Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Demographic And Clinical Characteristics Of Patients Prescribed Proprotein Convertase Subtilisin/kexin Type 9 Inhibitor Therapy And Patients Whose Current Lipid-Lowering Therapy Was Modified
Authors Baum SJ, Wade RL , Xiang P, Arellano J, Cerezo Olmos C, Nunna S, Chen CC, Carter CM, Desai NR
Received 23 May 2019
Accepted for publication 13 October 2019
Published 13 November 2019 Volume 2019:15 Pages 1325—1332
DOI https://doi.org/10.2147/TCRM.S216606
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Seth J Baum,1,2 Rolin L Wade,3 Pin Xiang,4 Jorge Arellano,4 Cesar Cerezo Olmos,5 Sasikiran Nunna,6 Chi-Chang Chen,6 Cathryn M Carter,7 Nihar R Desai8
1Department of Integrated Medical Sciences, Charles E Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL, USA; 2Preventive Cardiology Inc, Boca Raton, FL, USA; 3Medical and Scientific Services, IQVIA, Plymouth Meeting, PA, USA; 4Global Health Economics, Amgen Inc, Thousand Oaks, CA, USA; 5US Medical, Amgen Inc, Thousand Oaks, CA, USA; 6Real-World Evidence Solutions, IQVIA, Plymouth Meeting, PA, USA; 7Global Publications, Amgen Inc, Thousand Oaks, CA, USA; 8Section of Cardiovascular Medicine, Center for Outcomes Research and Evaluation, Yale School of Medicine, New Haven, CT, USA
Correspondence: Rolin L Wade
IQVIA, One IMS Drive, Plymouth Meeting, PA 19462, USA
Tel +1 215 434 812 2958
Email [email protected]
Purpose: Our objective was to describe the demographic and clinical characteristics of real-world patients in the US with elevated low-density lipoprotein cholesterol (LDL-C) whose lipid-lowering therapy (LLT) ─ both proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor and non-PCSK9 inhibitor ─ was actively modified.
Methods: This retrospective cohort study used linked laboratory (Prognos), pharmacy (IMS Formulary Impact Analyzer), and medical claims (IQVIA Dx/LRx or PharMetrics Plus) data. PCSK9 inhibitor–prescribed patients with LDL-C ≥70 mg/dL (multiply by 0.02586 for mmol/L) at the time of prescription were matched by LDL-C test date to patients whose non-PCSK9 inhibitor therapy was modified by intensifying statin therapy, switching statins without intensification, or augmenting with ezetimibe (N=12,345 in each cohort). Baseline demographics, use of LLT, LDL-C values, atherosclerotic cardiovascular disease (ASCVD) diagnoses and cardiovascular comorbidities, and occurrence of major adverse cardiovascular events (MACE) were assessed during the 2-year pre-index period.
Results: Mean age was 66.2 years in the PCSK9 inhibitor cohort and 64.1 years in the cohort whose LLT regimen was otherwise modified. Respectively, mean baseline LDL-C values were 150 and 121 mg/dL; 60.3% and 39.0% of patients had ASCVD diagnoses, and 9.6% and 5.1% had experienced a recent MACE. Prevalence of ASCVD diagnoses in the PCSK9 inhibitor and modified non-PCSK9 inhibitor cohorts, respectively, was 15.5% vs 9.1% for acute coronary syndrome, 20.7% vs 8.7% for coronary revascularization, and 22.2% vs 5.1% for possible familial hypercholesterolemia. In addition, 19.8% of patients in the PCSK9 inhibitor cohort were receiving both statins and ezetimibe vs 5.0% in the modified LLT cohort.
Conclusion: Physicians are prescribing PCSK9 inhibitor therapy to patients with markedly elevated LDL-C levels who also have comorbid risk factors for adverse cardiovascular events. These results may be of interest to payers and policymakers involved in devising access strategies for PCSK9 inhibitors.
Keywords: cardiovascular risk, lipid-lowering therapy, low-density lipoprotein, PCSK9 inhibitor, real-world treatment patterns
Introduction
In early 2018, it was estimated that in that year approximately 720,000 Americans would be hospitalized with a first myocardial infarction (MI) or would die because of coronary heart disease, and approximately 335,000 survivors would have a recurrent event.1 Similarly, an estimated 795,000 people experience a new (610,000) or recurrent (185,000) stroke annually; 87% of these events are ischemic in origin.1 Coronary heart disease is responsible for 43.8% of cardiovascular (CV)-related deaths in the US, followed by stroke (16.8%) and other cardiovascular diseases (CVDs; 17.9%).1 In 2016, approximately 544,800 people died of ischemic heart disease and 113,000 died of stroke.2 These premature deaths were associated with 7,605,300 and 1,139,800 years of life lost, respectively. In addition, the economic burden of CVD is substantial and increasing. The combined direct and indirect cost burden of CVD in 2016 was $555 billion (direct medical expenses, $318 billion; indirect costs, $237 billion).3 By 2035, 45.1% of adults in the US are projected to have some form of CVD, and this burden is expected to cost $1.1 trillion (direct, $749 billion; indirect, $368 billion).
Low-density lipoprotein cholesterol (LDL-C) plays a central role in the pathogenesis of atherosclerotic cardiovascular disease (ASCVD), and this relationship is both dose- and time-dependent.4,5 Although statins remain the cornerstone of lipid-lowering therapy (LLT), most patients with ASCVD do not achieve treatment goals with statins alone.6,7 The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor monoclonal antibodies represent an additional option for lowering of LDL-C levels in patients with ASCVD for whom maximally tolerated statin therapy, with or without augmentation with ezetimibe, is inadequate.8–10 For the first time, PCSK9 inhibitor therapies have been included, as Class IIa evidence for very high-risk patients with ASCVD, in the 2018 American College of Cardiology/American Heart Association (ACC/AHA) clinical practice guideline for the management of blood cholesterol.10 The 2018 ACC/AHA cholesterol guideline also introduces an LDL-C threshold of ≥70 mg/dL (≥1.8 mmol/L; multiply mg/dL by 0.02586 for mmol/L) as a trigger for treatment decisions in patients with very-high-risk ASCVD already receiving maximally tolerated statin and/or ezetimibe therapy.
Although early barriers to access and reimbursement for PCSK9 inhibitor therapy seem to be decreasing,11 overall approval rates for PCSK9 inhibitors were <50% between July 2015 and August 2016.12,13 A previous analysis of early adopters of PCSK9 inhibitor therapy in the US found that patients treated with PCSK9 inhibitors had higher CV risk in terms of LDL-C levels, CV comorbidities, statin intolerance, and intensity of LLT compared with patients treated with LLTs other than PCSK9 inhibitors.14 We aimed to describe the CV risk profiles of two distinct cohorts of patients—those prescribed PCSK9 inhibitor therapy and those whose non-PCSK9 inhibitor LLT had been recently modified (ie, intensified, switched, or augmented with ezetimibe). Characterization of these two profiles should answer current questions about the clinical appropriateness of PCSK9 inhibitor prescribing patterns in real-world clinical practice in the US.
Methods
Study Design And Patient Population
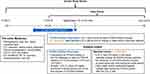
This retrospective cohort study used linked pharmacy adjudication status (IQVIA Formulary Impact Analyzer [FIA]; IQVIA, Plymouth Meeting, PA, USA), medical and prescription claims (IQVIA Dx/LRx or PharMetrics Plus [P+]), and laboratory data (Prognos, New York, NY, USA) from July 1, 2013, to December 31, 2017 (Figure 1). The FIA is a nationally representative transactional pharmacy claims database that captures complete transaction records for prescription transactions (approval, abandonment, and rejection). The LRx database contains information related to adjudicated dispensed prescriptions, which are sourced from retail, mail, long-term care, and specialty pharmacies. This database is sourced from pharmacies and represents >90% of all outpatient prescriptions dispensed in the US. The Dx database contains unadjudicated medical claims from office-based physicians, ambulatory facilities, and general health care sites. Dx data are supplemented with institutional claims, including claims from hospital-based physicians. This database is sourced from “clearing houses,” which are also referred to as “switches.” These data represent approximately 75% of all the physicians in the American Medical Association database and capture >1.3 billion claims per year. The P+ database contains adjudicated medical and pharmacy claims of patients in the US and is sourced from payers. This database contains standard fields such as inpatient and outpatient diagnoses and procedures, and retail and mail order prescription records; P+ has detailed information on the pharmacy and medical benefit (copayment, deductible), the inpatient stay (admission type and source, discharge status), and provider details (specialty, provider ID). The database includes >150 million unique individuals, with approximately 40 million active patients in the database in 2017.
Patients in all IQVIA databases and the Prognos LDL-C dataset were linked at a patient level using a deterministic encryption method that complies with Health Insurance Portability and Accountability Act (HIPAA) regulations. The PCSK9 inhibitor cohort included patients who submitted a prescription for PCSK9 inhibitor therapy. The cohort whose existing statin or ezetimibe regimen was modified included intensification of the current statin regimen by either increasing the dose of the current statin or switching statins at a higher intensity; by switching to a different statin, without an increase in statin intensity; or by augmenting statin therapy with ezetimibe. Supplementary Table S1 provides the statin intensity rules. Patients who switched statins without intensification were included in the modified non-PCSK9 inhibitor cohort, because studies have shown substantial LDL-C lowering among statin-treated patients who switched to a different statin at the same intensity.15,16 Included patients were ≥18 years old, had a pre-index LDL-C level ≥70 mg/dL at the time of prescription for PCSK9 inhibitor therapy or modification of other LLT, and had high-quality linkable data in the IQVIA claims databases (Figure 1; Supplementary Table S2). Patients in the modified LLT cohort were matched 1:1 to patients in the PCSK9 inhibitor cohort by the LDL test date (within the same calendar quarter; N=12,345 per cohort). The index event was the LDL-C test at the time of initial PCSK9 inhibitor request (PCSK9 inhibitor cohort) or the date-matched LDL-C test at the time of the change in statin and/or ezetimibe regimen (modified LLT cohort).
Pre-Index Measures And Index Event
All variables were measured during a 2-year baseline period prior to the index event. The index period was from July 1, 2015, to December 31, 2017, and the index event for each patient was the qualifying LDL-C test date (LDL-C ≥70 mg/dL).
Pre-index measures included demographics (age, sex, geographic region, insurance type), clinical characteristics (ASCVD diagnoses, CV comorbidities, LDL-C values), LLT utilization, and CV risk as measured by occurrence of recent major adverse CV events (MACE). MACE included events of MI, ischemic stroke (IS), coronary revascularization (coronary artery bypass graft or percutaneous coronary intervention), and unstable angina (UA). ASCVD diagnoses, CV comorbidities and risk factors, and MACE were identified using International Classification of Diseases (ICD)-9/ICD-10 diagnosis codes. Baseline demographic and clinical measures were summarized descriptively. This study was not designed to make statistical comparisons between cohorts and did not assess post-index outcomes.
Ethics
This study complied with all applicable laws regarding subject privacy, using HIPAA-compliant de-identified retrospective data sources. No direct subject contact or primary collection of individual human subject data occurred. Study results were in tabular form and aggregate analyses that omitted subject identification; therefore, informed consent, ethics committee approval, and institutional review board approval were not required.
Results
Demographics And Clinical Characteristics
Mean age was 66.2 years in the PCSK9 inhibitor cohort and 64.1 years in the cohort whose LLT regimen was modified; 58.4% and 49.0% of patients, respectively, were ≥65 years of age (Table 1). Regarding ezetimibe use, one in five patients (19.8%) in the PCSK9 inhibitor cohort and one in 20 (5.0%) in the cohort whose statin and/or ezetimibe regimen was modified were receiving both statins and ezetimibe.
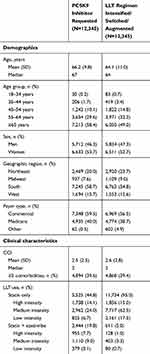 |
Table 1 Baseline Demographic And Clinical Characteristics: Matched Cohorts |
For the non-PCSK9 inhibitor cohort, there were no overt or unexpected directional differences in the constellation of demographic or clinical characteristics among the subgroups whose statin regimen was intensified, switched, or augmented with ezetimibe (Supplementary Tables S3 and S4).
Baseline LDL-C Values
Mean LDL-C levels at index were 150 mg/dL (SD, 48.9 mg/dL) in the PCSK9 inhibitor cohort and 121 mg/dL (SD, 37.5 mg/dL) in the cohort whose non-PCSK9 inhibitor regimen was modified (Figure 2). Baseline LDL-C levels were ≥130 mg/dL in 62.7% of patients in the PCSK9 inhibitor cohort and 36.2% in the modified LLT cohort; LDL-C levels were ≥100 mg/dL in 85.4% and 66.1% of patients, respectively. In the subset of patients of the modified LLT cohort who had their statin regimen augmented with ezetimibe (n=764), mean LDL-C was 115 mg/dL (SD, 38.2 mg/dL), and 57.5% of patients had LDL-C levels ≥100 mg/dL (Supplementary Table S4).
ASCVD Diagnoses And Comorbidities
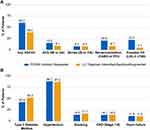
The percentages of patients with ASCVD diagnoses in the PCSK9 inhibitor and modified non-PCSK9 inhibitor cohorts, including any ASCVD diagnosis, acute coronary syndrome (defined by MI or UA), stroke (IS or transient ischemic attack), coronary revascularization, and possible familial hypercholesterolemia (defined as LDL-C ≥190 mg/dL any time during the baseline period), are summarized in Figure 3A. A history of ASCVD was present for 60.2% and 39.0% of patients, respectively. In the subgroup for which the LLT regimen was augmented with ezetimibe, 52.4% of patients had ASCVD (Supplementary Table S4). Prevalence of ASCVD diagnoses in the PCSK9 inhibitor and modified non-PCSK9 inhibitor cohorts, respectively, was 15.5% and 9.1% for acute coronary syndrome, 8.2% and 8.0% for stroke, 20.7% and 8.7% for coronary revascularization, and 22.2% and 5.1% for possible familial hypercholesterolemia. Type 2 diabetes and hypertension were the most common high-risk CV comorbidities in both groups (Figure 3B).
CV Risk
Nearly 10% of patients had at least one acute MACE (MI, IS, UA, or coronary revascularization) in the 2 years prior to their PCSK9 inhibitor prescription (Figure 4). In the cohort whose statin and/or ezetimibe LLT regimen was modified, 5.1% of patients had at least one acute MACE. Coronary revascularization was the most common MACE in both groups (7.4% in the PCSK9 inhibitor cohort vs 3.0% in the modified non-PCSK9 inhibitor cohort).
Discussion
This real-world analysis characterizing the CV risk of patients receiving a prescription for a PCSK9 inhibitor from July 2015 through 2017, prior to release of the 2018 ACC/AHA guideline,10 demonstrates that physicians were prescribing PCSK9 inhibitor therapy to patients with LDL-C values more than double the currently recommended target of <70 mg/dL for patients with very high-risk ASCVD. In addition to markedly elevated LDL-C, many patients also had clinical ASCVD, high-risk comorbidities, and/or a recent MACE. Compared with those whose non-PCSK9 inhibitor lipid-lowering regimen was otherwise modified, the CV risk profiles demonstrate that patients requesting access to PCSK9 inhibitor therapy had higher baseline LDL-C values and were older, with >50% of patients meeting the high-risk criteria of being ≥65 years old. In addition, patients prescribed PCSK9 inhibitors were generally more likely to have high-risk CV-related comorbidities, ASCVD diagnoses, and a recent MACE. More patients in the PCSK9 inhibitor cohort were receiving both statins and ezetimibe, suggesting greater LLT intensity in the PCSK9 inhibitor cohort. Our results are in accordance with a previous study, which found baseline LDL-C levels to be higher in patients prescribed PCSK9 inhibitor therapy compared with patients receiving other LLT.14 Interestingly, the earlier study found that higher LDL-C levels were not associated with higher PCSK9 inhibitor approval rates. Other variables that were significantly associated with approval of PCSK9 inhibitor therapy included age >65 years, history of ASCVD, prescription by a cardiologist or non-primary care provider, statin intolerance, longer statin duration, and noncommercial payer.13
Although CV risk is multifactorial, LDL-C level is a critical risk factor based on evidence that lower LDL-C levels are associated with a reduced risk of CV events along with improved patient outcomes, including for those with LDL-C levels averaging 70 mg/dL or less.6,8–10,17,18 The ACC/AHA recommendations to extend the use of PCSK9 inhibitors in very-high-risk patients with ASCVD and monitor patient response to statin therapy and lifestyle changes were based on perspectives gleaned from the outcome trials that demonstrated that lower LDL-C is better and safe, even at very low levels.10,19 Overall, the addition of a PCSK9 inhibitor to background statin therapy has been shown to further lower LDL-C levels by 43% to 64% and also to reduce the occurrence of CV events, including MI and IS.8,9,20,21
Despite declining CVD mortality rates in the 21st century, recent trends suggest that these rates are stabilizing and may even be on the rise because of shifting demographics, increasing prevalence of risk factors, and the lack of innovative treatments.22,23 One recent analysis predicts that if factors such as current treatment utilization remain unchanged, CVD mortality in the US will increase by 41% in 2040.23 The authors suggest that wide utilization of innovations as impactful as the introduction of statins is necessary to stabilize and potentially further reduce CVD mortality. For patients with LDL-C ≥70 mg/dL despite maximally tolerated statin therapy, current guidelines recommend considering ezetimibe for patients with clinical ASCVD and adding ezetimibe before a PCSK9 inhibitor for patients with very high-risk ASCVD.10 Results from our study are consistent with other real-world studies, suggesting that augmentation with ezetimibe may not be enough for some high-risk patients to achieve LDL-C levels <70 mg/dL.24,25 Addition of ezetimibe to statins has shown up to 25% reduction in LDL-C,26 and this reduction would not have been sufficient, on average, to lower the mean LDL-C from 115 to <70 mg/dL in the group whose LLT was augmented with ezetimibe. Therefore, modifying the current non-PCSK9 inhibitor LLT without adding a PCSK9 inhibitor, in general, may not provide optimal LDL-C outcomes, particularly in patients with LDL-C elevated to the extent observed in the study cohorts.
Although MI and stroke have a similar prevalence in the US,1 we observed a lower than expected percentage of patients with a history of IS in our sample of patients whose LLT was being actively managed. This finding suggests that the IS population may be undertreated with LLT. In a recently published study of recurrent MI and IS in Medicare beneficiaries, the rate of recurrent IS during the patients’ first year of survival after an event of IS (6.7%) was similar to that of patients with recurrent MI (7.2%), which further supports the need for aggressive intervention for secondary prevention in patients with MI or IS.27 Other important areas for future research will include investigating how the CV risk profile of patients in our cohort compares with the risk profiles defined in the 2018 ACC/AHA guideline, as well as assessing the impact of new guideline and the potential influence on physician prescribing practices for PCSK9 inhibitor therapy in future cohort analyses. Studies that assess the characteristics of filled vs abandoned prescriptions and impact on CV outcomes would also be of interest in the context of the changing value and access landscape for PCSK9 inhibitors.
Limitations
This was a retrospective analysis based on data linking different claims databases and is subject to limitations of conducting research using large administrative databases containing data not originally collected for research purposes. For example, it was not possible to determine whether patients were receiving maximally tolerated statin therapy or if any patients in the PCSK9 inhibitor cohort who were not receiving statins at index had been rechallenged for statin intolerance. In addition, retrospective analyses can only identify relationships; they cannot establish inference. This analysis was descriptive; thus, possible confounding factors may not be controlled for in the results. Although the analysis included both privately insured patients and Medicare beneficiaries, results should be interpreted in the context of the study sample and may not necessarily be generalizable to the US population. It was not possible to identify patients who may have received samples of PCSK9 inhibitor therapy during the pre-index period; therefore, only patients with LDL-C ≥70 mg/dL were included in the analysis. Finally, the study population was not restricted to patients with ASCVD; however, many patients not receiving statins in the PCSK9 inhibitor cohort possibly were statin intolerant and many had probable familial hypercholesterolemia, as might be expected for early adopters of PCSK9 inhibitor therapy.
Conclusions And Relevance
Physicians are prescribing PCSK9 inhibitor therapy to patients with risk profiles consistent with recent guideline recommendations for use of PCSK9 inhibitors, including LDL-C elevations and comorbid risk factors for adverse CV events that suggest a higher risk profile than patients on other LLTs. Since the PCSK9 inhibitor outcomes trials and the 2018 ACC/AHA guideline support the “lowest is best” concept regarding LDL-C levels and CV risk, augmentation with ezetimibe may not be enough to achieve LDL-C levels <70 mg/dL in many high-risk patients with ASCVD. These results may be of interest to payers and policymakers involved in devising access strategies for PCSK9 inhibitors.
Abbreviations
ACC/AHA, American College of Cardiology/American Heart Association; ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; CV, cardiovascular; CVD, cardiovascular disease; FIA, Formulary Impact Analyzer; HIPAA, Health Insurance Portability and Accountability Act; ICD, International Classification of Diseases; IS, ischemic stroke; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy; MACE, major adverse CV events; MI, myocardial infarction; P+, PharMetrics Plus; PCSK9, proprotein convertase subtilisin/kexin type 9; UA, unstable angina.
Data Sharing Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
SJB: president of Excel Medical Clinical Trials, LLC, and Preventive Cardiology, Inc; consultant/advisory board member/principal investigator for Merck, Akcea, Amgen Inc, Regeneron, Sanofi, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Esperion, Gemphire, Madrigal, Novartis, Gerson Lehrman Group, Novo Nordisk and Guidepoint Global; secretary/treasurer of the FH Foundation; immediate past president of the American Society for Preventive Cardiology. RLW, SN, C-CC: employees of IQVIA, which was hired by Amgen Inc to conduct this study. PX, JA, CCO, CMC: employees of Amgen Inc and own Amgen Inc stock. NRD: research grants from Medtronic, Johnson & Johnson; consulting fees from Amgen Inc, Relypsa, OPKO, scPharmaceuticals, Cytokinetics. The authors report no other conflicts of interest in this work.
References
1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association [published correction appears in Circulation. 2018;137(12):e493]. Circulation. 2018;137(12):e67–e492. doi:10.1161/CIR.0000000000000558
2. The US Burden of Disease Collaborators. The State of US Health, 1990–2016: burden of diseases, injuries, and risk factors among US States. JAMA. 2018;319(14):1444–1472. doi:10.1001/jama.2018.0158
3. American Heart Association & American Stroke Association. Cardiovascular Disease: A Costly Burden for America — Projections through 2035. American Heart Association website. Available from: https://healthmetrics.heart.org/wp-content/uploads/2017/10/Cardiovascular-Disease-A-Costly-Burden.pdf. Accessed February 14, 2019.
4. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. doi:10.1093/eurheartj/ehx144
5. Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161(1):161–172. doi:10.1016/j.cell.2015.01.036
6. Boekholdt SM, Hovingh GK, Mora S, et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64(5):485–494. doi:10.1016/j.jacc.2014.02.615
7. Wong ND, Chuang J, Zhao Y, Rosenblit PD. Residual dyslipidemia according to low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B among statin-treated US adults: National Health and Nutrition Examination Survey 2009–2010. J Clin Lipidol. 2015;9(4):525–532. doi:10.1016/j.jacl.2015.05.003
8. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi:10.1056/NEJMoa1615664
9. Szarek M, White HD, Schwartz GG, et al. Alirocumab reduces total nonfatal cardiovascular and fatal events: the ODYSSEY OUTCOMES trial. J Am Coll Cardiol. 2019;73(4):387–396. doi:10.1016/j.jacc.2018.10.039
10. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285–e359. doi:10.1016/j.jacc.2018.11.003
11. Zafrir B, Jubran A. Lipid-lowering therapy with PCSK9-inhibitors in the real-world setting: two-year experience of a regional lipid clinic. Cardiovasc Ther. 2018;36(5):e12439. doi:10.1111/1755-5922.12439
12. Baum SJ, Toth PP, Underberg JA, Jellinger P, Ross J, Wilemon K. PCSK9 inhibitor access barriers-issues and recommendations: improving the access process for patients, clinicians and payers. Clin Cardiol. 2017;40(4):243–254. doi:10.1002/clc.22713
13. Hess GP, Natarajan P, Faridi KF, Fievitz A, Valsdottir L, Yeh RW. Proprotein convertase subtilisin/kexin type 9 inhibitor therapy: payer approvals and rejections, and patient characteristics for successful prescribing. Circulation. 2017;136(23):2210–2219. doi:10.1161/CIRCULATIONAHA.117.028430
14. Rane PB, Patel J, Harrison DJ, et al. Patient characteristics and real-world treatment patterns among early users of PCSK9 inhibitors. Am J Cardiovasc Drugs. 2018;18(2):103–108. doi:10.1007/s40256-017-0246-z
15. Lewis SJ, Olufade T, Anzalone DA, Malangone-Monaco E, Evans KA, Johnston S. LDL cholesterol levels after switch from atorvastatin to rosuvastatin. Curr Med Res Opin. 2018;34(10):1717–1723. doi:10.1080/03007995.2017.1421147
16. Schuster H, Barter PJ, Stender S, et al. Effects of switching statins on achievement of lipid goals: measuring effective reductions in cholesterol using rosuvastatin therapy (MERCURY I) study. Am Heart J. 2004;147(4):705–713. doi:10.1016/j.ahj.2003.10.004
17. Cholesterol Treatment Trialists' (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi:10.1016/S0140-6736(10)61350-5
18. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289–1297. doi:10.1001/jama.2016.13985
19. Rubenfire M. Latest in Cardiology. American College of Cardiology website. Available from: https://www.acc.org/latest-in-cardiology/ten-points-to-remember/2018/11/09/14/28/2018-guideline-on-management-of-blood-cholesterol. Accessed January 7, 2019.
20. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–1509. doi:10.1056/NEJMoa1500858
21. Cannon CP, Cariou B, Blom D, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36(19):1186–1194. doi:10.1093/eurheartj/ehv028
22. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association [published correction appears in Circulation. 2017; 135(10):e646and Circulation. 2017;136(10):e196]. Circulation. 2017;135(10):e146–e603. doi:10.1161/CIR.0000000000000485
23. Ortendahl JD, Diamant AL, Toth PP, Cherepanov D, Harmon AL, Broder MS. Protecting the gains: what changes are needed to prevent a reversal of the downward cardiovascular disease mortality trend? Clin Cardiol. 2019;42(1):47–55. doi:10.1002/clc.23097
24. Menzin J, Aggarwal J, Boatman B, et al. Ezetimibe use and LDL-C goal achievement: a retrospective database analysis of patients with clinical atherosclerotic cardiovascular disease or probable heterozygous familial hypercholesterolemia. J Manag Care Spec Pharm. 2017;23(12):1270–1276. doi:10.18553/jmcp.2017.16414
25. Chen CC, Rane PB, Hines DM, Patel J, Harrison DJ, Wade RL. Low-density lipoprotein cholesterol outcomes post-non-PCSK9i lipid-lowering therapies in atherosclerotic cardiovascular disease and probable heterozygous familial hypercholesterolemia patients. Ther Clin Risk Manag. 2018;14:2425–2435. doi:10.2147/TCRM.S180783
26. Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 focused update of the 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 2017;70(14):1785–1822. doi:10.1016/j.jacc.2017.07.745
27. Li S, Peng Y, Wang X, et al. Cardiovascular events and death after myocardial infarction or ischemic stroke in an older Medicare population. Clin Cardiol. 2019;42(3):391–399. doi:10.1002/clc.23160
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.