Back to Journals » Clinical Ophthalmology » Volume 17
Delphi Panel Consensus Regarding Current Clinical Practice Management Options for Demodex blepharitis
Authors Farid M, Ayres BD, Donnenfeld E , Gaddie IB, Gupta PK , Holland E, Lindstrom R, Pflugfelder SC, Karpecki PM , Nichols KK , Starr CE, Yeu E
Received 3 December 2022
Accepted for publication 13 February 2023
Published 27 February 2023 Volume 2023:17 Pages 667—679
DOI https://doi.org/10.2147/OPTH.S399989
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Marjan Farid,1 Brandon D Ayres,2 Eric Donnenfeld,3 Ian Benjamin Gaddie,4 Preeya K Gupta,5 Edward Holland,6 Richard Lindstrom,7 Stephen C Pflugfelder,8 Paul M Karpecki,9 Kelly K Nichols,10 Christopher E Starr,11 Elizabeth Yeu12
1Gavin Herbert Eye Institute, UC-Irvine, Irvine, CA, USA; 2Wills Eye Hospital, Philadelphia, PA, USA; 3Ophthalmic Consultants of Long Island, Long Island, NY, USA; 4Gaddie Eye Centers, Louisville, KY, USA; 5Triangle Eye Consultants, Raleigh, NC and Tulane University, New Orleans, LA, USA; 6University of Cincinnati, Cincinnati, OH, USA; 7University of Minnesota, Minneapolis, MN, USA; 8Baylor College of Medicine, Houston, TX, USA; 9Kentucky Eye Institute, Lexington, KY, USA; 10University of Alabama at Birmingham, School of Optometry, Birmingham, AL, USA; 11Weill Cornell Medicine, New York, NY, USA; 12Virginia Eye Consultants, Norfolk, VA, USA
Correspondence: Marjan Farid, University of California, 850 Health Sciences Road, Mail Code: 4375, Irvine, CA, 92697, USA, Tel +1 949 824-0327, Email [email protected]
Purpose: To obtain consensus on Demodex blepharitis (DB) treatment using a modified Delphi panel process.
Methods: Literature search identified gaps in knowledge surrounding treatment of DB. Twelve ocular surface disease experts comprised the Demodex Expert Panel on Treatment and Eyelid Health (DEPTH). They completed a live roundtable discussion in addition to 3 surveys consisting of scaled, open-ended, true/false, and multiple-choice questions pertaining to the treatment of DB. Consensus for scaled questions using a 1 to 9 Likert scale was predefined as median scores of 7– 9 and 1– 3. For other question types, consensus was achieved when 8 of 12 panelists agreed.
Results: The experts agreed that an effective therapeutic agent for treatment of DB would likely decrease the necessity of mechanical intervention, such as lid scrubs or blepharoexfoliation (Median = 8.5; Range 2– 9). When treating DB, panelists believed that collarettes serve as a surrogate for mites, and that eliminating or reducing collarettes should be the main clinical goal of treatment (Median = 8; Range 7– 9). The panelists would treat patients with at least 10 collarettes, regardless of other signs or symptoms and agreed that DB can be cured, but there is always the possibility for a reinfestation (n = 12). There was also consensus that collarettes, and therefore mites, are the primary treatment target and the way by which clinicians can monitor patient response to therapy (Median = 8; Range 7– 9).
Conclusion: Expert panelists achieved consensus on key facets of DB treatment. Specifically, there was consensus that collarettes are pathognomonic for DB, that DB patients with > 10 collarettes should be treated even in the absence of symptoms, and that treatment efficacy can be tracked by collarette resolution. By increasing awareness about DB, understanding the goals of and monitoring treatment efficacy, patients will receive better care and, ultimately, better clinical outcomes.
Keywords: collarettes, cylindrical dandruff, ocular surface disease, eyelid disease
Introduction
Blepharitis
Lid and ocular surface diseases often share a constellation of signs, including lid margin erythema, meibomian gland inspissation, conjunctivitis, conjunctival erythema, tear film instability, and keratitis. Blepharitis is a common inflammatory ocular disease that affects the eyelid margin, frequently resulting in disruption of the ocular surface and tear film.1 When present, blepharitis can damage the ocular surface and exacerbate concurrent ocular surface disorders.1–3 Because of its chronic nature and frequent comorbidity with other ocular surface diseases, managing these patients can be difficult.2 There are no FDA-approved treatments specifically for blepharitis, so the goal is often palliative, providing short-term management of signs and symptoms, with strategies such as warm compresses and lid hygiene, topical anti-inflammatory agents, and topical and oral antibiotics.2 While these options may temporarily relieve patient symptoms, they rarely address the root cause of the disease.
Demodex blepharitis
One of the most common etiologies of blepharitis is known to be Demodex infestation, representing up to 70% of all patients diagnosed with blepharitis.4–9 Demodex is a microscopic parasite commonly found on the human body.5,10 There are more than 1600 species of Demodex mites, with Demodex folliculorum (Figure 1) prevalent in the lash follicle and Demodex brevis usually in the meibomian glands.11 Both Demodex folliculorum and Demodex brevis have been associated with Demodex blepharitis (DB).12–14 These species of Demodex were first reported near the eye more than 50 years ago, but to date there have been no treatments approved by the FDA with the indication of treating Demodex blepharitis (DB).15
 |
Figure 1 Microscopic views of Demodex mites. (A) Multiple Demodex mites on and around a lash. (B) Highly magnified Demodex mite. |
Recent findings from a comprehensive study of 1032 participants from 7 geographically different clinical sites showed that nearly 58% of all-comers had Demodex blepharitis, a strikingly high percentage. When looking at specific subsets of patients, even higher proportions of patients with blepharitis (69.1%) and glaucoma (64.8%) had DB.16 Because of this high prevalence across both optometry and ophthalmology practices, understanding the role of Demodex is crucial to the overall understanding of blepharitis management.
Since many symptoms overlap with other ocular surface conditions, Demodex blepharitis is commonly underdiagnosed or misdiagnosed.17,18 With the challenges of signs and symptoms overlapping with other ocular surface disorders, such as dry eye disease (DED) and meibomian gland dysfunction, there has been a lack of consensus among clinicians surrounding the diagnosis and management of DB.16,17 Moreover, there is currently no “gold standard” for management of Demodex blepharitis which, when coupled with poor patient compliance using current treatment options, demonstrates a need for developing consensus on, and spreading awareness of, dedicated management strategies to improve treatment outcomes.
The Delphi Methodology
Since the 1960s, the Delphi panel methodology has been used as a way to gain consensus on a given topic. First used by the RAND Corporation in collaboration with the US Air Force, the Delphi methodology is a process by which a group of experts can come to consensus utilizing a series of questionnaires.19 While primarily relying on surveys, the process may be modified to include face-to-face meetings. This methodology has been used across multiple professions, including numerous areas of medicine and, specifically, eyecare.20–33
The current group of experts, Demodex Expert Panel on Treatment and Eyelid Health (DEPTH), previously convened and obtained consensus about aspects of DB, including pathophysiology, patient profile, signs and symptoms, diagnosis, and comorbidities associated with Demodex blepharitis.34 To the authors’ knowledge, that was the first Delphi panel that had been convened to address Demodex blepharitis. While consensus was obtained about a variety of aspects of DB, consensus was not achieved surrounding aspects of treatment of this condition. This paper, therefore, describes the second Delphi methodology process undertaken by this group, focusing on the management of Demodex blepharitis.
Materials and Methods
This Delphi Panel process was funded by an unrestricted grant from Tarsus Pharmaceuticals, Inc. (Irvine, CA, USA) and administered independently by i2Vision (San Diego, CA, USA), a third-party medical communications company. As this study does not involve patients or study subjects, and it does not submit people to actions or impose specific behaviors on them, according to the Medical Research in Human Subjects Act (WMO), an ethical approval was not needed. At the beginning of the first Delphi Panel, a team from i2Vision identified and invited experts in ocular surface disease to participate in the process. Following literature review, individuals at i2Vision wrote survey questions, developed the online surveys, instructed the panelists and administered the surveys, compiled the anonymous survey results, analyzed survey data, wrote subsequent survey questions, developed the agenda for the live meeting, provided pre-reading material to panelists ahead of the meeting, collated and analyzed the final data, and organized and assisted the authors in the writing of the paper. To minimize bias, the interim results were masked from the panelists until the end of the process, and there was a firewall between i2Vision and the study sponsor until the manuscript of study results was written. The second phase of the process focusing on management/treatment was administered in the same way in order to prevent potential bias.
The DEPTH panel was composed of ophthalmologists and optometrists from across the US practicing in a range of settings, including private practice and academia, for varying durations. All panelists were identified as having expertise in external disease, blepharitis, and ocular surface disease. To determine expertise, the number of publications, history of podium presentations, and membership and involvement in organizations and committees related to these ocular surface conditions were considered. Prior to the first Delphi panel, a comprehensive literature search was conducted using the keywords “Demodex”, “demodicosis + eye”, “blepharitis”, “collarette”, and “cylindrical dandruff” for publications with clinical evidence about Demodex blepharitis. Results were limited to papers published between 2015 and 2021. The search generated 95 papers related to Demodex and Demodex blepharitis that were used to create the surveys. A repeat literature search was performed prior to the second Delphi panel process on management/treatment using additional keywords “Demodex + treatment” to identify papers focusing on treatment and any newer publications published since the initial literature search. Search dates included May 2021 through January 2022, and 4 additional papers were identified.
As with the first DEPTH Delphi panel, surveys included questions based on a Likert scale from 1 to 9 in order to produce quantifiable results. Approximately one-third of the questions in Surveys 1 and 2 were structured to use the scale, along with about three-fourths of the Survey 3 questions. Additionally, there were true/false, yes/no, multiple-choice, and open-ended questions in the surveys. Demographic data was collected from panelists during the first Delphi panel so it was not included again. The same definition of consensus was used for this second Delphi process: for the 1 to 9 scaled questions, a median score between 7 and 9 or between 1 and 3 indicated consensus achieved at the high and low ends of the scale, respectively, and a median score between 4 and 6 indicated that consensus was not achieved. Additionally, if more than one-third of the panel members selected 1 to 3 and more than one-third of the panel members selected 7 to 9, this was considered disagreement (Figure 2). For the forced choice and open-ended questions, consensus was defined as agreement between at least 8 of the 12 panelists.
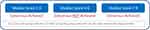 |
Figure 2 DEPTH guidelines for consensus and disagreement. |
DEPTH panelists completed 3 electronic surveys with questions presented in a randomized order to each participant to minimize bias from “survey fatigue.” The i2Vision team was also masked to survey respondents, as the results were collected anonymously. Surveys were structured so that all questions had to be answered; no blank answers were allowed. Similar to the prior Delphi panel, these surveys intentionally repeated some questions with variations in wording in order to assess consistency of panelist responses. The respondents were provided the instructions shown in Figure 3.
 |
Figure 3 Survey instructions provided to DEPTH panelists. |
All 3 surveys covered a range of topics primarily related to the treatment and management of Demodex blepharitis. Survey 1 consisted of 40 scaled, open-ended, and closed-ended questions (yes/no, multiple-choice, true/false), some of which allowed the respondent to include free-text elaboration. The results of Survey 1 were compiled and analyzed, and these results were used to develop Survey 2 based on topics about Demodex blepharitis treatment that did not achieve consensus.
The goal of Survey 2 was to confirm areas of consensus obtained in Survey 1 and to move toward consensus on additional topics. This survey contained 25 scaled, multiple-choice, or true/false questions developed based, in part, on panelist answers from the Survey 1 open-ended questions. The results were then compiled and analyzed, using areas of non-consensus to develop discussion questions for the live meeting.
Prior to the live meeting, 3 articles related to Demodex blepharitis treatment were sent to the panel as pre-reading material.35–37 The articles chosen for distribution were published since the prior Delphi panel and had a focus on treatment and management of Demodex blepharitis. A video conference was then held to discuss aspects of DB management. As detailed in the first paper, the original Delphi methodology from the RAND Corporation exclusively utilized surveys in order to maintain participant anonymity.34 The “modified Delphi methodology”, however, includes face-to-face meetings and has become more common in recent years. This modified process has proven particularly useful in the healthcare setting.38–41
The goal of the meeting was to facilitate discussion among the DEPTH panelists after having completed the first 2 surveys and the pre-reading materials provided before the video conference. During the moderated meeting, high-level results from the first 2 surveys were presented, and panelists discussed general themes from them as well as the recent literature relating to the treatment of DB. It was made clear to the panelists that the goal of the meeting was not to achieve further consensus, but rather to have meaningful discussion about the treatment and management of Demodex blepharitis.42 While the discussion was captured, there was no analysis performed on the content of the meeting.
Following the face-to-face meeting, panelists completed the final 15-question survey with true/false and scaled questions based on areas still lacking consensus after the first 2 surveys and discussion at the live meeting. The results of Survey 3 were compiled and analyzed, and the data from all the surveys were combined. The results are presented here.
Results
Demographic Information of the Expert Panelists
The 3 surveys had a response rate of 100%. Nine ophthalmologists and 3 optometrists comprised the DEPTH panel. The mean age of the panelists was 53.7 years (SD 10.5 years; Range 40–73 years), with an average of 23.9 years (SD 10.7; Range 10–43 years) in practice. Four panelists were women (33.3%) and 8 were men (66.7%). Half of the participants practiced in an academic or academic referral setting, while the other half were in private practice (including office-based private practice, single specialty ophthalmology group, private practice, multi-specialty private practice, and MD/OD practice). All panelists reside and practice in the US.
In the following results, the parenthetical numbers indicate how many of the 12 panelists agreed on closed-ended questions. For scaled questions, the median and the range are reported. The open-ended questions, while not yielding a numeric score, did result in some emerging patterns which are also noted.
Specific Management Modalities
All of the DEPTH panelists (n = 12) have managed DB patients using tea tree oil (TTO) in various forms, including gel, ointment, wipes, face wash, or in-office treatment. However, the majority of experts (n = 10) agreed that patient tolerability, treatment efficacy, and potential damage to the meibomian glands are all of concern when treating with TTO. Other over-the-counter (OTC) management strategies have also been used to varying degrees (Table 1).
 |
Table 1 Currently Used Over-the-Counter Therapies |
When asked about the necessity of mechanical intervention, such as home lid scrubs or in-office blepharoexfoliation, in the treatment of DB, the panelists did not reach consensus (Median = 5.5; Range 1–8). In a subsequent question, however, the DEPTH experts agreed that, in the future when there is an effective therapeutic agent for treatment of DB, mechanical intervention may not be necessary (Median = 8.5; Range 2–9).
Timing of and Threshold for Treatment
When determining the point at which a patient should be treated, initially there was no consensus about specific disease thresholds for treatment. In an open-ended question, though, there was a trend toward the treatment of patients with moderate collarettes, or cylindrical dandruff, which are the pathognomonic clinical sign of DB even without other signs or symptoms. In subsequent closed-ended questions, panelists unanimously agreed that they would treat patients with >10 collarettes/cylindrical dandruff plus additional signs (eg, lid erythema, loss of lashes, misdirected lashes) but no symptoms (n = 12), as well as patients with >10 collarettes plus symptoms (redness, itching, tearing, dry eye, irritation) but no additional signs (n = 12). They also unanimously agreed that they would NOT treat a patient with 0 to 2 collarettes and no symptoms (n = 12). For the converse, a patient with 0 to 2 collarettes with symptoms but with no additional signs, the DEPTH panel agreed that they would treat in those circumstances (n = 11).
When asked in a different way, the experts indicated that they would initiate treatment for DB in a patient that had at least 10 collarettes even in the absence of symptoms. (Median = 7; Range 1–9) They would also initiate treatment for DB in a patient with 0 to 2 collarettes per lid margin and who suffered from itching, lid erythema, and/or other signs or symptoms that may be attributed to Demodex (Median = 8; Range 2–9).
Clinical Findings
When asked the extent to which they agreed with the statement, “Complete eradication of mites is necessary when treating Demodex blepharitis”, the DEPTH panelists’ responses had a median of 3 (Range 1–8), indicating the experts felt that complete eradication of mites is not necessary. In another question, the experts agreed that collarettes or cylindrical dandruff are a clinical surrogate for mites, thus eliminating the need to clinically count mites and/or epilate lashes (Median = 9; Range 8–9).
While initially there was no consensus surrounding which patients should be evaluated for DB, following the background reading and discussion at the live meeting, panelists came to agreement that, as part of the routine slit-lamp exam performed on every patient, clinicians should check for evidence of Demodex in the form of collarettes as they examine the lids and lashes (Median = 9; Range 8–9). The DEPTH panelists also agreed that the most effective way to visualize collarettes is to have the patient look down during the slit-lamp exam and look for collarettes at the upper eyelash base (Median = 9; Range 3–9).
Successful Treatment
A number of questions centered around the definition of success and what determines a “successfully treated patient.” In a series of yes/no questions, panelists agreed unanimously that a DB-treated patient experiencing no itching would be a treatment success (n = 12). Similarly, nearly all of the experts felt that minimal to no lid erythema (n = 11) and improved or no symptoms (n = 11) would be considered a success. In terms of collarettes, 10 panelists agreed that a patient with 0 to 2 collarettes would be successfully treated, and 8 felt that a decrease in collarettes would define treatment success. The role of mites was unclear from this series of questions, with no consensus about treatment success when presented with differing scenarios (patients with no mites, no more than 1 mite per lash, or no more than 10 total mites).
Luo et al found that a reduction of 1 mite per lash could be considered a DB treatment success.37 When queried about this paper provided as part of the pre-meeting reading materials, however, the DEPTH panelists did not obtain consensus on this point (Median = 4; Range 1–8). Following discussion at the face-to-face meeting, the group did, however, reach consensus about the following statement in the final survey: “The primary goal of treatment for DB is the elimination/significant decrease of collarettes, from which we extrapolate that mites would also be eliminated/significantly decreased, and from which we would expect other signs and symptoms to improve. In other words, collarettes may be the primary target of treatment and the way in which ‘treatment success’ is measured” (Median = 8, Range 7-9).
Cure
In the first questionnaire, an open-ended question was asked about whether or not DEPTH panelists felt that DB can be cured. Consensus was not reached, with 6 experts indicating that a cure is possible and 6 indicating that it is not. There was consensus, however, that, whether or not panelists felt DB can be cured, reinfestation or recurrence is likely (n = 10). Table 2 shows panelist responses when asked a series of true/false questions indicating under what circumstances they would consider a DB patient cured.
 |
Table 2 Clinical Findings That Could Indicate Demodex blepharitis Was Cured “A Cure for DB Would Require Resolution or Improvement of…” |
Demodex blepharitis cure was discussed at length during the live meeting. Some of the topics included: the definition of the word “cure”; how other medical specialties define “cure”; whether elimination of symptoms equates to a cure, or if it requires elimination of the root cause of DB; and whether there is a difference between clinical trials and clinical practice when thinking about a cure. During the discussion, panelists came to agree that, although there was initially lack of consensus about whether DB can be cured, a cure is possible. The non-consensus centered around differing opinions about the duration of mite eradication they would consider a “cure.” Ultimately, however, the DEPTH panelists unanimously agreed with the statement: “Demodex blepharitis can be cured, but there is always the potential for recurrence or reinfestation” (n = 12).
Goals of Treatment
The DEPTH panelists came to consensus that the primary objective when treating DB is the elimination or significant decrease of collarettes (Median = 7; Range 4–9). Similarly, when asked, “True or false: The single most important goal of treatment for Demodex blepharitis is elimination/significant reduction in collarettes”, there was consensus that this statement is true (n = 9). On the other hand, there was also consensus that mite eradication should not be the goal of treatment (n = 9). While it may seem like clinicians would think that eradicating mites would be of paramount importance, discussion at the live meeting shed light on the seeming contradiction. In this discussion, the panelists clarified that although mite elimination is important, most clinicians treating DB are using collarettes, not mites, as the clinical marker for treatment success.
During the video conference, the panelists discussed the concept of collarettes serving as a surrogate for mites, and the elimination of one necessarily means the elimination of the other. Therefore, by eliminating or dramatically reducing collarettes, the mite burden is also eliminated or reduced. There was also discussion about being able to monitor the resolution of DB by looking for collarettes rather than needing to epilate lashes to look for mites. This discussion reinforced the suggestion for clinicians to perform routine slit-lamp evaluations, in which checking for the presence of collarettes should be an essential step.
The final survey helped quantify the content of the live meeting discussion. The DEPTH panelists concurred that when diagnosing and treating Demodex blepharitis, collarettes are a reasonable surrogate for mites, thereby eliminating the need to count mites and/or epilate lashes (Median = 9; Range 3–9). In another question, the group reached consensus about the statement, “The primary goal when treating DB is the elimination or significant decrease of collarettes, from which it can be extrapolated that mites would also be eliminated or decreased, leading to improvement in other signs and symptoms” (Median = 8; Range 7-9). In other words, collarettes, and therefore mites, are the primary target of treatment in clinical practice and the way by which clinicians can monitor patient response to therapy.
Barriers to Treatment
The panel of experts reached consensus that the biggest barrier to the treatment of DB is its underdiagnosis (Median = 9; Range 7–9). And while there was no consensus on any single barrier, other challenges to treatment included patient intolerance of interventions, limited efficacy of management strategies, recurrence of disease, non-compliance with prescribed treatment regimens, and cost.
Educational Strategies
In one survey and again in the live meeting, the ways in which clinicians could educate patients about DB arose. Among possible educational analogies were: Demodex blepharitis is most like cancer that can be cured by going into remission but can return; most like head lice that can be cured, but reinfestation can occur; most like an infection that can be cured, but you can be reinfected; and most like allergies in which you can control symptoms but occasional “flare-ups” will always occur. While there was no consensus when these items were presented individually in a series of questions, a subsequent question brought the experts to consensus that Demodex blepharitis is similar to head lice in that it can be cured with treatment, but there is the potential for reinfestation. (Median = 8; Range 1–9) Areas of consensus across key scaled questions of all 3 surveys are summarized in Table 3.
 |
Table 3 Key Areas of Consensus on Scaled Questions |
Discussion
Blepharitis can often be a non-remitting, chronic, progressive disease process that is difficult to stabilize using currently available management options. Based on findings in the literature, it is likely that a significant proportion of blepharitis has a Demodex infestation component.5–7,16,43 The available OTC treatment options provide temporary DB symptom relief at best, but none address the root cause and some are not well tolerated. In the prior study by the DEPTH panel, consensus was reached across a range of topics pertaining to DB including diagnosis, and this second Delphi process brings to light specific aspects of treatment and management.34
The Delphi method has been proven a successful way to achieve consensus, historically using written surveys and more recently incorporating electronic surveys, as were used in this process. Studies have shown that online surveys have similar validity to written questionnaires.31,44 As with the first Delphi procedure undertaken by this group, this second process utilized 3 rounds of surveys, a number consistent with reports in the literature as sufficient to reach consensus.19,34,45 The inclusion of the live meeting, as in the current process, has also been found beneficial in reaching consensus by allowing panelists to engage in discourse with peers.42
The focus for this second DEPTH Delphi panel was the treatment and management of DB in a clinical practice setting, and a number of topical areas were addressed. Consensus was achieved early about existing OTC management options. Panelists largely agreed that while tea tree oil and terpinen-4-ol-containing products are probably the most effective of the currently available OTC options, there are drawbacks to their use and limitations to their efficacy. While more work needs to be done to assess this, recent evidence suggests that even low concentrations of terpinen-4-ol can be toxic to meibomian gland epithelial cells in culture.46 Hirsch-Hoffman et al assessed several therapies in DB patients, including TTO ointment and TTO foam. At the end of 2 months, mean mite count from 10 epilated lashes was assessed. The TTO ointment and foam patients had 13.3 and 12.0 mites per 10 lashes, respectively. This was similar to the oral ivermectin group (12.8 mites per 10 lashes), better than the oral metronidazole group (22 mites per 10 lashes), and worse than the metronidazole ointment group (9.8 mites per 10 lashes).47 Ultimately, the study concluded that none of the options were effective in eradicating mites in DB patients.
Another aspect of current treatment modalities was the inclusion of mechanical intervention in the form of lid scrubs or microblepharoexfoliation. By the third survey, the experts agreed that with no approved treatments currently available, there is still a place for lid hygiene, but when an FDA-approved therapy becomes available, lid scrubs may no longer be a necessary part of management of DB. A recent meta-analysis of current DB treatments also suggests that pharmacological interventions, including those which are TTO-derived, are more effective than non-pharmacological interventions; however, adverse reactions are more commonly observed in TTO-derived treatments.48
Panelists were asked about when they would initiate treatment in DB patients, and most of the questions centered around specific thresholds of collarettes in combination with signs and/or symptoms. Ultimately, this group of experts would treat patients with at least 10 collarettes, regardless of other signs or symptoms. If patients have 0 to 2 collarettes, DEPTH panelists would treat if patients also had symptoms. This demonstrates the importance of purposefully looking for collarettes on every patient and specifically considering a diagnosis of DB in patients who present with redness, itching, tearing, dry eye, and/or irritation along with the presence of collarettes.17,18
In the first Delphi process undertaken by the DEPTH panelists, consensus was reached that collarettes or cylindrical dandruff are pathognomonic for DB.34 The current treatment-focused Delphi method yielded additional consensus that collarettes are a surrogate for mites. This allows for a shift in thinking about how DB can be diagnosed based on clinical findings. With collarettes indicating the presence of mites, clinicians are able to move away from the practice of epilating lashes for light microscopy examination to detect mites and simply have patients look in downgaze during slit-lamp examination to assess for collarettes. Counting collarettes instead of mites is not only more comfortable for patients, but it is a more clinically efficient way to diagnose DB.
After diagnosis and treatment initiation, there must be a way to determine whether a therapy is working. The surveys contained several questions about characteristics or findings of a successfully treated DB patient. Ultimately, the DEPTH panelists agreed that collarettes are the key factor: if collarettes are significantly decreased or eliminated, there is high confidence that the mites that caused them are gone. With collarettes and mites eliminated, one would expect the symptoms to improve accordingly. Therefore, treatment efficacy can be tracked by the resolution of collarettes and symptoms.
In a concept related to successful treatment, much of the discussion during the live meeting surrounded the definition of “cure.” One of the articles provided as pre-reading to the panelists prior to the video conference described the recent Phase 2a study of 0.25% lotilaner for the treatment of DB. In it, Gonzalez-Salinas et al report results from a study called Io in which patients were treated with lotilaner drops twice daily for 42 days. The outcome measures were collarette elimination (≤2 lashes with collarettes) and mite eradication (0 mites). Of the 18 subjects in the study, 72.2% experienced elimination of collarettes, and 77.8% achieved eradication of mites by day 42.36 These findings taken together – eradication of mites and elimination of collarettes – were defined as a clinical cure of Demodex blepharitis, with this being the first paper to report such findings.
These findings were consistent with prior pilot studies, Mars and Jupiter, in which patients were treated twice daily for 28 days.49,50 Because the life cycle of a mite is about 3 weeks, it was thought that lengthening the duration of treatment from 4 to 6 weeks, or 2 full mite life cycles, could improve outcomes.10,51,52 Thus, the Io study was completed, demonstrating that the longer duration of treatment did, indeed, improve outcomes from mite eradication rates of 66.7% in Jupiter and 57.1% in Mars. This 6-week treatment strategy was also utilized in the Saturn-1 study.
Saturn-1 was a phase 2b/3 randomized, controlled, double-masked trial in which 421 patients received either lotilaner ophthalmic solution 0.25% or vehicle twice daily for 6 weeks. The primary efficacy endpoint, assessed on day 43, was the proportion of patients reaching complete collarette cure (0 to 2 collarettes on the upper eyelid). Additional endpoints as well as safety were also assessed.53
Collarette cure at day forty-three was seen in 44% (n = 92 of 209) of patients treated with lotilaner compared to 7% (n = 14 of 204) of the vehicle-treated patients. Mites were eradicated in 68% (n = 142 of 209) of study patients compared to 18% (n = 37 of 204) of vehicle patients. These comparisons were both statistically significant (P < 0.0001).50,53
Prior to the live meeting, the expert panel had not come to consensus on what characteristics would define a successfully treated patient or a cure. The DEPTH panelists had a robust discussion about cure vs control as well as other phrases such as “durable resolution”, “episodic cure”, “clinical resolution”, “temporary elimination”, “remission of disease”, and “clinically meaningful cure”. There was dialogue about whether a cure relates to resolution of symptoms or if the symptoms are more incidental and the true cure involves elimination of the root cause, in this case Demodex mites. The panelists also talked about what infectious disease specialists would consider a cure to be and how cure is defined in clinical trials for antibiotics and other anti-infectives.
Since the goal of the face-to-face meeting was not to gain additional consensus, the final survey had follow-up questions about the definition of cure, in part based on the discussion from the live meeting. The Gonzalez-Salinas paper defined “clinical cure” for DB as collarette elimination (<2 lashes with collarettes) and mite eradication (0 mites).36 The group came to consensus that they felt this definition is a reasonable definition for cure. Additionally, the DEPTH panelists came to consensus that DB is both a chronic and recurrent inflammatory condition of the eyelid caused by mites, and that the active infestation can be cured.
When looking critically at this study, some limitations emerge. The limited size of the DEPTH panel with 12 clinicians means that the findings here may not translate to all clinicians in varying geographies, different practice settings, and diverse patient populations. Similarly, the findings from this particular expert panel may not be repeatable with other experts in ocular surface disease. The survey process itself has the potential to introduce bias; however, steps were taken to minimize this potential. For example, the questions were randomized when presented to the panelists, and consensus was predefined in both scaled and closed-ended questions.
Conclusion
The DEPTH panel convened a second time with the aim of achieving consensus around aspects of treatment and management of patients with Demodex blepharitis in a clinical setting. Demodex blepharitis is a common condition and currently, there is no single therapeutic strategy for its management. The panelists concurred that some blepharitis maintenance therapies may provide temporary symptom relief but likely do not address the root cause of the disease. In the future, with a well-tolerated FDA-approved therapy, the experts believe that mechanical intervention such as lid scrubs may no longer be as necessary. The panel unanimously agreed that the key treatment goal for DB patients is the reduction or elimination of collarettes, with collarettes being a surrogate for Demodex mites. They also concurred that treatment success may be measured by the degree to which collarettes are eliminated or significantly decreased, indicating the elimination or decrease of Demodex mites. DEPTH panelists achieved consensus that, in order to assess patients for collarettes, a simple and effective technique that can easily be incorporated into the routine eye examination is as simple as having the patient look down during slit-lamp exam. Ultimately, the panel agreed, it is possible to cure Demodex blepharitis for some duration of time, although reinfestation is possible. The information from both phases of this Delphi panel can be used to increase awareness about Demodex blepharitis, leading to better care received by patients and better clinical outcomes.
Acknowledgments
The authors would like to thank the team at i2Vision for designing and administering this Delphi Panel and supporting the writing of this paper. The authors would also like to thank Tarsus Pharmaceuticals, Inc. for providing an unrestricted grant to fund this endeavor.
Disclosure
All of the authors serve as consultants to Tarsus Pharmaceuticals, Inc. Dr Brandon D Ayres is a consultant for Alcon, Tarsus, Bausch and Lomb, and Carl Zeiss Meditech; a consultant and speaker bureau for Allergan, outside the submitted work. Dr Eric Donnenfeld reports personal fees from Tarsus and Blephex, during the conduct of the study. Dr Ian Benjamin Gaddie reports personal fees from Tarsus, during the conduct of the study; personal fees from Bausch and Lomb, Orasis, and Ocusoft, outside the submitted work. Dr Preeya K Gupta reports personal fees from Tarsus, during the conduct of the study. Dr Richard Lindstrom reports personal fees from Tarsus and is also a less than 0.1% equity owner. Dr Paul M Karpecki reports personal fees from Tarsus Pharmaceuticals, Oasis Medical, Azura Pharmaceuticals, OcuSoft, Bruder Healthcare, BioTissue, Thea, B+L, and Alcon, outside the submitted work. Dr Kelly K Nichols reports personal fees from Tarsus, during the conduct of the study; personal fees from Allergan/AbbVie, Aerie, Alderya, Bruder, B+L, Dompé, HanAll Bio, Iveric, Kala Pharmaceuticals, Novartis/Shire, Osmotica/RVL Pharmaceuticals, Oyster Point Pharma, Inc., Palatin, SightSciences, Inc., Tarsus, Tear Film Innovations/Alcon/Acquiom, TearSolutions, Thea, Versea, Visionology, Xequel, and YuYu Pharmaceuticals; research grants from Aramis, Kowa, Sylentis, TearScience, and Science Based Health, outside the submitted work. Dr Christopher E Starr reports personal fees from i2Vision, during the conduct of the study. Dr Elizabeth Yeu is a consultant and participated in research for AcuFocus, BioTissue, New World Medical; consultant for and owns equity from Advanced Vision Group, BlephEx, Expert Opinion, Tarsus, Visus; speaker bureau and research for Alcon; consultant for Allergan, Bausch & Lomb/Valeant, BVI, Bruder, Dompe, EyePoint Pharmaceuticals, Glaukos, Guidepoint, Iveric, J & J Vision, Kala Pharmaceuticals, LENSAR, Merck, Novartis, Ocusoft, Omeros, Sight Sciences, STAAR, Surface, Thea, Zeiss; consultant for and owns equity from Aurion, Avellino, CorneaGen, LayerBio, Melt, Ocular Science; owns equity from Centricity, Equinox, Mati, Modernizing Medicine, Orasis, Science Based Health, outside the submitted work. Elizabeth Yeu, MD, is also on the Board of Directors of Tarsus Pharmaceuticals, Inc. The authors report no other conflicts of interest in this work.
References
1. Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7(2 Suppl):S1–S14. doi:10.1016/s1542-0124(12)70620-1
2. Amescua G, Akpek EK, Farid M, et al. Blepharitis preferred practice pattern®. Ophthalmology. 2019;126(1):P56–P93. doi:10.1016/j.ophtha.2018.10.019
3. Arrúa M, Samudio M, Fariña N, et al. Comparative study of the efficacy of different treatment options in patients with chronic blepharitis. Arch Soc Esp Oftalmol. 2015;90(3):112–118. doi:10.1016/j.oftal.2013.09.003
4. Lee SH, Chun YS, Kim JH, Kim ES, Kim JC. The relationship between demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51(6):2906–2911. doi:10.1167/iovs.09-4850
5. Biernat MM, Rusiecka-Ziółkowska J, Piątkowska E, Helemejko I, Biernat P, Gościniak G. Occurrence of Demodex species in patients with blepharitis and in healthy individuals: a 10-year observational study. Jpn J Ophthalmol. 2018;62(6):628–633. doi:10.1007/s10384-018-0624-3
6. Kabataş N, Doğan AŞ, Kabataş EU, Acar M, Biçer T, Gürdal C. The effect of demodex infestation on blepharitis and the ocular symptoms. Eye Contact Lens. 2017;43(1):64–67. doi:10.1097/ICL.0000000000000234
7. Wesolowska M, Knysz B, Reich A, et al. Prevalence of Demodex spp. in eyelash follicles in different populations. Arch Med Sci. 2014;10(2):319–324. doi:10.5114/aoms.2014.42585
8. Teo A, Rosenberg E, Jacobson A. Prevalence of demodex colonization in patients presenting to an outpatient clinic. Invest Ophthalmol Vis Sci. 2021;62(8):1236.
9. Sadri E, Yeu E, Trattler W, Holdbrook M, Baba S. The prevalence of collarettes and Demodex blepharitis in ophthalmology and optometry practices (Titan study).
10. Liu J, Sheha H, Tseng SCG. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010;10(5):505–510. doi:10.1097/ACI.0b013e32833df9f4
11. Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58(1):169–177. doi:10.2307/3278267
12. Nicholls SG, Oakley CL, Tan A, Vote BJ. Demodex species in human ocular disease: new clinicopathological aspects. Int Ophthalmol. 2017;37(1):303–312. doi:10.1007/s10792-016-0249-9
13. Kim JH, Chun YS, Kim JC. Clinical and immunological responses in ocular demodecosis. J Korean Med Sci. 2011;26(9):1231–1237. doi:10.3346/jkms.2011.26.9.1231
14. Zhang AC, Muntz A, Wang MTM, Craig JP, Downie LE. Ocular Demodex: a systematic review of the clinical literature. Ophthalm Physiol Opt. 2020;40(4):389–432. doi:10.1111/opo.12691
15. Coston TO. Demodex folliculorum blepharitis. Trans Am Ophthalmol Soc. 1967;65:361–392.
16. Trattler W, Karpecki P, Rapoport Y, et al. The prevalence of demodex blepharitis in US eye care clinic patients as determined by collarettes: a pathognomonic sign. Clin Ophthalmol. 2022;16:1153–1164. doi:10.2147/OPTH.S354692
17. Fromstein SR, Harthan JS, Patel J, Opitz DL. Demodex blepharitis: clinical perspectives. Clin Optom. 2018;10:57–63. doi:10.2147/OPTO.S142708
18. Rabensteiner DF, Aminfar H, Boldin I, et al. Demodex mite infestation and its associations with tear film and ocular surface parameters in patients with ocular discomfort. Am J Ophthalmol. 2019;204:7–12. doi:10.1016/j.ajo.2019.03.007
19. Dalkey NC. The Delphi method: an Experimental Study of Group Opinion. RAND Corporation; 1969. Available from: https://www.rand.org/pubs/research_memoranda/RM5888.html.
20. Santos MS, Alves MR, Freitas D, et al. Ocular allergy Latin American consensus. Arq Bras Oftalmol. 2011;74(6):452–456. doi:10.1590/s0004-27492011000600016
21. Ma Quintana J, Escobar A, Bilbao A. Explicit criteria for prioritization of cataract surgery. BMC Health Serv Res. 2006;6:24. doi:10.1186/1472-6963-6-24
22. Buchan JC, Dean WH, Foster A, Burton MJ. What are the priorities for improving cataract surgical outcomes in Africa? Results of a Delphi exercise. Int Ophthalmol. 2018;38(4):1409–1414. doi:10.1007/s10792-017-0599-y
23. Douglas RS, Tsirbas A, Gordon M, et al. Development of criteria for evaluating clinical response in thyroid eye disease using a modified Delphi technique. Arch Ophthalmol. 2009;127(9):1155–1160. doi:10.1001/archophthalmol.2009.232
24. Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844–851. doi:10.1016/j.ophtha.2012.10.036
25. Lee PP, Sultan MB, Grunden JW, Cioffi GA. Assessing the importance of IOP variables in glaucoma using a modified delphi process. J Glaucoma. 2010;19(5):281–287. doi:10.1097/IJG.0b013e3181b4ca8d
26. Lakhani BK, Giannouladis K, Leighton P, King AJ. Seeking a practical definition of stable glaucoma: a Delphi consensus survey of UK glaucoma consultants. Eye. 2020;34(2):335–343. doi:10.1038/s41433-019-0540-x
27. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
28. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25(8):900–907. doi:10.1097/01.ico.0000214802.40313.fa
29. Tallouzi MO, Mathers JM, Moore DJ, et al. COSUMO: study protocol for the development of a core outcome set for efficacy and effectiveness trials in posterior segment-involving uveitis. Trials. 2017;18(1):576. doi:10.1186/s13063-017-2294-8
30. Thompson DA, Ali RR, Banin E, et al. Advancing therapeutic strategies for inherited retinal degeneration: recommendations from the Monaciano Symposium. Invest Ophthalmol Vis Sci. 2015;56(2):918–931. doi:10.1167/iovs.14-16049
31. Sodi A, Banfi S, Testa F, et al. RPE65-associated inherited retinal diseases: consensus recommendations for eligibility to gene therapy. Orphanet J Rare Dis. 2021;16(1):257. doi:10.1186/s13023-021-01868-4
32. Dana R, Farid M, Gupta PK, et al. Expert consensus on the identification, diagnosis, and treatment of neurotrophic keratopathy. BMC Ophthalmol. 2021;21(1):327. doi:10.1186/s12886-021-02092-1
33. Gomes JAP, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359–369. doi:10.1097/ICO.0000000000000408
34. Ayres BD, Donnenfeld E, Farid M, et al. Clinical diagnosis and management of demodex blepharitis: the demodex expert panel of treatment and eyelid health (DEPTH). Eye. In Press 2023.
35. Shah PP, Stein RL, Perry HD. Update on the management of demodex blepharitis. Cornea. 2022;41(8):934–939. doi:10.1097/ICO.0000000000002911
36. Gonzalez-Salinas R, Yeu E, Holdbrook M, et al. Collarette elimination and demodex mite eradication with topical lotilaner ophthalmic solution, 0.25. J Ocul Pharmacol Ther. 2021;37(8):479–484. doi:10.1089/jop.2021.0011
37. Luo KS, Xie A, Yang JJ, Shen EP. Critical value of Demodex count per lash for symptomatic and clinical improvement of Demodex blepharitis. Eye. 2022;36(3):663–665. doi:10.1038/s41433-021-01442-z
38. Eubank BH, Mohtadi NG, Lafave MR, et al. Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Med Res Methodol. 2016;16:56. doi:10.1186/s12874-016-0165-8
39. Niederberger M, Spranger J. Delphi technique in health sciences: a map. Front Public Health. 2020;8:457. doi:10.3389/fpubh.2020.00457
40. Fletcher AJ, Marchildon GP. Using the delphi method for qualitative, participatory action research in health leadership. Int J Qual Methods. 2014;13(1):1–8. doi:10.1177/160940691401300101
41. Hsu CC, Sandford B. The delphi technique: making sense of consensus. Pract Assess Res Eval. 2019;12(1). doi:10.7275/pdz9-th90
42. Khodyakov D, Chen C. Response changes in Delphi processes: why is it important to provide high-quality feedback to Delphi participants? J Clin Epidemiol. 2020;125:160–161. doi:10.1016/j.jclinepi.2020.04.029
43. Gonzalez-Salinas R, Yeu E, Holdbrook M, et al. Safety and efficacy of topical lotilaner ophthalmic solution 0.25% for the treatment of demodex blepharitis: a pilot study. J Ophthalmol. 2021;2021:3862684. doi:10.1155/2021/3862684
44. Eberhardt M, Rammohan G. Blepharitis. StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459305/.
45. Kongsved SM, Basnov M, Holm-Christensen K, Hjollund NH. Response rate and completeness of questionnaires: a randomized study of Internet versus paper-and-pencil versions. J Med Internet Res. 2007;9(3):e25. doi:10.2196/jmir.9.3.e25
46. Brown B, Cochran SW, Dalkey NC. The DELPHI method, II: structure of experiments. RAND Corporation; 1969. Available from: https://www.rand.org/pubs/research_memoranda/RM5957.html.
47. Chen D, Wang J, Sullivan DA, Kam WR, Liu Y. Effects of terpinen-4-ol on meibomian gland epithelial cells in vitro. Cornea. 2020;39(12):1541–1546. doi:10.1097/ICO.0000000000002506
48. Hirsch-Hoffmann S, Kaufmann C, Bänninger PB, Thiel MA. Treatment options for demodex blepharitis: patient choice and efficacy. Klin Monbl Augenheilkd. 2015;232(4):384–387. doi:10.1055/s-0035-1545780
49. Martínez-Pulgarín DF, Ávila MY, Rodríguez-Morales AJ. Interventions for Demodex blepharitis and their effectiveness: a systematic review and meta-analysis. Cont Lens Anterior Eye. 2021;44(6):101453. doi:10.1016/j.clae.2021.101453
50. Gonzalez-Salinas R, Karpecki P, Yeu E, et al. Safety and efficacy of lotilaner ophthalmic solution, 0.25% for the treatment of blepharitis due to demodex infestation: a randomized, controlled, double-masked clinical trial. Cont Lens Anterior Eye. 2022;45(4):101492. doi:10.1016/j.clae.2021.101492
51. Cheng AMS, Sheha H, Tseng SCG. Recent advances on ocular Demodex infestation. Curr Opin Ophthalmol. 2015;26(4):295–300. doi:10.1097/ICU.0000000000000168
52. Rather PA, Hassan I. Human demodex mite: the versatile mite of dermatological importance. Indian J Dermatol. 2014;59(1):60–66. doi:10.4103/0019-5154.123498
53. Yeu E, Wirta DL, Karpecki P, Baba SN, Holdbrook M; Saturn I Study Group. Lotilaner ophthalmic solution, 0.25%, for the treatment of demodex blepharitis: results of a prospective, randomized, vehicle-controlled, double-masked, pivotal trial (Saturn-1). Cornea. 2022. doi:10.1097/ICO.0000000000003097
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
