Back to Journals » International Journal of Nanomedicine » Volume 17
Dental Implant Healing Screws as Temporary Oral Drug Delivery Systems for Decrease of Infections in the Area of the Head and Neck
Authors Pokrowiecki R , Szałaj U, Fudala D, Zaręba T, Wojnarowicz J , Łojkowski W , Tyski S , Dowgierd K , Mielczarek A
Received 16 August 2021
Accepted for publication 31 January 2022
Published 12 April 2022 Volume 2022:17 Pages 1679—1693
DOI https://doi.org/10.2147/IJN.S333720
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Rafał Pokrowiecki,1,2 Urszula Szałaj,3,4 Damian Fudala,3 Tomasz Zaręba,5 Jacek Wojnarowicz,3 Witold Łojkowski,3 Stefan Tyski,5 Krzysztof Dowgierd,6 Agnieszka Mielczarek7
1Department of Cranio- Maxillofacial Surgery, Oral Surgery and Implantology, Medical University of Warsaw, Warsaw, Poland; 2Head and Neck Surgery Department—Maxillofacial Surgery Department, Craniofacial Center, Regional Specialized Children’s Hospital, Olsztyn, 10-561, Poland; 3Institute of High Pressure Physics, Polish Academy of Sciences, Warsaw, Poland; 4Faculty of Materials Engineering, Warsaw University of Technology, Warsaw, Poland; 5Department of Antibiotics and Microbiology, National Medicines Institute, Warsaw, Poland; 6Head and Neck Surgery Clinic for Children and Young Adults, Department of Clinical Pediatrics, University of Warmia and Mazury, Olsztyn, 10-719, Poland; 7Department of Conservative Dentistry, Medical University of Warsaw, Warsaw, Poland
Correspondence: Agnieszka Mielczarek, Department of Conservative Dentistry, Medical University of Warsaw, Warsaw, Poland, Tel +48 691226414 ; +48 22 116 64 46, Email [email protected]
Background: Periimplantitis is continuously one of major threats for the uneventful functioning of dental implants. Current approaches of drug delivery systems are being more commonly implemented into oral- and maxillofacial biomaterials in order to decrease the risk of implant failure due to bacterial infection. Silver nanoparticles and their compounds have been proven in eradicating oral bacteria responsible for peri-implant infections. Nevertheless, their evaluation as coating for implant abutments has not been extensively evaluated so far. This article describes a novel coating consisting of zinc oxide (ZnO) and silver (Ag) nanoparticles (NPs). This coating was used to modify healing abutments that could be used as drug delivery systems in oral implantology.
Materials and Method: Nanoparticles with a ZnO + 0.1% Ag composition were produced by microwave solvothermal synthesis and then incorporated into the surface of titanium healing abutments by high-power ultrasonic deposition. Surface morphology, roughness, wettability were evaluated. Ability of biofilm formation inhibition was tested against S. mutans, S. oralis, S. aureus and E. coli.
Results: ZnO+0.1%Ag NPs were sufficiently deposed on the surface of the abutments creating nanostructured coating which increased surface roughness and decreased wettability. Modified abutments significantly decreased bacterial biofilm formation. Bacteria present in SEM studies were unlikely to settle and replicate on the experimental abutments as their cells were rounded, insufficiently spread on the surface and covered with released NPs.
Conclusion: Experimental nanostructured abutments were easily manufactured by high-power ultrasonic deposition and provided significant antibacterial properties. Such biomaterials could be used as temporary drug delivery abutments for prevention and treatment of intra- and extraoral peri-implant infections in the area of the head and neck.
Keywords: dental implants, periimplantitis, nanotechnology, nanoparticles, NPs, drug delivery, zinc oxide, ZnO, silver, Ag
Introduction
Over the past 40 years, regenerative medicine has made major steps forward in the improvement of the quality of life of patients and increased their life expectancy. Developments have been made in the field of biomedical implants which have contributed significantly to the current state of the art of modern healthcare systems. Implants are used in almost all procedures but fields that fully cover the application of metallic and non-metallic materials with the use of permanent or temporary biomaterials are: orthopedics, maxillofacial and cranial surgery, ophthalmology, neurosurgery and vascular surgery. Yet, biomaterials represent a group of materials prone to bacterial adhesion and biofilm formation, regardless of the anatomical site where the implant has been implanted. The factors influencing bacterial settlement on the implant surface may be divided into patient-related, microbiome-related and material-related factors.1,2 Dental screws are commonly used for the reconstruction of lost tissues in the oral cavity. As they function as permucosal implants, being exposed into environment of saliva, they are under the risk of contamination by oral bacteria responsible for formation of the biofilm (early colonizers eg Streptococcus oralis, S. mitis, S mutans) and subsequent by periodontopathogenic species when early biofilm is settled. Also, due to relatively small size and favorable biomechanical properties, dental implants are more frequently used for extraoral bone-anchored prosthesis in the reconstruction of the orbital, auricular and nasal area in the patients after ablative surgeries or post-traumatic defects.3–5 Peri-implantitis (PI), which describes infections of the soft and hard tissues around oral and extraoral implants, poses a threat in contemporary oral and maxillo-facial implantology.6,7 Therefore, in order to decrease the risk of surgical site infections (SSIs) and implant loss, general antibiotic therapy is recommended in the peri- and postoperative period.
However, it may develop in the early postoperative period but also years after implantation and frequently exhibits a slow, chronic course. When left untreated, peri-implantitis contributes to implant and surrounding bone loss, but may also initiate infections in the head and neck area of significant magnitude such as osteomyelitis, sinusitis, abscesses, phlegmon and pathological fractures of the jawbones. Such complications are more likely to develop in patients with immunodeficiencies, osteoporosis, cancer, uncontrolled diabetes, obesity or after radio- and/or chemotherapy.8–10 Moreover, antimicrobial resistance proliferation can lead to development of peri-implant superinfections unlikely to be treated by contemporary protocols.11 Oftenly, even early diagnosed, infections result in implant failure and the necessity of secondary procedures: implant removal (Figure 1), bone regeneration, re-implantation or even implant treatment discontinuation in favor for removable prosthetic dentures. Additional procedures, implant debridement, drainage, etc. and any necessity of re-operation contribute to higher morbidity and increased treatment costs.11,12
Therefore, the need for “smart biomaterials” that can reduce the risk of postoperative infections is indisputable. Along with nanotechnology, regardless of the surface science aiming at intensification of the osseointegration process, antibacterial approaches were taken into concern in order to decrease the risk of peri-implant infections. Such approaches were based upon local drug delivery (LDD) models and required certain surface modifications in order to sufficiently incorporate the antibacterial agents on the surface and their subsequent delivery into the specific anatomical areas when the implant was placed.13,14 With time, studies showed that such devices exhibited some limitations, mostly due to a restricted effective release rate leading to decrease of antibacterial potential over time. An initial burst release of the drug which led to cytotoxicity, as well as different, unknown interactions of nanoparticles (NPs) with the host’s biomolecules were reported.15–17 The most troublesome part of the design of an antibacterial implant is adjustment of the effective drug release to the required period of the implant’s functioning in the organism.13,18 In 2018, the first classification of antibacterial implants for maxillofacial surgery and the requirements that should be fulfilled for their clinical applicability were described.19 Consequently, dental implants are partially external, permucosal, permanent implants and are of the highest risk of bacterial peri-implant infections across the whole human body. In the past, attempts were made to improve implant-prosthesis interlock. The idea was however based strictly on biomechanical aspects of implant-abutment fit, and further tissue adaptation in order to minimize the micro-gap between the implant and the prosthetic superstructure. Various types of connections between the implant body and the abutment were tested with regard to bacterial infiltration. However, most recent studies have shown that despite biomechanical design, bacterial infiltration is almost inevitable.20–22 As current local drug delivery is restricted by time, it is obvious that none of the permanent implants can provide sustained drug release over years of functioning. On the other hand, it would be beneficial to protect the implant from the very beginning from bacterial leakage and biofilm formation. Partially external implants, which are usually temporal implants being under the greatest risk of the bacterial settlement are likely to be the most applicable also as temporary drug delivery systems.
Dental implant healing abutments are partially external, permucosal, temporal implants used as the seal of the upper part of the implant during its osseointegration or/and soft tissue seal formation.19 At this time, implant is more prone to bacterial infection and risk of failure. Therefore, it is beneficial to enhance the healing process by decreasing the percentage of the bacteria in the near proximity of the biomaterial. Due to simple design and structure of the healing abutments, they may be easily modified and used as temporary local drug delivery devices,15,23,24 which may decrease the bacterial infiltration during implant healing and thus, prevent from material infection or creation of poor implant-tissue interface caused by bacterial leakage during early period of implant tissues healing.25,26
Silver nanoparticles as well as Zinc oxide nanoparticles have already proven track of antibacterial activity against oral bacteria (early colonizers and periodontopathogenic species)27–31 in the mechanism of silver ions release and direct interactions of the nanoparticles with bacteria cell wall leading to multiple-level cell disruption to the bacterial cell through oxidative stress, protein dysfunction, membrane and DNA damage.32,33 The aim of this study was to create experimental healing abutments coated in a silver – zinc oxide nanoparticle formula that could provide a local antibacterial environment against early colonizing species for better soft tissue adaptation and the creation of a healthy soft tissue seal around future implant-embedded superstructures.
Materials and Methods
Nano-Coatings on Healing Abutments Preparation
Nanoparticle formulations based on zinc oxide (ZnO)-silver (Ag) mixed at ZnO+0.1%Ag in compounds further used as coatings, were prepared by microwave solvothermal co-synthesis and characterized in accordance with the procedures at the Laboratory of Nanostructures, Institute of High Pressure Physics (UNIPRESS), which the Polish Academy of Sciences described in an earlier publications.27,34 The advantages of microwave synthesis of nano zinc oxide are discussed in detail in an earlier review publication.35 Then, titanium dental implant healing abutments (4.0x4.5x6 mm, titanium 6-aluminium 4-vanadium alloy (Ti-6Al-4V), Alpha Bio) were coated with ZnO+0.1%Ag NPs using a modified high-power ultrasound technique. The implant abutments fixed vertically on a Teflon stand were placed in a 900 mL steel vessel with a cooling and magnetic stirring system. 750 mL of an aqueous suspension of ZnO+0.1%Ag NPs with a concentration of 1 wt% was poured into the vessel using a peristaltic pump (Ismatec, BVP Standard, Wertheim, Germany) and magnetically mixed. An ultrasonic titanium horn (Ø 18 mm) was immersed 15 mm from the top surface of the implant abutments. The high-power ultrasonic coating process was performed at a frequency of 20 kHz. The ultrasonic energy was provided by a UIP500hdT generator (500W, Hielscher Ultrasonics GmbH, Germany). The high-power ultrasonic coating process was carried out for 6 min. The temperature of the process was stabilized at 30 ± 1 C by means of a cooling system. After the coating process, the implant abutments were rinsed with 100 mL of deionized water and dried in a vacuum dryer.
X-Ray Powder Diffraction
Diffraction patterns of the X-ray powder diffraction (XRD) were gathered at the RT within the range of 2 theta angle from 10° to 100° with the step of 0.02°, using the X-ray powder diffractometer (CuKα1) (X’Pert PRO, Panalytical). The full width at half maximum (FWHM) was determined by the Pearson VII function implemented in Fityk software, version 0.9.8. Based on the diffraction patterns, the size of crystallites was determined in the direction of the crystallographic axes a and c using Scherrer equation.36
Crystallite Size Distribution
The analysis of XRD peak profile was performed using the analytical formula for polydispersive powders. This technique provides four parameters: average crystallite size, deviation of the average crystallite size, dispersion of size and deviation of dispersion of sizes. For calculating the crystallite diameter and size distribution, the Nanopowder XRD Processor Demo web application was used which employs equations dedicated to spherical crystallites.37
Density and Specific Surface Area
Skeleton density (pycnometric density) measurements were carried out using the helium pycnometer (AccuPyc II 1340, FoamPyc V1.06, Micromeritics), the measurements were carried out in accordance with ISO 12154:2014 at the temperature of 25±2°C. The specific surface area of NPs was determined using the surface analyser (Gemini 2360, V 2.01, Micromeritics) by the nitrogen adsorption-desorption method based on the linear form of the BET (Brunauer-Emmett-Teller) isotherm equation, in accordance with ISO 9277:2010. Prior to performing measurements of density and specific surface area, the ZnO samples were subjected to 2h desorption (VacPrep 061, Micromeritics), under vacuum (0.05 mbar) at the temperature of 150°C. Based on the determined specific surface area and skeleton density, the average size of particles defining their diameter was determined using the equation, with the assumption that all particles are spherical and identical.34,37
Particle Size Distribution in Saliva Suspension
The particle size distribution was measured by the dynamic light scattering (DLS) method (λ=633 nm, Zetasizer Nano-ZS ZEN 3600, Malvern Instruments Ltd., UK). The tests were carried out with the following parameters: temperature 24°C, measurement angle 173° (backscattering), analysis model: auto mode, number of measurements 6.
The suspension sample for DLS analysis was obtained as follows. Sample suspension of NPs in deionized water (100 mL, 1000 ppm) was subjected to the ultrasonic homogenization process (UP200, Hielscher, Teltow, Germany) with the following parameters: duration 3 minutes, amplitude 0.7, cycle 1, sonotrode diameter 14 mm, without temperature stabilization. The suspension sample (2mL) after reaching room temperature was diluted to a concentration of 200 ppm using human saliva.
Scanning Electron Microscopy (SEM)
Before and after modification, the characteristics of the implant abutments were investigated by scanning electron microscopy (SEM) (Ultra Plus, Carl Zeiss Meditec AG, Jena, Germany) with an in-column detector (Immersion Lens detector (InLens) and Angle Selective Backscatter detector (AsB)). Elemental analysis was mapped with the use of additional microscope equipment – an Energy-dispersive X-ray spectroscopy (EDS) microanalysis system (Bruker, Quantax 400 with an ultrafast detector with an energy resolution of 127 eV and an active surface of 30 mm2). SEM images were obtained at magnifications of 1000, 25,000 and 100,000.
Atomic Force Microscopy (AFM)
Topography imaging and scratch tests were performed using an Atomic Force Microscope (AFM) (Asylum Research Oxford MFP-3D-Bio, Santa Barbara, CA USA). Imaging was carried out using the Asylum Research software (Version 16). Surface topography imaging and detection of ZnO+0.1% Ag on the tested implant abutment surfaces were carried out using Contact mode for phase contrast imaging. The scratch test was performed using AFM with a diamond tip (Nanosensors, Type: ATEC-NC-10) measured at various locations on the (n=15). The average values and standard deviations (SDs) were calculated.
Surface Wettability
Measurement of the contact angle was performed by placing a drop of deionized water (conductivity 0.9 µS/cm) on the surface of flat titanium (grade 5) plates – clean and coated with ZnO+0.1% Ag. The drop shape was recorded directly by a digital camera and processed with the Krüss ADVANCE computer program (Krüss, Germany). The Young-Laplace equation was used in the drop shape analysis measured at various locations on the (n=15). The average values and standard deviations (SDs) were calculated. Coating of the flat titanium plates was performed in the same way as previous (point 2.1).
Patient Enrolment Saliva Sampling and Bacteria Isolation
The study was conducted in accordance with the Declaration of Helsinki. Generally healthy patients (n=15) aged 18–65, after signing an informed consent, were enrolled into the study (Warsaw Medical University Research Ethics Committee Agreement no. KB/150/2018). The inclusion criteria were: Approximal Plaque Index (API) > 15%, positive bleeding on periodontal probing test, generally healthy. Samples were harvested by the unstimulated method into SalivaBio Oral Swabs (SOSs), 1 mL per patient, and then transported using standardized kits (Oral Swab Collection Kit and Cryovial 2 Cryostorage Box, Salimetrics, USA). A standardized protocol for bacteria sampling from saliva, culturing, identification, storage and antibacterial tests for the pharmaceutical microbiology was conducted at the Department of Antibiotics and Microbiology, National Medicines Institute (Official Medicines Control Laboratory) (ISO PN-EN ISO/IEC 17025:2005) Warsaw, PL. The saliva samples were spread and cultured on microbiological substrates in order to extract and identify oral bacteria. Then, the bacteria were subcultured, divided and grouped as described before.27,28 The strains were stored at −80°C as per the requirement for the analysis. Prior to material testing, the saliva-derived streptococcal strains, control S. aureus and E. coli strains, were subcultured twice on Columbia Blood Agar (CBA) with a 5% sheep blood (bioMérieux SA, Marcy l’Etoile, France) medium for 24–48 h at 37 °C to ensure viability.
Microbiological Studies
The ability to form a biofilm on the implants was carried out for 2 strains of oral streptococci: Streptococcus oralis and Streptococcus mutans, and for Staphylococcus aureus and Escherichia coli. The strains were grown on Columbia 5% blood agar. For each strain, a cell suspension was prepared by diluting the 7-McFarland standardized suspension 20 times in medium. A tryptic soy broth (BioMerieux) with 0.25% glucose (AppliChem) was used. The prepared working suspensions in the broth were applied in 1 mL aliquots to a 40-well cell culture plate. Four wells were filled for each strain. In the first three, titanium implants coated with zinc oxide and silver ions were placed, and in the fourth, a titanium implant without additional finishing. The plates were incubated in a shaking incubator (Shaker-Thermostat DTS-4, ELMI Sky Line) at 37°C with the plate rotating at 350 rpm for 24 hours. After the end of incubation, the medium was removed and plates were washed three times with 0.9% NaCl and dried at 37 °C. The implants were transferred to a new plate and stained with 0.1% aqueous crystal violet (Merck) for 3 minutes. Unbound dye was rinsed 3 times with sterile water and the implants were transferred to new wells and the washing repeated. The dye bound by the biofilm on the implants was dissolved with a mixture of ethanol (POCH) and acetone (POCH) in the proportion 4:1. The absorbance was measured at 570 nm against a blank. The mean absorbance value was determined for each strain. The obtained results were compared to the absorbance value obtained for the implant without additional finishing. Tests were performed in triplicate for each bacterial specie.
Statistical Analysis
All numerical data were analyzed by using the STATISTICA PL 20 software. The results of the roughness, wettability, and antibacterial assays were statistically analyzed by using the analysis of variance and Tukey’s honest significant difference. A P-value of <0.05 was considered statistically significant in all the tests.
Results
Nanoparticles
The characteristics of the obtained nanoparticles are shown in Figure 2 and Table 1. The average size of the ZnO+0.1%Ag NPs was 28 nm ± 9 nm. The average particle size was equal to the average crystallite size which means that the nanoparticles obtained were monocrystalline (Table 1). After their exposure to the environment of either deionized water, artificial saliva or human saliva (Figure 3A), the formation of agglomerates of different size distributions was observed as in previous studies. The average size of the agglomerates formed from ZnO+0.1%Ag NPs in the human saliva suspension was 342 nm ± 16 nm, which was about 12 times larger than the average size of a single NPs (Figure 3).
 |
Table 1 Characterization of ZnO+0.1% Ag Nanoparticles |
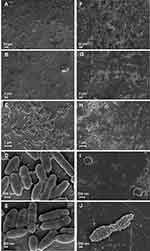
The high-power ultrasound technique provided sufficient incorporation of the ZnO+0.1%Ag NPs on the implant surface. The gradient texture of the coating was designed to maximize the percentage of the coating in the upper part of the abutment and minimize its volume at the lower part connecting with the implant body (Figure 4). Layers of ZnO+0.1%Ag NPs with an island structure were obtained (Figure 4D–L).
 |
Figure 4 Comparison of SEM images of a pure implant abutment (A–C) and an implant abutment coated with ZnO+0.1% Ag (D–L). Abbreviations: Ag, silver; ZnO, zinc oxide; nm, nanometer; µm, micrometer. |
SEM studies with elemental mapping and EDS confirmed the chemical composition of the obtained surface enriched with ZnO and Ag nanoparticles (Figures 5A–F and 6B).
The coating of ZnO+0.1% Ag nanoparticles increased the surface roughness. Unmodified, stock abutments exhibited a mean Ra value of 26.0 nm ± 5.3 nm. In the experimental group, the mean Ra value was 74.0 nm ± 28.9. The upper part of the coated abutment had a surface roughness of mean Ra value was 87.7 nm ± 16.1 nm, while the lower part of the coated abutment, 32.8 nm ± 6.0 nm. Modification significantly increased the abutment roughness in the upper part (p<0.005) (Figure 7). The coating of ZnO+0.1%Ag NPs also changed the surface wettability. It was found that the mean contact angle θ of the unmodified surface was 80.3° ± 16.8° while the modified surface exhibited 63.5° ± 7.1° (p<0.005) (Figure 8).
 |
Figure 8 Example of contact angle analysis results (A) of the pure implant abutment surface and (B) of the implant abutment surface after modification with ZnO+0.1%Ag. |
The microbiological studies confirmed that the ZnO+0.1%Ag coated surfaces decreased the adhesion and growth of all tested bacteria on the implant surface (Figure 9) (p<0.005). Early colonizing oral bacteria which are responsible for initiation of biofilm formation on the implant surface were reduced in the range from 30 to 70%. Maximum reduction of attached bacteria was observed in the S. aureus (p=0.001) and E. coli (p=0.003) subgroups respectively, which are commonly found in the infection of the metallic biomaterials.
 |
Figure 9 Absorbance test indicating a significant reduction in bacterial adhesion on the titanium surface modified with nanoparticles (p<0.005). |
In the SEM studies of the retrieved abutments, the control abutments were covered with multiple, widely spread out bacterial colonies (Figure 10A–E). On the modified surface, only single, rounded bacterial cells were observed which indicated that the implant surface was unlikely to be settled by bacteria, regardless of the total bacteria mass persisting on the surface after microbiological studies (Figure 10F–J).
Moreover, some cells were fully covered with nanoparticles which indicated the interaction of the implant coating with bacteria and their subsequent surrounding by NPs (Figure 10J).
Discussion
Peri-implant infections and peri-implantitis are an ongoing problem worldwide, and one of increasing prevalence. As there is still no consensus regarding treatment of already infected implants, more approaches should be investigated with regard to the prophylaxis of peri-implantitis.1,38
Local drug delivery in the treatment of periodontal diseases has well established protocols, however its application in biomaterials used in the orofacial area is still in the experimental phase. This is mostly due to difficulty in the design of materials that can provide sustained and prolonged diffusion of the drug into the peri-implant space, and even more importantly, are able to provide anti-adhesive properties against bacterial biofilms while also having biocompatible properties providing undisturbed osseointegration.39,40 Early investigations focused on the modification of the intraosseous part of dental implants and showed promising results. In our previous research, silver nanoparticles were tested against oral bacteria as well. It was shown that silver in the form of nanoparticles exhibits high antibacterial activity in a liquid medium, similarly to other researchers. However, uncontrolled release of NPs and their ions in the mechanism described as “burst release” may contribute to certain toxicity against human osteoblasts and in turn, affect osseointegration.15,28,41
Potential toxicity of the NPs against human cells are based on three mechanisms where nanoparticles or their ions penetrate the cell such as: clathrin-mediated endocytosis of the protein-NPs complexes, internalization of the metal ions through metal ion transporters and Trojan horse mechanism where NPs are internalized through scavenger receptor endocytosis.42
As these may occur simultaneously, release rate and total drug concentration in the tissues must be weighed against desired bacterial activity. Therefore, as peri-implantitis is preceded by inflammation of the soft tissue collar around the dental implant, it is logical to design permucosal drug delivery systems in the form of antibacterial abutments that could prevent peri-implantitis. Such an approach could reduce the percentage of peri-implant infections and simultaneously solve the problem of osseointegration and potential disturbance in the implant-bone interlock caused by drug release.42,43 Dental implant healing screws are removable implants, used temporary (from 2 weeks to several months) during implant healing. They are replaced by final prosthetic superstructure when implant is integrated with surrounding tissues (both, bone and gingiva). Therefore it was stated, that they may serve as drug carriers which improve tissue adaptation by decreasing the bacterial leakage during early period of implant adaptation, providing better implant- tissue interface seal and in turn- better resistance to infections in the subsequent period of implant functioning when loaded with final prosthetic superstructure.22,44,45
Iwanczyk et al described a specially designed healing abutment as an antibiotic carrier. In their work, the abutment contained an inner chamber for a drug that was diffused from drug release openings (pores) into the peri-implant area. In their work, the device provided sustained diffusion of the antibiotics (clindamycin or tetracycline) that inhibited the growth of S. Aureus and S. Epidermidis. However, the authors did not evaluate their materials against common oral bacterial responsible for peri-implant infections, nor did they evaluate the release profiles from the device into a biological medium.25 In the work of Xing et al,15 an electrochemical method of cathodic polarization to coat doxycycline onto the outer surface of a dental abutment was evaluated and showed inhibition of s. epidermidis in a broth culture and on agar plates. The authors concluded that such a coating could be used in the prevention of peri-implant mucositis, but again, they did not test their coatings against oral bacteria. The work of Xu et al described that their experimental dental abutments coated with natural antibacterial totarol inhibited the early oral colonizer S. gordonii and a multispecies oral bacterial culture.46 This, in turn, could support the formation of a tight epithelial seal and prevent further bacterial infiltration. Their observations are in agreement with ours, as the prevention of early biofilm formation may tip the scales in favor of tissue adaptation in the “race for the surface” described by Gristina in 1987, where tissues and bacteria compete in settlement on the implant surface.47 Also, Brunello et al48 claimed that improving soft tissue attachment and reducing bacterial colonization on titanium abutments are key factors for the long-term maintenance of healthy soft and hard peri-implant tissues. In their work, anodized surfaces coated with titanium nitride (TiN) or zirconium nitride (ZrN) were tested against clinical strains of oral bacteria: Streptococcus salivarius, S. sanguinis, S. mutans, S. sobrinus, and S. oralis isolated from clinical specimens, similarly to our investigations. In their study, the TiN coatings significantly inhibited the growth of the tested oral bacteria.48 However, the presence of the coating induced significant changes in the surface properties such as roughness, which was also seen in this work, as well as in our previous study28 Nascimiento et al evaluated implant abutments coated either with iodoform or nanosilver as a sealant between the abutment and the implant screw. A leakage test showed a significant reduction of clinically isolated multispecies oral bacteria in the test group than in the control (no sealant) showing that drug delivery at the implant-abutment, abutment-tissue interface is an important factor that may decrease the percentage of implant failure due to infection or the development of a defective tissue seal.44 In another study, De Avila et al described LbL coating assembly as a sufficient method for surface optimization that reduced the adhesion of P. gingivalis on the titanium surface. Their method also increased the surface roughness and changed its topography.16
In the present study, ZnO+0.1%Ag coated titanium healing abutments significantly decreased biofilm formation of early colonizers (streptococci) and also S. Aureus and E. Coli. The decrease of the ability to form biofilm may be contributed to the multilevel activity of the nanoparticle base implant coatings. The interaction of NPs on the bacterial cell may occur in the mechanism of bacterial clathrin-mediated endocytosis (CME) of the protein corona complexes that are formed by the interaction of nanoparticles with proteins of the surrounding biological environment, interaction of the ions released from the NPs with the bacterial cell membrane and their eventual internalization through metal ion transporters or endocytosis of the NPs by scavenger receptors.49,50 Therefore, the multilevel action of the NPs deposed on the titanium healing abutment against bacteria is related to adhesion decrease on the surface and direct toxicity against bacterial cells by disruption of the membrane and internal de-arrangement of vital biochemical processes due to the toxicological impact of the released ions that leads to activation of reactive oxygen species, enzyme activity and cell structure damage. It was also shown in SEM studies that bacterial cells that persisted on the surface were characterized by round shape and non-spread appearance on the surface which indicates its unfavorable action on bacterial settlement.
Conclusion
In this work, we described sonocoating as an efficient method of modification of the outer surface of a dental abutment. The designed coating, based on the application of a mixture of zinc oxide and silver nanoparticles, is easily obtained and could be used for the modification of dental implant abutments without the modification of conventional surgical equipment. The ZnO+0.1%Ag NPs coating provides antibacterial and antiadhesive properties against oral early colonizers responsible for biofilm formation. Presented abutments could be used as temporary drug delivery systems in the prevention of peri-implant mucositis or in the treatment of already established infections of the peri-implant tissues. The described coating also inhibited S. aureus responsible for the majority of percutaneous implant infections, therefore could be considered as a coating for other than dental implants, such as metallic biomaterials for craniofacial surgery.
Institutional Review Board Statement
Study was approved by Warsaw Medical University Research Ethics Committee Agreement no. KB/150/2018.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. The study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The study was financed by AAID Foundation (2018). Part of the research project was carried out with the use of equipment funded by the project CePT, reference: POIG.02.02.00-14-024/08, financed by the European Regional Development Fund within the Operational Programme “Innovative Economy” for 2007–2013. The Authors would like to thank Jan Mizeracki for SEM studies, Maciej Łojkowski and Wojciech Święszkowski for AFM studies. The authors would like to thank Ewelina Kuzniewska for graphic design and data curation and analysis.
Funding
The study was financed by AAID Foundation (2018). Part of the research project was carried out with the use of equipment funded by the project CePT, reference: POIG.02.02.00-14-024/08, financed by the European Regional Development Fund within the Operational Programme “Innovative Economy” for 2007–2013. The study was partially funded by Medical University of Warsaw no. 1S18/3/PU/N/22. The study was partially funded by National Medicines Institute.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pokrowiecki R, Mielczarek A, Tomasz zareba ST. Oral microbiome and peri-implant diseases: where are we now? Ther Clin Risk Manag. 2017;13:1529–1542. doi:10.2147/TCRM.S139795
2. Belibasakis GN. Microbiological and immuno-pathological aspects of peri-implant diseases. Arch Oral Biol. 2014;59(1):66–72. doi:10.1016/j.archoralbio.2013.09.013
3. Eo MY, Cho YJ, Nguyen TTH, Seo MH, Kim SM. Implant-supported orbital prosthesis: a technical innovation of silicone fabrication. Int J Implant Dent. 2020;6(1):1–6. doi:10.1186/S40729-020-00248-0
4. Federspil PA. Implant-retained craniofacial prostheses for facial defects. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2009;8:Doc03. doi:10.3205/CTO000055
5. Yeganeh F, Haghighat A, Amini-Pozveh M. Dental implant-retained auricular prosthesis. Dent Res J. 2018;15(6):444. doi:10.4103/1735-3327.245227
6. Monje A, Pons R, Insua A, et al. Morphology and severity of peri-implantitis bone defects. Clin Implant Dent Relat Res. 2019. doi:10.1111/cid.12791
7. Khoury F, Keeve PL, Ramanauskaite A, et al. Surgical treatment of peri-implantitis – consensus report of working group 4. Int Dent J. 2019;69(S2):18–22. doi:10.1111/idj.12505
8. Guobis Z, Pacauskiene I, Astramskaite I. General diseases influence on peri-implantitis development: a systematic review. J Oral Maxillofac Res. 2016;7(3). doi:10.5037/JOMR.2016.7305
9. Hashim D, Cionca N. A comprehensive review of peri-implantitis risk factors. Curr Oral Heal Rep. 2020;7(3):262–273. doi:10.1007/S40496-020-00274-2
10. Shugaa-Addin B, Al-Shamiri HM, Al-Maweri S, Tarakji B. The effect of radiotherapy on survival of dental implants in head and neck cancer patients. J Clin Exp Dent. 2016;8(2):e194. doi:10.4317/JCED.52346
11. Verdugo F, Laksmana T, Uribarri A. Systemic antibiotics and the risk of superinfection in peri-implantitis. Arch Oral Biol. 2016;64:39–50. doi:10.1016/j.archoralbio.2015.12.007
12. Daubert DM, Weinstein BF, Bordin S, Leroux BG, Flemmig TF. Prevalence and predictive factors for peri-implant disease and implant failure: a cross-sectional analysis. J Periodontol. 2015;86(3):337–347. doi:10.1902/jop.2014.140438
13. Dukhin SS, Labib ME. Theory of effective drug release from medical implants based on the Higuchi model and physico-chemical hydrodynamics. Colloids Surf A Physicochem Eng Asp. 2012;409:10–20. doi:10.1016/j.colsurfa.2012.04.040
14. Gimeno M, Pinczowski P, Pérez M, et al. A controlled antibiotic release system to prevent orthopedic-implant associated infections: an in vitro study. Eur J Pharm Biopharm. 2015;96:264–271. doi:10.1016/j.ejpb.2015.08.007
15. Xing R, Witso IL, Jugowiec D, et al. Antibacterial effect of doxycycline-coated dental abutment surfaces. Biomed Mater. 2015;10(5):055003. doi:10.1088/1748-6041/10/5/055003
16. de Avila ED, Castro AG, Tagit O, et al. Anti-bacterial efficacy via drug-delivery system from layer-by-layer coating for percutaneous dental implant components. Appl Surf Sci. 2019;488:194–204. doi:10.1016/j.apsusc.2019.05.154
17. Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73(2–3):121–136. doi:10.1016/S0168-3659(01)00248-6
18. Zhang L, Zhang L, Yang Y, et al. Inhibitory effect of super-hydrophobicity on silver release and antibacterial properties of super-hydrophobic Ag/TiO2 nanotubes. J Biomed Mater Res - Part B Appl Biomater. 2016;104(5):1004–1012. doi:10.1002/jbm.b.33454
19. Pokrowiecki R. The paradigm shift for drug delivery systems for oral and maxillofacial implants. Drug Deliv. 2018;25(1):1504–1515. doi:10.1080/10717544.2018.1477855
20. Harder S, Dimaczek B, Açil Y, Terheyden H, Freitag-Wolf S, Kern M. Molecular leakage at implant-abutment connection–in vitro investigation of tightness of internal conical implant-abutment connections against endotoxin penetration. Clin Oral Investig. 2010;14(4):427–432. doi:10.1007/S00784-009-0317-X
21. Canullo L, Penarrocha-Oltra D, Soldini C, Mazzocco F, Penarrocha M, Covani U. Microbiological assessment of the implant-abutment interface in different connections: cross-sectional study after 5 years of functional loading. Clin Oral Implants Res. 2015;26(4):426–434. doi:10.1111/clr.12383
22. Mishra SK, Chowdhary R, Kumari S. Microleakage at the different implant abutment interface: a systematic review. J Clin Diagn Res. 2017;11(6):ZE10–ZE15. doi:10.7860/JCDR/2017/28951.10054
23. Negahdari R, Ghavimi MA, Barzegar A, et al. Antibacterial effect of nanocurcumin inside the implant fixture: an in vitro study. Clin Exp Dent Res. 2021;7(2):163–169. doi:10.1002/CRE2.348
24. Odatsu T, Kuroshima S, Sato M, et al. Antibacterial properties of nano-ag coating on healing abutment: an in vitro and clinical study. Antibiotics. 2020;9(6):1–11. doi:10.3390/antibiotics9060347
25. Iwańczyk B, Wychowański P, Minkiewicz-Zochniak A, Strom K, Jarzynka S, Oledzka G. Bioactive healing abutment as a potential tool for the treatment of peri-implant disease-in vitro study. Appl Sci. 2020;10(15):5376. doi:10.3390/APP10155376
26. Wychowanski P, Osiak M, Morawiec T, Laubitz D, Czerniuk M, Wolinski J. The Bioactive Healing Abutment (BHA) for controlling microflora in periimplantitis. Clin Oral Implants Res. 2019;30(S19):247. doi:10.1111/clr.203_13509
27. Pokrowiecki R, Wojnarowicz J, Zareba T, et al. Nanoparticles and human saliva: a step towards drug delivery systems for dental and craniofacial biomaterials. Int J Nanomedicine. 2019;14:9235–9257. doi:10.2147/IJN.S221608
28. Pokrowiecki R, Zaręba T, Szaraniec B, Pałka K, Mielczarek A, Elżbieta Menaszek ST. In vitro studies of nanosilver doped Ti-implants for oral and maxillofacial surgery. Int J Nanomed. 2017;12:4285–4297. doi:10.2147/IJN.S131163
29. Cabal B, Cafini F, Esteban-Tejeda L, et al. Inhibitory effect on in vitro streptococcus oralis biofilm of a soda-lime glass containing silver nanoparticles coating on Titanium Alloy. PLoS One. 2012;7(8):1–9. doi:10.1371/journal.pone.0042393
30. Wang J, Du L, Fu Y, Jiang P, Wang X. ZnO nanoparticles inhibit the activity of Porphyromonas gingivalis and Actinomyces naeslundii and promote the mineralization of the cementum. BMC Oral Health. 2019;19(1):1–11. doi:10.1186/S12903-019-0780-Y/FIGURES/6
31. Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015;7(3):219. doi:10.1007/S40820-015-0040-X
32. Anees Ahmad S, Sachi Das S, Khatoon A, et al. Bactericidal activity of silver nanoparticles: a mechanistic review. Mater Sci Energy Technol. 2020;3:756–769. doi:10.1016/J.MSET.2020.09.002
33. Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;7:1831. doi:10.3389/FMICB.2016.01831/BIBTEX
34. Wojnarowicz J, Chudoba T, Koltsov I, Gierlotka S, Dworakowska S, Lojkowski W. Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis. Nanotechnology. 2018;29(6):065601. doi:10.1088/1361-6528/aaa0ef
35. Wojnarowicz J, Chudoba T, Lojkowski W. A review of microwave synthesis of zinc oxide nanomaterials: reactants, process parameters and morphologies. Nanomater. 2020;10(6):1086. doi:10.3390/NANO10061086
36. Wojnarowicz J, Opalinska A, Chudoba T, et al. Effect of water content in ethylene glycol solvent on the size of ZnO nanoparticles prepared using microwave solvothermal synthesis. J Nanomater. 2016;2016:1–15. doi:10.1155/2016/2789871
37. Szałaj U, Świderska-ś Sroda A, Chodara A, Gierlotka S, Łojkowski W. Nanoparticle size effect on water vapour adsorption by hydroxyapatite. Nanomaterials. 2019;9(7):1005. doi:10.3390/NANO9071005
38. Sakka S, Baroudi K, Nassani MZ, et al. Factors associated with early and late failure of dental implants. Cochrane Database Syst Rev. 2012;3(9):Cd004970. doi:10.1111/j.2041-1626.2012.00162.x
39. Bose S, Robertson SF, Bandyopadhyay A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018;66:6–22. doi:10.1016/j.actbio.2017.11.003
40. Cheng H, Chawla A, Yang Y, et al. Development of nanomaterials for bone-targeted drug delivery. Drug Discov Today. 2017;6446(17). doi:10.1016/j.drudis.2017.04.021
41. Zhao Y-N, Xu X, Wen N, et al. A Drug Carrier for Sustained Zero-Order Release of Peptide Therapeutics. Sci Rep. 2017;7(1):5524. doi:10.1038/s41598-017-05898-6
42. Pokrowiecki R, Pałka KMA. Nanomaterials in dentistry: a cornerstone or a black box? Nanomedicine. 2018;13(6):639–667. doi:10.2217/nnm-2017-0329
43. Thakral G, Thakral R, Sharma N, Seth J, Vashisht P. Nanosurface - the future of implants. J Clin Diagn Res. 2014;8(5):ZE07–ZE10. doi:10.7860/JCDR/2014/8764.4355
44. Do Nascimento C, Nogueira Fernandes FHC, Teixeira W, Pedrazzi V. Iodoform and silver-coated abutments preventing bacterial leakage through the implant-abutment interfaces: in vitro analysis using molecular-based method. Arch Oral Biol. 2019;105:65–71. doi:10.1016/j.archoralbio.2019.06.009
45. Sasada Y, Cochran D. Implant-abutment connections: a review of biologic consequences and peri-implantitis implications. Int J Oral Maxillofac Implants. 2017;32(6):1296–1307. doi:10.11607/JOMI.5732
46. Xu Z, Krajewski S, Weindl T, et al. The application of natural antibacterial coating for the surface modification of dental implants and abutments. Clin Oral Implants Res. 2019;30(S19):132. doi:10.1111/clr.90_13509
47. Gristina AG, Naylor P, Myrvik Q. Infections from biomaterials and implants: a race for the surface. Med Prog Technol. 1988;14(3–4):205–224.
48. Brunello G, Brun P, Gardin C, et al. Biocompatibility and antibacterial properties of zirconium nitride coating on titanium abutments: an in vitro study. PLoS One. 2018;13(6):e0199591. doi:10.1371/journal.pone.0199591
49. Besinis A, De Peralta T, Tredwin CJ, Handy RD. Review of nanomaterials in dentistry: interactions with the oral microenvironment, clinical applications, hazards, and benefits. ACS Nano. 2015;9(3):2255–2289. doi:10.1021/nn505015e
50. Chandran P, Riviere JE, Monteiro-Riviere NA. Surface chemistry of gold nanoparticles determines the biocorona composition impacting cellular uptake, toxicity and gene expression profiles in human endothelial cells. Nanotoxicology. 2017;11(4):507–519. doi:10.1080/17435390.2017.1314036
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.