz diffusion cells.30 Briefly, all kinds of SLNs and NLCs were placed evenly on the surface of the membrane (molecular weight cutoff =30,000 Da) mounted between the donor and receiver compartments of Franz diffusion cells. The receiver compartment was filled with pH 7.4 PBS, stirred at 300 rpm and maintained at 32°C±1°C using circulating water bath. Then, 0.5 mL of samples was collected at predetermined time points (1, 2, 4, 6, 8, 12, 24 and 48 h) from the receiver compartment and replaced with fresh pH 7.4 PBS. The amount of drugs released was determined by HPLC as described in the “Drug encapsulation efficiency and loading capacity” section.
In vitro cellular viability
In vitro cellular viability was evaluated using the MTT reduction test performed with mouse BALB/c-3T3 cells.31,32 Cells were seeded at ~2×104 into 96-well plates and incubated for 48 h. The cells were exposed for 24 h to samples of free LID, free PRI, free LID and PRI (LID/PRI), and LID- and/or PRI-loaded SLNs and NLCs. The drug concentrations ranged from 0.1 to 1.6 mg/mL. Untreated cells were used as control. Cells were then detected by incubation in the presence of MTT solution (25 μL, 5 mg/mL) for 2 h at 37°C. The amount of MTT converted to formazan was measured to determine the percentage of viable cells, using a microplate reader at a wavelength of 630 nm.
Ex vivo skin permeation study
Ex vivo permeation study experiments of LID- and/or PRI-loaded SLNs and NLCs were carried out on Franz diffusion cells.27,33 Firstly, rat abdominal skin was prepared as follows. Wistar rats were sacrificed and the fur on the abdominal area of the rats was removed. The skin was excised from the abdominal surface and the adherent fat and subcutaneous tissue was removed. The excised rat skin was mounted between donor and receptor compartments of the Franz diffusion cells with a surface area of 2.2 cm2 and a receptor volume of 20 mL. Samples of free LID and/or PRI, LID- and/or PRI-loaded SLNs and NLCs containing 25 mg of LID and 25 mg of PRI were placed in the donor compartment. In the receptor compartment, 20 mL of PBS (pH 7.4) was placed. At predetermined time intervals (0, 0.5, 1, 2, 4, 6, 8, 12, 24, and 48 h), 1 mL of receptor medium was taken from the receiver and was replaced with 1 mL of PBS. The amount of drugs in the receptor medium was assayed by HPLC as described in the “Drug encapsulation efficiency and loading capacity” section. The cumulative amount of LID and PRI permeated was calculated as follows:
|
where Ct represents the drug concentration of the receptor medium at each sampling time interval, Ci the drug concentration of the ith sample, V0 the volume of the receiver solution (20 mL), Vi the volume of the sample (1 mL) and A is the effective diffusion surface area (2.2 cm2).
In vivo anesthesia analgesic effect evaluation
Radiant heat tail-flick latency (TF latency) test was employed for evaluating the in vivo anesthetic effect of LID- and/or PRI-loaded SLNs and NLCs.34 Wistar rats were randomly divided into 10 groups, with each group consisting of eight animals, and samples (containing 25 mg of LID and/or 25 mg of PRI) were applied on the tail. The samples included: LID/PRI SLNs, LID SLNs, PRI SLNs, LID/PRI NLCs, LID NLCs, PRI NLCs, free LID/PRI, free LID, free PRI and 0.9% saline control. Rats were placed in a plastic box; the ventral surface of the distal 5–6 cm of the tail was placed over a 0.5 cm hole, beneath which an infrared radiant bulb was placed. A 10 s cutoff was used to minimize the risk of tissue damage. TF latency was converted to represent the maximum possible effect (MPE) according to the following formula:
|
The baseline latency was calculated as the mean of three different measurements taken at 15 min intervals. Baseline latencies typically ranged from 2.5 to 3.0 s.
Statistical analysis
All data are expressed as mean ± SD. Statistical analyses were performed using Student’s t-test. Differences were considered significant when the P-value was <0.05 (P<0.05).
Results
Preparation and characterization of SLNs and NLCs
SLNs and NLCs were characterized by particle size, size distribution, zeta potential, EE and DL (Table 1). Particle size of SLN systems was about 120 nm, which was smaller than that of NLC systems (175 nm). The size distribution of SLNs and NLCs was around 0.2. The zeta potential of LID/PRI SLNs and LID/PRI NLCs was +29 and +21 mV, respectively. The EE of both SLNs and NLCs was above 90%. The DL of SLN systems was higher than that of NLC systems (P<0.05).
Stability of SLNs and NLCs
The stabilities of SLNs and NLCs were evaluated over a period of 120 days. For both SLNs and NLCs, the average particle diameter remained almost constant throughout the period (Figure 1A). There were no significant changes with time in the PDIs of all the formulations tested (Figure 1B). For both formulations, the zeta potential was positive and showed no major changes during the 90 days of storage (Figure 1C).
In vitro drug release
In vitro LID and PRI release from SLN and NLC systems is illustrated in Figure 2A and 2B. Sustained-release patterns were found with both SLN and NLC systems during the 48 h of testing. Compared with SLNs, NLCs showed a faster release before the first 8 h when more than half of the drugs were released from the vectors. However, SLNs exhibited a more sustained drug release behavior.
In vitro cellular viability
In vitro cellular viability data obtained using BALB/c-3T3 fibroblast cells incubated with free drugs and drug-loaded SLNs and NLCs are shown in Figure 3. At all the studied concentrations, LID- and/or PRI-loaded NLCs had moderate effect on cellular viability (higher or around 80%). Decreases in viability of cells occurred in the LID- and/or PRI-loaded SLN formulas and free drug solutions. The significance among the systems was also considered and is illustrated in the figure. The IC50 values of free drug solutions were between 0.7 and 1.0 mg/mL, which are significantly higher than those of LID- and/or PRI-loaded NLCs and SLNs.
Ex vivo skin permeation
The skin permeation abilities of the various systems were evaluated by ex vivo permeation study. Ex vivo drug permeation behaviors of LID- and/or PRI-loaded SLNs and NLCs were calculated and compared with free LID and/or PRI (Figure 4). Increases in permeation of LID and/or PRI were observed with time. The LID- and/or PRI-loaded SLNs and NLCs were much more sufficient than the free drug solutions (P<0.05). During the first 6 h, the drug permeation of NLCs was more effective than that of SLNs. However, after 6 h, the SLNs showed better skin permeation capacity (P<0.05).
In vivo anesthesia analgesia effect
In vivo anesthesia analgesia effect of drug-loaded SLNs and NLCs were evaluated by TF latency test. It can be found in Figure 5 that all the SLNs, NLCs and free drugs increased the TF latency significantly. There were no significant differences in baseline TF latency among all the groups tested. LID- and/or PRI-loaded SLNs and NLCs showed effective and long-lasting effect than the free drug groups. Compared with LID- and/or PRI-loaded SLNs, the NLC systems induced more remarkable anesthetic effect (P<0.05). Most importantly, the dual drugs–coloaded carriers exhibited significant anesthesia analgesia effect than the single drug-loaded carriers (P<0.05).
Discussion
Lipid-based delivery systems can be utilized for skin delivery in topical anesthesia.35 SLNs and NLCs are the most suitable ones because of their high adhesion to the skin, thus bringing about a pleasant esthetic character and skin feel, and controlled release of the drug leading to persistent anesthetic effect. In this study, LID and PRI were co-encapsulated in SLNs as well as NLCs. These two kinds of delivery systems were evaluated and compared throughout the research.
SLNs and NLCs were characterized by their particle size, size distribution, zeta potential, EE and DL (Table 1). Size is critical to topical drug delivery systems, since it was reported that particles smaller than 300 nm have the ability to deliver the drugs into deeper layers of the skin.36 The average diameter of SLNs and NLCs in this study was below 200 nm; this may be the evidence that the formulation had the potential to deliver the drugs across the skin. Particle sizes of SLN systems were smaller than those of NLC systems (P<0.05). This may be explained by the fact that only solid lipids are used in SLN formulation; on the contrary, the lipids used in NLC formulation include liquids and solid lipids. Future studies are required in order to find out if this helps with the delivery of the drugs. In addition, PDI values of the SLNs and NLCs were obtained as around 0.2, suggesting homogenous size distribution in all formulations.37 The zeta potential of LID/PRI SLNs and LID/PRI NLCs was positive due to the presence of amine groups of DDAB. The incorporation of the cationic lipid (DDAB) induced a positive zeta potential of the carriers.38 The lower surface charge of the blank SLNs and NLCs than that of the corresponding drug-loaded particles may be explained by some of the positive drugs sitting near the surface of the particles. This phenomenon may cause faster release in the first few hours. We may observe this in the In vitro drug release section. The EE of both SLNs and NLCs was above 90%. Compared to other kinds of NLCs published in the literature, the EE values of LID were in the range 37%–44%, while lower values of around 33%–37% were obtained for PRI.11 This confirmed that in our study, the drug dissolved in the lipid matrix, remained associated with the matrix and there was no obvious drug diffusion.39
Stability evaluation of the SLNs and NLCs was essential to confirm that the structural properties were preserved over storage time, since disruption of nanocarriers in the drug delivery systems could affect their therapeutic potential.11 The stabilities of nanocarriers were evaluated over a period of 120 days in terms of the mean particle diameter, PDI and zeta potential. For SLNs and NLCs, no aggregate was found, as the average particle diameter remained almost constant throughout the period. There were no significant changes with time in the PDIs of all the formulations tested. The value of the zeta potential also provides an indication of the stability of carriers in suspension. Both SLN and NLC systems were stable during the 4 months of storage time.
In vitro release of LID and PRI from the SLN and NLC systems was in a sustained-release pattern during the 48 h of testing. The sustained-release behavior is an important prerequisite for successful drug delivery.40 This phenomenon could be explained by the entrapment of the drugs by the lipid materials, which could slow down the release of the drugs. NLCs showed a faster release before the first 8 h than SLNs; this may be due to the fact that the liquid lipid used in the NLCs lets the drugs be released from the carriers easier than the solid lipid used in the SLNs. The faster drug release during the first 12 h may be explained by the fact that there are some drugs near the surface of the particles, and thus, they could be released faster initially.
In vitro cellular viability data of free drugs and drug-loaded SLNs and NLCs were different. Firstly, the samples tested in the study followed a dose-dependent manner. The cytotoxicity increased with the increase of drug concentrations. Secondly, the drug-loaded SLNs and NLCs had no obvious effect on cell viability, compared with free drugs (P<0.05). Lower toxicity to the cells may be explained by the protective effects of the carriers to the drugs, thus letting less drugs be exposed to the cells.31 Finally, drug-loaded NLCs showed obvious higher cell viability compared to drug-loaded SLNs (P<0.05). This may be explained by better encapsulation and protection of drugs by NLCs than their SLNs counterpart; also, the size of the particles may influence toxicity.
Ex vivo skin permeation efficiency of LID- and/or PRI-loaded SLNs and NLCs was much more than that of free drug solutions (P<0.05). The results illustrated that both SLN and NLC systems have better skin permeation capacity, which may be due to the nanoscopic size and the biocompatibility for long-term skin administration.41 The skin permeation capacities of the two systems were also compared with those of some similar systems reported. The LID- and PRI-loaded nanoemulsion had a cumulative skin permeation of <250 μg/cm2 for PRI and 200 μg/cm2 for LID.4,10 However, in this study, the cumulative skin permeation of PRI and LID was 521 and 547 μg/cm2, respectively. During the first 6 h, the drug permeation of NLCs was more effective than that of SLNs; this may be due to the faster release of NLCs during the first few hours. After 6 h, the SLNs showed better skin permeation capacity. Thus, it can be concluded that SLNs had better permeation efficiency than NLCs during the experiments. The particle sizes of SLN systems were smaller than those of NLC systems (P<0.05), which could be the explanation for better ex vivo permeation of the SLN systems. Could SLNs also get better efficiency in vivo? We applied the in vivo study to verify this.
In vivo TF latency test is the most frequently used method. This test uses radiant heat to both measure the latency of the response to thermal noxious stimuli and assess the pain threshold and effect of anesthesia. The time of MPE in the saline group showed no obvious change during the experiment. LID- and/or PRI-loaded SLNs and NLCs showed long-lasting effect than the free drug groups. The results indicated that the drug-loaded nanocarriers revealed a more interesting anesthetic effect in the first few minutes and also displayed sustained anesthetic activity, compared with free drugs. The more remarkable anesthetic effect of the NLC systems than the SLNs illustrates that the impressive anesthetic effect of the NLC systems could bring about better LA therapy effects than SLNs. LID based ethosomes and liposomes were found to have weaker and relatively shorter-lasting anesthetic effect than the SLN and NLC systems in this study.42 Significantly better anesthesia analgesic effect of the dual drugs–coloaded carriers than the single drug-loaded carriers could prove the synergistic effect of the double drugs co-delivered by the same vector. We could also conclude from the results that SLN systems exhibited outstanding permeation efficiency and could deliver the drugs more effectively through the skin; also, the NLC systems could better enhance the in vivo anesthesia analgesia effect of the drugs when applied on the rats.
Conclusion
In summary, LID- and PRI-coloaded SLNs and NLCs can enhance the skin permeation and anesthesia analgesic effect of LID and PRI. Combination delivery of the dual drugs exhibited more remarkable efficiency than signal drug-loaded systems. LID/PRI SLNs have smaller particle size than LID/PRI NLCs, and thus get better ex vivo skin permeation ability. The release of LID/PRI NLCs appeared faster during the first several hours than that of LID/PRI SLNs, and also revealed stronger in vivo anesthesia analgesic effect than SLN systems. Both SLNs and NLCs are promising dual drugs delivery systems for topical anesthetic analgesic therapy with different aspects of advantages. Our further research will mainly focus on how to combine the superiority of SLNs and NLCs together and construct a more efficient system.
Disclosure
The authors report no conflicts of interest in this work.
References
de Araújo DR, da Silva DC, Barbosa RM, et al. Strategies for delivering local anesthetics to the skin: focus on liposomes, solid lipid nanoparticles, hydrogels and patches. Expert Opin Drug Deliv. 2013;10(11):1551–1563. | ||
Musawi AA, Andersson L. Use of topical as only anesthetic for suturing a traumatic facial laceration. Dent Traumatol. 2010;26(3):292–293. | ||
Meechan JG, Howlett PC, Smith BD. Factors influencing the discomfort of intraoral needle penetration. Anesth Prog. 2005;52(3):91–94. | ||
Negi P, Singh B, Sharma G, Beg S, Katare OP. Biocompatible lidocaine and prilocaine loaded-nanoemulsion system for enhanced percutaneous absorption: QbD-based optimization, dermatokinetics and in vivo evaluation. J Microencapsul. 2015;32(5):419–431. | ||
Friedman PM, Mafong EA, Friedman ES, Geronemus RG. Topical anesthetics update: EMLA and beyond. Dermatol Surg. 2001;27(12):1019–1026. | ||
Tadicherla S, Berman B. Percutaneous dermal drug delivery for local pain control. Ther Clin Risk Manag. 2006;2(1):99–113. | ||
Eichenfield LF, Funk A, Fallon-Friedlander S, Cunningham BB. A clinical study to evaluate the efficacy of ELA-Max (4% liposomal lidocaine) as compared with eutectic mixture of local anesthetics cream for pain reduction of venipuncture in children. Pediatrics. 2002;109(6):1093–1099. | ||
Koh JL, Harrison D, Myers R, Dembinski R, Turner H, McGraw T. A randomized, double-blind comparison study of EMLA and ELA-Max for topical anesthesia in children undergoing intravenous insertion. Paediatr Anaesth. 2004;14(12):977–982. | ||
Buckley MM, Benfield P. Eutectic lidocaine/prilocaine cream. A review of the topical anaesthetic/analgesic efficacy of a eutectic mixture of local anaesthetics (EMLA). Drugs. 1993;46(1):126–151. | ||
Negi P, Singh B, Sharma G, Beg S, Raza K, Katare OP. Phospholipid microemulsion-based hydrogel for enhanced topical delivery of lidocaine and prilocaine: QbD-based development and evaluation. Drug Deliv. 2016;23(3):951–967. | ||
Ribeiro LN, Franz-Montan M, Breitkreitz MC, et al. Nanostructured lipid carriers as robust systems for topical lidocaine-prilocaine release in dentistry. Eur J Pharm Sci. 2016;93:192–202. | ||
Jain S, Patel N, Shah MK, Khatri P, Vora N. Recent advances in lipid-based vesicles and particulate carriers for topical and transdermal application. J Pharm Sci. 2017;106(2):423–445. | ||
Vahabi S, Eatemadi A. Nanoliposome encapsulated anesthetics for local anesthesia application. Biomed Pharmacother. 2017;86:1–7. | ||
Zhai Y, Zhao L, Wang Z, Zhai G. RETRACTED ARTICLE: Preparation and characterization of novel lipid nanocapsules of ropivacaine for transdermal delivery. Drug Deliv. 2016;23(2):619–628. | ||
Wang Y, Wang S, Shi P. Transcriptional transactivator peptide modified lidocaine-loaded nanoparticulate drug delivery system for topical anesthetic therapy. Drug Deliv. 2016;23(9):3193–3199. | ||
Arora R, Katiyar SS, Kushwah V, Jain S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: a comparative study. Expert Opin Drug Deliv. 2017;14(2):165–177. | ||
Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54(Suppl 1):S131–S155. | ||
Doktorovová S, Kovačević AB, Garcia ML, Souto EB. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2016;108:235–252. | ||
Weber S, Zimmer A, Pardeike J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 2014;86(1):7–22. | ||
Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366(1–2):170–184. | ||
Jiang Z, Sun C, Yin Z, et al. Comparison of two kinds of nanomedicine for targeted gene therapy: premodified or postmodified gene delivery systems. Int J Nanomedicine. 2012;7:2019–2031. | ||
Singh I, Swami R, Pooja D, Jeengar MK, Khan W, Sistla R. Lactoferrin bioconjugated solid lipid nanoparticles: a new drug delivery system for potential brain targeting. J Drug Target. 2016;24(3):212–223. | ||
Qu J, Zhang L, Chen Z, et al. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv. 2016;23(9):3408–3416. | ||
Wang L, Jia E. Ovarian cancer targeted hyaluronic acid-based nanoparticle system for paclitaxel delivery to overcome drug resistance. Drug Deliv. 2016;23(5):1810–1817. | ||
Thakkar A, Chenreddy S, Thio A, Khamas W, Wang J, Prabhu S. Preclinical systemic toxicity evaluation of chitosan-solid lipid nanoparticle-encapsulated aspirin and curcumin in combination with free sulforaphane in BALB/c mice. Int J Nanomedicine. 2016;11:3265–3276. | ||
Makwana V, Jain R, Patel K, Nivsarkar M, Joshi A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharm. 2015;495(1):439–446. | ||
Vidlářová L, Hanuš J, Veselý M, Ulbrich P, Štěpánek F, Zbytovská J. Effect of lipid nanoparticle formulations on skin delivery of a lipophilic substance. Eur J Pharm Biopharm. 2016;108:289–296. | ||
Chen C, You P. A novel local anesthetic system: transcriptional transactivator peptide-decorated nanocarriers for skin delivery of ropivacaine. Drug Des Devel Ther. 2017;11:1941–1949. | ||
de Melo NF, Grillo R, Guilherme VA, et al. Poly(lactide-co-glycolide) nanocapsules containing benzocaine: influence of the composition of the oily nucleus on physico-chemical properties and anesthetic activity. Pharm Res. 2011;28(8):1984–1994. | ||
Shah PP, Desai PR, Channer D, Singh M. Enhanced skin permeation using polyarginine modified nanostructured lipid carriers. J Control Release. 2012;161(3):735–745. | ||
Silva de Melo NF, Campos EV, Gonçalves CM, et al. Development of hydrophilic nanocarriers for the charged form of the local anesthetic articaine. Colloids Surf B Biointerfaces. 2014;121:66–73. | ||
Ni S, Qiu L, Zhang G, Zhou H, Han Y. Lymph cancer chemotherapy: delivery of doxorubicin-gemcitabine prodrug and vincristine by nanostructured lipid carriers. Int J Nanomedicine. 2017;12:1565–1576. | ||
Wang J, Zhang L, Chi H, Wang S. An alternative choice of lidocaine-loaded liposomes: lidocaine-loaded lipid-polymer hybrid nanoparticles for local anesthetic therapy. Drug Deliv. 2016;23(4):1254–1260. | ||
Ouchi K, Sekine J, Koga Y, Nakao S, Sugiyama K. Establishment of an animal model of sedation using epidural anesthesia that uses the tail-flick test for evaluating local anesthetic effects in rats. Exp Anim. 2013;62(2):137–144. | ||
Basha M, Abd El-Alim SH, Kassem AA, El Awdan S, Awad G. Benzocaine loaded solid lipid nanoparticles: Formulation design, in vitro and in vivo evaluation of local anesthetic effect. Curr Drug Deliv. 2015;12(6):680–692. | ||
Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003;258(1–2):141–151. | ||
Saengkrit N, Saesoo S, Srinuanchai W, Phunpee S, Ruktanonchai UR. Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surf B Biointerfaces. 2014;114:349–356. | ||
Tabatt K, Sameti M, Olbrich C, Müller RH, Lehr CM. Effect of cationic lipid and matrix lipid composition on solid lipid nanoparticle-mediated gene transfer. Eur J Pharm Biopharm. 2004;57(2):155–162. | ||
Iqbal N, Vitorino C, Taylor KM. How can lipid nanocarriers improve transdermal delivery of olanzapine? Pharm Dev Technol. 2017;22(4):587–596. | ||
Feng C, Li X, Dong C, Zhang X, Zhang X, Gao Y. RGD-modified liposomes enhance efficiency of aclacinomycin A delivery: evaluation of their effect in lung cancer. Drug Des Devel Ther. 2015;9:4613–4620. | ||
Vitorino C, Almeida J, Gonçalves LM, Almeida AJ, Sousa JJ, Pais AA. Co-encapsulating nanostructured lipid carriers for transdermal application: from experimental design to the molecular detail. J Control Release. 2013;167(3):301–314. | ||
Zhu X, Li F, Peng X, Zeng K. Formulation and evaluation of lidocaine base ethosomes for transdermal delivery. Anesth Analg. 2013;117(2):352–357. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.