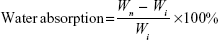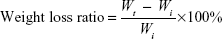Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Degradability, bioactivity, and osteogenesis of biocomposite scaffolds of lithium-containing mesoporous bioglass and mPEG-PLGA-b-PLL copolymer
Authors Cai Y, Guo L, Shen H, An X, Jiang H, Ji F, Niu Y, Qiu C
Received 15 February 2015
Accepted for publication 6 April 2015
Published 24 June 2015 Volume 2015:10(1) Pages 4125—4136
DOI https://doi.org/10.2147/IJN.S82945
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Lei Yang
Yanrong Cai,1 Lieping Guo,2 Hongxing Shen,2 Xiaofei An,2 Hong Jiang,3 Fang Ji,2 Yunfei Niu2
1The College of Basic Science of Medicine, Hunan University of Traditional Chinese Medicine, Changsha, 2Department of Orthopaedics, Changhai Hospital, Second Military Medical University, 3School of Materials Science and Engineering, University of Shanghai for Science and Technology, Shanghai, People’s Republic of China
Abstract: Biocomposite scaffolds of lithium (Li)-containing mesoporous bioglass and monomethoxy poly(ethylene glycol)-poly(D,L-lactide-co-glycolide)-poly(L-lysine) (mPEG-PLGA-b-PLL) copolymer were fabricated in this study. The results showed that the water absorption and degradability of Li-containing mesoporous bioglass/mPEG-PLGA-b-PLL composite (l-MBPC) scaffolds were obviously higher than Li-containing bioglass/mPEG-PLGA-b-PLL composite (l-BPC) scaffolds. Moreover, the apatite-formation ability of l-MBPC scaffolds was markedly enhanced as compared with l-BPC scaffolds, indicating that l-MBPC scaffolds containing mesoporous bioglass exhibited good bioactivity. The cell experimental results showed that cell attachment, proliferation, and alkaline phosphatase activity of MC3T3-E1 cells on l-MBPC scaffolds were remarkably improved as compared to l-BPC scaffolds. In animal experiments, the histological elevation results revealed that l-MBPC scaffolds significantly promoted new bone formation, indicating good osteogenesis. l-MBPC scaffolds with improved properties would be an excellent candidate for bone tissue repair.
Keywords: MBG, copolymer, degradability, bioactivity, osteogenesis, cell proliferation, bone repair
Introduction
Some biodegradable polymers have been applied as biomedical materials for tissue repair and drug delivery, such as polylactide, poly(D,L-lactide-co-glycolide) (PLGA), polycaprolactone, and monomethoxy poly(ethylene glycol)-poly(D,L-lactide-co-glycolide) (mPEG-PLGA).1–4 Recently, an amphiphilic block copolymer of monomethoxy (polyethylene glycol)-poly(D,L-lactide-co-glycolide)-poly(L-lysine) (mPEG-PLGA-b-PLL) was synthesized,5,6 and the studies indicated that mPEG-PLGA-b-PLL with good biocompatibility and degradability would be a promising and effective carrier for the delivery of drugs and genes.7,8
In the past few years, the applications of mesoporous bioglasses (MBGs) for bone tissue regeneration have been proposed, because their large surface area and high pore volume may enhance their bioactivity and promote new bone formation.9,10 The large surface area and high pore volume of MBG result in higher surface chemical reactivity compared with normal bioglasses (BGs) without mesopores.11,12 Lithium (Li) has been widely used as a long-term mood stabilizer in the treatment of bipolar and depressive disorders for 50 years.13 Li+ ions can stimulate the osteogenic differentiation of bone mesenchymal stem cells (BMSCs) through activation of the canonical Wnt signaling pathway in vitro.14 Previous studies suggested that Li+ ions can protect cartilage by increasing the level of autophagy, and Li+ ions might biologically fulfill the disparate requirements for bone and cartilage regeneration in osteochondral defects.15 Recently, Li-containing mesoporous bioglass (Li-MBG) scaffold has been developed for promoting regeneration of osteochondral defects, which was investigated with both in vitro cell culture and in vivo animal-based osteoarthritis models.16
Inorganic bioactive materials, such as hydroxyapatite, tricalcium phosphate, and bioglass, have been incorporated into polymers, such as poly(lactide-co-glycolide), polyamide, chitosan, and collagen, to develop biocomposites that could improve the hydrophilicity and bioactivity of the polymer-based composites.17,18 Therefore, in this study, Li-MBG as the bioactive filler was incorporated into biodegradable copolymer of mPEG-PLGA-b-PLL to prepare composite (Li-containing mesoporous bioglass/mPEG-PLGA-b-PLL composite [l-MBPC]) scaffolds, and the properties of degradability, bioactivity, cell responses in vitro, and osteogenesis in vivo of the scaffolds were investigated.
Materials and methods
Preparation of Li-MBG
Li-MBGs were prepared by incorporating Li (molar: 5%) into mesoporous BG using sol–gel method.15,16 The nonionic block polymer Pluronic P-123 (EO20PO70EO20, 5800) was used as co-templates to produce mesoporous structures.19 The prepared Li-MBG has the chemical composition of Li, Ca, P, and Si with molar ratio of Li:Ca:P:Si at 5:10:5:80. All the chemicals were purchased from Sigma-Aldrich Co., St Louis, MO, USA. The mesoporous structure of Li-MBG was observed using transmission electron microscope (JEM-2010; JEOL, Tokyo, Japan). The Li-containing bioglass (Li-BG; as a control) with the same composition was also prepared using the same method without addition of P-123.
Preparation of mPEG-PLGA-b-PLL block copolymer
The mPEG-PLGA-PLL copolymer (Mn =26,000) was synthesized19 according to the following steps: 1) synthesis of the hydroxyl-terminated mPEG-PLGA through ring-opening polymerization of D,L-lactide and glycolide, which was initiated by mPEG and catalyzed by Sn(OCt)2; 2) synthesis of Boc-L-Phe end-capped mPEG-PLGA through the hydroxyl end group of the mPEG-PLGA copolymer converted to Boc-l-Phe; 3) synthesis of amino-terminated mPEG-PLGA through the removal of the t-butoxycarbonyl end group from the Boc-l-Phe end-capped mPEG-PLGA; 4) synthesis of mPEG-PLGA-poly(Nε-(Z)-L-lysine) through ring-opening polymerization of the initiated N-carboxyanhydrides by the amino-terminated mPEG-PLGA; and 5) synthesis of amino-terminated mPEG-PLGA-PLL through the removal of the Nε-(carbonylbenzoxy) end group of the mPEG-PLGA-poly(Nε-(Z)-L-lysine) block copolymer.7,8
Preparation of l-MBPC scaffolds
l-MBPC scaffolds with Li-MBG content of 35 wt% were prepared by solvent casting and particulate leaching method. The mPEG-PLGA-b-PLL was added into the solvent (chloroform), followed by continuous stirring to disperse uniformly. The Li-MBG powders and sodium chloride particulates (used as the porogens), with the size of around 400 μm, were added into the copolymer solution and stirred for 1 hour. The mixture was then cast into the molds (Φ10×2 mm and Φ6×6 mm). The obtained samples were dried at 50°C for over 24 hours to remove the solvent. Then, the samples were soaked into water for 48 hours to leach out the particulates, and then, the samples were dried at 50°C for 24 hours to obtain l-MBPC scaffolds. The Li-containing bioglass/mPEG-PLGA-b-PLL composite (l-BPC) scaffolds (as a control) with BG content of 35 wt% were prepared by the same method.
Water absorption of l-MBPC scaffolds
Water absorption of l-MBPC scaffolds (l-BPC scaffolds used as controls) was evaluated by measuring the weight changes after the samples were soaked into water for different time points. The samples (Φ10×2 mm) were weighed and then immersed into water for 1 hour, 2 hours, 3 hours, 6 hours, 12 hours, and 24 hours. At the predetermined time points, the specimens were removed from water and the weights of the samples were measured. The water absorption ratio of the samples was determined by the following equation:
|
|
where Wi is the initial weight and Wn is the weight of the samples at time n.
Degradation of l-MBPC scaffolds
The degradation of l-MBPC scaffolds (l-BPC scaffolds used as controls) was evaluated by measuring the weight loss ratio of the samples soaked in phosphate-buffered saline (PBS). The samples (Φ10×2 mm) were weighed and then immersed into PBS using a shaking water bath at 37°C for 1 week, 2 weeks, 4 weeks, 6 weeks, 8 weeks, 10 weeks, and 12 weeks. At different time points, the specimens were washed thoroughly with deionized water, followed by drying at 50°C for 24 hours. The weight loss ratio of the samples was determined by the following equation:
|
|
where Wi is the initial weight and Wt is the weight of samples soaking for time t.
Bioactivity of l-MBPC scaffolds
The bioactivity of l-MBPC scaffolds (l-BPC scaffolds used as controls) was determined by observing apatite formation on the specimen surfaces after soaking in simulated body fluid (SBF) at 37°C for different time points. The samples (Φ10×2 mm) were placed in the bottles filled with 80 mL SBF using a shaking water bath at 37°C. At 1 day, 2 days, 3 days, 5 days, and 7 days, the specimens were washed with deionized water, followed by drying at 37°C for 24 hours. The surface morphology and composition of the samples were characterized by scanning electron microscope (SEM; S-3400N; Hitachi Ltd., Tokyo, Japan) and energy-dispersive X-ray spectroscopy (EDS; Falcon, New York, USA), respectively. The changes in the ion concentrations (Si, Ca, and P ions) in SBF after soaking the samples at different time points were determined by inductively coupled plasma atomic emission spectroscopy (IRIS 1000; Thermo Elemental, Massachusetts, USA).
Cell culture
MC3T3-E1 cells (ATCC; Chinese Academy of Sciences, Shanghai, People’s Republic of China) were cultured with Dulbecco’s Modified Eagle’s Medium supplemented with glutamine (Thermo Fisher Scientific, Waltham, MA, USA), containing 10% (v/v) fetal calf serum (Sijiqing, Hangzhou, People’s Republic of China) and 1% (v/v) antibiotics in the 100% standard humidified atmosphere with 5% CO2 at 37°C. The culture medium was replaced every 2 days. The trypsin and EDTA solution (Thermo Fisher Scientific, 0.5 g/L and 0.2 g/L, respectively) were used to harvest cells before the cells reached confluence. The cells were resuspended in fresh culture medium before seeding on specimens and tissue culture plate (TCP) as control, and the cell density was calculated for later experiments. The samples of l-MBPC and l-BPC scaffolds (Φ10×2 mm) were sonicated in ethanol and sterilized in autoclave at 100°C for 30 minutes.
Cell attachment
The MC3T3-E1 cells (2×105 cells/50 mL) were seeded on the specimens (Φ10×2 mm) located into 24-well TCPs, and the TCP was used as a control. The cell samples were maintained at 37°C under 5% CO2 condition for 4 hours, and then, the culture medium was removed. Then, the residual cultured medium and unattached cells were removed by washing with PBS three times. After the attached cells on the samples were digested by trypsin, the adherent cells were counted with a hemacytometer, and the cell attachment efficiency was determined by counting the number of cells remaining in the wells.
Cell proliferation and morphology
MC3T3-E1 cells (2×105 cells/50 mL) were seeded on the specimens in 24-well plates, and the cell proliferation was determined using a Cell Counting Kit-8 at 1 day, 4 days, and 7 days. At the specific time point, the specimens were gently rinsed three times with PBS. Standard culture media with and without cells were used as positive control and blank control for cell viability, respectively. All samples were tested in triplicate, and the results were expressed in mean absorbance values (OD), which were obtained from a microplate reader (Synergy HT; Bio-Tek, Vermont, USA) at 450 nm.
The cell morphology of MC3T3-E1 cells was examined by visualizing the filamentous actin of the cytoskeleton using a confocal laser scanning microscopy (Leica TCS SP2; Leica Microsystems, Wetzlar, Germany). The samples were then put into 24 wells, and the cells were seeded on the samples at a density of 2.0×104 cells/well. After incubation for 4 days, the specimens were washed gently with PBS to remove the unattached cells. According to the protocol, the cells on specimens were fixed with 2.5% glutaraldehyde for 15 minutes at the room temperature and permeabilized with 0.1% Triton X-100 in PBS for another 15 minutes. After washing three times with PBS, the cells were stained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich Co.) and fluorescein isothiocyanate (Sigma-Aldrich Co.).
Alkaline phosphatase activity
MC3T3-E1 cells (2×104 cells/50 mL) were seeded on the specimens, which were placed in 24-well plates, and then cultured at 37°C and 100% humidity atmosphere with 5% CO2. The alkaline phosphatase (ALP) activity of the cells was measured with the ALP assay in osteogenic medium at 7 days and 10 days. At the specific time point, the cell lysates were obtained by adding 1 mL of 0.2% Nonidet P-40 solution to each well at room temperature for 1 hour. Then, 50 μL of 1 mg/mL p-nitrophenylphosphate (Sigma-Aldrich Co.) substrate solution (pH =9), which contains 0.1 mol/L glycine and 0.5 mmol/L MgCl2 in 1 M diethanolamine buffer, was added to each well and incubated at 37°C for 15 minutes. The reaction was stopped by 100 μL of 0.1 M sodium hydroxide. Then, OD value was quantified with a microplate reader (SpectraMax 384; Molecular Devices LLC, Sunnyvale, CA, USA) at the wavelength of 405 nm. The ALP activity was expressed as OD value per total protein. The total protein content was determined using bicinchoninic acid protein assay kits and a series of bovine serum albumin standards. The ALP activity of cell lysates was normalized to the total protein content of each lysate.
Implantation in vivo
Animal surgical procedures
This study was approved by the Research Center for Laboratory Animal of Shanghai University of Traditional Chinese Medicine. The New Zealand White rabbits with an average weight of 2 kg and age of 5 months were used. The 18 rabbits were randomly divided into three groups and anesthetized by intramuscular injection of Zoletil 50 (25 mg/kg body weight) and 2% Rompon (0.15 mL/kg body weight), and the surgery was carried out under sterile condition. Lateral and medial approaches were performed in left shaved knees to expose the distal femoral epiphysis, and the defects were created with a medium speed burr. After the defects were washed with physiological saline, the specimens (Φ6×6 mm) were implanted. The wounds of the rabbits were sutured, and prophylactic antibiotic was given continuously for 3 days.
Histological elevation
The rabbits were sacrificed successively by an overdose of pentobarbital at the time points of 1 month, 2 months, and 3 months to obtain animal experimental samples. After fixation with 4% neutral buffered formalin for 48 hours, the animal experimental samples (extracted femora) were decalcified in 12.5% EDTA, dehydrated in a graded series of alcohol, and embedded in paraffin. Serial cross-sections with 5 mm thickness were made using a microtome. After hematoxylin and eosin (H&E) staining, each section was observed under light microscope, and at least five images were randomly obtained in the same section. Using image analytical software Image-Pro Plus (Media Cybernetics, Florida, USA), new bone area was expressed as the percentage of newly formed bone area within the bone defect area.
Statistical analysis
All data were analyzed with Origin 8.0 (OriginLab Corporation, Northampton, USA) and expressed as the mean ± standard deviation (M ± SD). Statistical comparisons were carried out using one-way analysis of variance with post hoc test.
Results
Characterization of Li-MBG and composite scaffolds
Figure 1 shows the transmission electron microscope images of the morphology and the microstructure of Li-MBG. It is found that Li-MBG had highly ordered mesoporous channel and uniform pore size of about 5 nm.
  | Figure 1 TEM images of Li-MBG. |
Figure 2 presents the SEM images of the surface morphology of l-MBPC and l-BPC scaffolds. It is found that no obvious difference was found for both l-MBPC and l-BPC scaffolds. In high magnification of the images, some BG particles were found on the surface of l-BPC scaffolds, while no obvious Li-MBG particles were found on l-MBPC scaffolds.
Water absorption of l-MBPC scaffolds
Figure 3 shows the water absorption of l-MBPC and l-BPC scaffolds after immersing into water for 1 hour, 2 hours, 3 hours, 6 hours, 12 hours, and 24 hours. It is found that the water absorption ratio of both the l-MBPC and l-BPC scaffolds increased with time, and the water absorption ratio of l-MBPC scaffolds was 235%, while that of l-BPC scaffolds was 172% after immersing into water for 24 hours. The results revealed that the water absorption ratio of l-MBPC scaffolds was obviously higher than that of l-BPC scaffolds.
Degradation of l-MBPC scaffolds
Figure 4 shows the weight loss ratio of l-MBPC and l-BPC scaffolds after soaking into PBS. It is found that the weight loss of both the samples increased with time, and the weight loss of l-MBPC scaffolds was approximately 67 wt% after immersing in PBS for 12 weeks, which was significantly higher than that of l-BPC scaffolds, which was 48 wt%.
Bioactivity of l-MBPC scaffolds
Figure 5 shows the SEM images of the surface morphology of l-MBPC and l-BPC scaffolds after soaking in SBF for 7 days. It is found that both l-MBPC and l-BPC scaffold surfaces were covered by many spherical-shaped apatites, indicating that both l-MBPC and l-BPC scaffolds could induce apatite formation on their surfaces. For l-MBPC scaffolds, numerous agglomerates of elongated particles appeared, while the precipitation on l-BPC scaffolds was less evident than that on l-MBPC scaffolds, revealing that l-MBPC scaffolds could induce more apatite formation on their surface than l-BPC scaffolds.
Figure 6 shows the EDS of the surfaces of l-MBPC and l-BPC scaffolds after soaking into SBF for 7 days. It is found that both Ca and P peaks were detected, and the Ca/P mole ratios of l-MBPC and l-BPC scaffolds were 1.61 and 1.53, respectively. The results indicated that the Ca/P ratio of the formed apatite on l-MBPC was higher than that on l-BPC scaffolds. The EDS results were consistent with SEM. Figure 7 shows the changes in ion concentration of Si, Ca, and P in solution with time after l-MBPC and l-BPC scaffolds were soaked into SBF. It is found that the Si ion concentrations in SBF for l-MBPC continued to increase for both the scaffolds at a slower rate up to 7 days. In addition, the Ca ion concentrations in SBF for l-MBPC scaffolds exhibited faster increase than l-BPC scaffolds within the first 3 days, and then, decreased gradually up to 7 days. The P ion concentrations in SBF for both l-MBPC and l-BPC scaffolds showed slow decrease from 1 day to 7 days. More importantly, the decrease in Ca and P ion concentrations for l-MBPC was obviously faster than l-BPC scaffolds.
In vitro cytocompatibility
Cell attachment
The results of cell attachment efficiency are profiled in Figure 8. After cultured for 4 hours, it is found that the cell attachment percentage on l-MBPC (138%) scaffolds was significantly higher than that on l-BPC scaffolds (113%) and TCP (100%). The results suggested that l-MBPC scaffolds containing Li-MBG facilitated cell adhesion.
Cell proliferation and cell morphology
Figure 9 shows the proliferation of MC3T3-E1 cells on l-MBPC and l-BPC scaffolds at different time points. It is found that the OD values for both l-MBPC and l-BPC scaffolds increased with time, indicating good cytocompatibility. At 1 day and 4 days, there was no obvious difference of OD value for both l-MBPC and l-BPC scaffolds. However, the OD value for l-MBPC scaffolds was significantly higher than l-BPC scaffolds at 7 days. The results indicated that l-MBPC scaffolds significantly promoted the MC3T3-E1 cell proliferation than l-BPC scaffolds.
Figure 10 shows the cytoskeletal morphology of the MC3T3-E1 cells cultured on both l-MBPC and l-BPC scaffolds. It is found that the MC3T3-E1 cells grew into both l-MBPC and l-BPC scaffolds, and the number of cells in l-MBPC scaffolds was significantly higher than that in l-BPC scaffolds at 4 days. The results indicated that l-MBPC scaffolds obviously enhanced MC3T3-E1 cells viability as compared with l-BPC scaffolds.
ALP activity
Figure 11 shows the ALP activity of MC3T3-E1 cells cultivated on both l-MBPC and l-BPC scaffolds at 7 days and 10 days. It is found that the ALP activity was expressed at low levels at 7 days, and no significant differences were detected for both l-MBPC and l-BPC scaffolds. However, the ALP activity of the cells on l-MBPC scaffolds was significantly higher than that on l-BPC scaffolds at 10 days. The results revealed that l-MBPC scaffolds could promote cell differentiation as compared with l-BPC scaffolds.
Histological elevation after scaffolds implanted in vivo
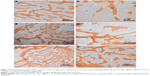
Figure 12 shows the photos of histological sections (H&E staining) for the l-MBPC and l-BPC scaffolds implantation in vivo for 1 month, 2 months, and 3 months, respectively. At 1 month, a few new bone tissues were found to grow into l-MBPC and l-BPC scaffolds. At 2 months, new bone tissue gradually increased, while the materials reduced accordingly for both l-MBPC and l-BPC scaffolds. In addition, the new bone tissue formation in l-MBPC scaffolds was significantly higher than that in l-BPC scaffolds. At 3 months, a large number of new bone tissues formed, and bone marrow was found in l-MBPC scaffolds, which was surrounded by new bone tissues composed of the mature bone tissues. Meanwhile, some new bone tissues formed but no mature bone tissue was found in l-BPC scaffolds. Obviously, new bone tissues in l-BPC scaffolds were significantly less than those in l-MBPC scaffolds.
Figure 13 shows the quantitative analysis of the new bone area by histological sections. The results showed that the new bone area gradually increased with time for both l-MBPC and l-BPC scaffolds, and the new bone area for l-MBPC scaffolds was markedly higher than that for l-BPC scaffolds at both 2 months and 3 months.
Discussions
MBG had superior bone-forming activities, but it is difficult to use MBG as scaffolds for bone tissue repair because of its low mechanical properties and brittleness.20 Biocomposites containing BG and polymers have attracted increasing attention as promising biomaterials for bone tissue regeneration.21,22 In this study, Li-MBG was incorporated into copolymer of mPEG-PLGA-b-PLL to form biocomposite scaffolds by solvent casting and particulate leaching method.
Generally, the degradation rate of the scaffolds should correlate with the growth of new bone tissue and gradually replaced by the new bone tissue in vivo.23 In this study, it was found that the weight loss ratio of l-MBPC scaffolds was 67 wt% after immersing into PBS for 12 weeks, which was significantly higher than that of l-BPC scaffolds, which was 48 wt%. The results showed that the degradation rate of l-MBPC scaffolds was faster than that of l-BPC scaffolds, indicating that addition of Li-MBG into mPEG-PLGA-b-PLL obviously improved the degradability of the composite scaffolds. It could be suggested that Li-MBG with large surface area and high pore volume leads to more water being absorbed into the composite scaffolds, which resulted in an increased degradation of l-MBPC scaffolds as compared with l-BPC scaffolds.
The in vitro bioactivity of the biomaterials can be determined by testing the apatite formation on their surfaces in SBF, which could predict the in vivo bone bioactivity of the biomaterials.24 In this study, both l-MBPC and l-BPC scaffolds could induce apatite formation on their surface, and the ability of apatite formation on l-MBPC scaffolds was faster than that on l-BPC scaffolds after soaking into SBF for 7 days, indicating that incorporation of Li-MBG into mPEG-PLGA-b-PLL significantly enhanced the apatite-formation ability of l-MBPC scaffolds as compared with l-BPC scaffolds. The results suggested that Li-MBG with large specific surface area and high pore volume in l-MBPC scaffolds might greatly accelerate the deposition of apatite, and therefore, enhance the bioactivity of l-MBPC scaffolds as compared with l-BPC scaffolds containing Li-BG.
The dissolution of BG from both l-MBPC and l-BPC scaffolds initially increased the local Si, Ca, and P ion concentrations, which resulted in apatite precipitation. The apatite formed on both l-MBPC and l-BPC scaffold surfaces, which subsequently caused the decrease in the Ca and P ion concentrations of SBF. According to the mechanism of apatite formation on BG,25,26 it can be drawn that the processes of apatite precipitation on l-MBPC scaffolds largely depended on the ion deposition rate and dissolution velocity on the specimen surfaces. Furthermore, Li-MBG with large surface area and high pore volume lead to a faster release rate of Si, Ca, and P ions from l-MBPC than l-BPC scaffolds, which might improve the apatite-formation ability of l-MBPC scaffolds as compared with l-BPC scaffolds.
Cell attachment and proliferation are obviously influenced by the surface characteristics of the biomaterials, such as surface morphology and surface chemistry.26 In this study, the adhesion of MC3T3-E1 cells on l-MBPC scaffolds was significantly higher than that on l-BPC scaffolds, and the proliferation of MC3T3-E1 cells on l-MBPC and l-BPC scaffolds increased with time, indicating good cytocompatibility. l-MBPC scaffolds are not highly degrading materials (the weight loss was 4 wt% at 1 week). Therefore, the degradation of l-MBPC scaffolds did not affect cell attachment and proliferation on its surface. Moreover, the proliferation of the cells on l-MBPC scaffolds was obviously higher than that on l-BPC scaffolds at 7 days. The results suggested that Li-MBG with large surface and high pore volume on l-MBPC scaffolds might provide more chances for the interaction between MC3T3-E1 cells and composite surfaces, and thus, enhanced cell adhesion and proliferation.
The cytoskeletal morphology of the MC3T3-E1 cells cultured on both l-MBPC and l-BPC scaffolds was visualized using a confocal laser scanning microscopy. It was found that the MC3T3-E1 cells could attach and grow into l-MBPC and l-BPC scaffolds. Moreover, the number of cells in l-MBPC scaffolds was significantly higher than that in l-BPC scaffolds at 4 days, suggesting that l-MBPC scaffolds containing Li-MBG obviously promoted cell adhesion and growth and proliferation as compared to l-BPC scaffolds containing Li-BG. The ALP of MC3T3-E1 cells on the biomaterials could reflect the influence of the material on cells ability to form new bone both in vitro and in vivo.27 In this study, the ALP activity of the MC3T3-E1 cells on l-MBPC scaffolds was significantly higher than that on l-BPC scaffolds at 10 days, revealing that l-MBPC scaffolds containing Li-MBG obviously promoted cell differentiation as compared to l-BPC scaffolds containing Li-BG.
Some studies have shown that ionic products (Si and Ca) dissolution from bioglass could stimulate osteoblast proliferation.28 A study reported that sol–gel BG had significant osteogenic effects by releasing a higher level of Si, and the stimulatory effects of increased extracellular calcium on osteoblast-like cells has also been demonstrated.29 In this study, it was found that Si and Ca ions were released continuously from both l-MBPC and l-BPC scaffolds into SBF with time, and the release rate of Si and Ca ions from l-MBPC scaffolds was significantly faster than that from l-BPC scaffolds. In addition, the results showed that MC3T3-E1 cells proliferated and differentiated more quickly after being cultured on l-MBPC scaffolds than on l-BPC scaffolds. Therefore, it can be suggested that the continuous dissolution of the composite scaffolds produced a microenvironment with rich Si and Ca ions that might be responsible for stimulating cell responses, such as growth, proliferation, and differentiation, and a fast release of Si and Ca ions from l-MBPC scaffolds, which obviously promoted the cell responses as compared with l-BPC scaffolds.
Ideal biomaterials not only interact actively with cells but also stimulate tissue regeneration and repair bone defects.30 The transition from newly formed bone to a mature one is a gradual process where there is a dynamic interaction between scaffolds and the invasion of bone cells/tissues.31–33 In this study, it was found that new bone tissues gradually increased and the materials continued to reduce for both l-MBPC and l-BPC scaffolds after implanting at different time points. At 3 months, a large quantity of new bone tissues formed in l-MBPC scaffolds, which was significantly higher than in l-BPC scaffolds.
As compared with l-BPC scaffolds containing Li-BG, it is likely that Li-MBG with large surface area and high pore volume in l-MBPC scaffolds provided a suitable microenvironment for the interaction between cell/tissue and implants, and thus, enhanced cell/tissue growth into the scaffolds in vivo. Moreover, the fast release of Si and Ca ions from the dissolution of l-MBPC scaffolds produced a microenvironment, which might be responsible for stimulating cell growth/new tissue formation in the scaffolds in vivo. Furthermore, Li-MBG with large surface area and high pore volume in l-MBPC scaffolds might promote degradation of scaffolds in vivo, which might enhance tissue regenerations. It can be suggested that l-MBPC scaffolds as the bioactive implants might have special biofunctions to stimulate tissue regeneration, which would be an excellent candidate for bone tissue engineering and orthopedic applications.
Conclusion
Bioactive scaffolds of l-MBPC were fabricated by the addition of Li-MBG into mPEG-PLGA-b-PLL copolymer. The results showed that incorporation of Li-MBG into mPEG-PLGA-b-PLL significantly improved water absorption and degradability of l-MBPC scaffolds as compared with l-BPC scaffolds. Moreover, l-MBPC scaffolds exhibited good bioactivity, and the apatite formation on their surfaces was markedly higher than that on l-BPC scaffolds. Cell culture and histological elevation results indicated that l-MBPC scaffolds not only improved cell functions (attachment, proliferation, and differentiation) but also promoted new bone tissue regeneration, which were obviously better than l-BPC scaffolds. The results suggested that the incorporation of Li-MBG into mPEG-PLGA-b-PLL was a useful approach to obtain biocomposite scaffolds with improved properties.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No 31271031) and the National Natural Science Fund of Shanghai (No 13ZR1427900) and Shanghai Education Development Foundation (No Slgl4050).
Disclosure
The authors report no conflicts of interest in this work.
References
Xiao RZ, Zeng ZW, Zhou GL, Wang JJ, Li FZ, Wang AM. Recent advances in PEG-PLA block copolymer nanoparticles. Int J Nanomedicine. 2010;5:1057–1065. | ||
Szlek J, Paclawski A, Lau R, Jachowicz R, Mendyk A. Heuristic modeling of macromolecule release from PLGA microspheres. Int J Nanomedicine. 2013;8:4601–4611. | ||
Feng RL, Song ZM, Zhai GX. Preparation and in vivo pharmacokinetics of curcumin-loaded PCL-PEG-PCL triblock copolymeric nanoparticles. Int J Nanomedicine. 2012;7:4089–4098. | ||
Kim MS, Seo KS, Hyun H, Kim SK, Khang G, Lee HB. Sustained release of bovine serum albumin using implantable wafers prepared by MPEG-PLGA diblock copolymers. Int J Pharm. 2005;304(1):165–177. | ||
Rangari D, Vasanthan N. Study of strain-induced crystallization and enzymatic degradation of drawn poly(L-lactic acid) (MPEG-PLGA-B-PLL) films. Macromolecules. 2012;45(18):7397–7403. | ||
Mattioli S, Kenny JM, Armentano I. MPEG-PLGA-b-PLLsma surface modification of porous MPEG-PLGA-B-PLL films: analysis of surface properties and in vitro hydrolytic degradation. J Appl Polym Sci. 2012;125(S2):239–247. | ||
Liu PF, Qin LB, Wang Q, et al. cRGD-functionalized mPEG-PLGA-PLL nanoparticles for imaging and therapy of breast cancer. Biomaterials. 2012;3(28):6739–6747. | ||
Liu PF, Sun YM, Wang Q, Sun Y, Li H, Duan YR. Intracellular trafficking and cellular uptake mechanism of mPEG-PLGA-PLL and mPEG-PLGA-PLL-Gal nanoparticles for targeted delivery to hepatomas. Biomaterials. 2014;3(2):760–770. | ||
Wu CT, Miron R, Sculean A, et al. Proliferation, differentiation and gene expression of osteoblasts in boron-containing associated with dexamethasone deliver from mesoporous bioactive glass scaffolds. Biomaterials. 2011;32(29):7068–7078. | ||
Zhu YF, Li XL, Yang JH, Wang SL, Gao H, Hanagata N. Composition-structure-property relationships of the CaO-MxOy-SiO2-P2O5 (M=Zr, Mg, Sr) mesoporous bioactive glass (MBG) scaffolds. J Mater Chem. 2011;21(25):9208–9218. | ||
Cicuendez M, Portoles MT, Izquierdo-Barba I, Vallet-Rego M. New nanocomposite system with nanocrystalline apatite embedded into mesoporous bioactive glass. Chem Mater. 2012;24(6):1100–1106. | ||
Wu XH, Wei J, Lu X, Lv Y. Chemical characteristics and hemostatic performances of ordered mesoporous calcium-doped silica xerogels. Biomed Mater. 2010;5(3):035006. | ||
Clement-Lacroix P, Ai MR, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406–17411. | ||
Day TF, Guo XZ, Garrett-Beal L, Yang YZ. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005; 8(5):739–750. | ||
Han PP, Wu CT, Chang J, Xiao Y. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/beta-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials. 2012;33(27):6370–6379. | ||
Wu Y, Zhu SA, Wu CT, et al. A bi-lineage conducive scaffold for osteochondral defect regeneration. Adv Funct Mater. 2014;24:4473–4483. | ||
Lacroix J, Jallot E, Lao J. Gelatin-bioactive glass composites scaffolds with controlled macroporosity. Chem Eng J. 2014;256(1):9–13. | ||
Serra T, Planell JA, Navarro M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013;9(3):5521–5530. | ||
Shen YH, Liu WC, Lin KL, et al. pH effects on solid/liquid interface the critical factor to stimulate osteoporotic bone regeneration. Langmuir. 2011;27(6):2701–2708. | ||
Liu PF, Yu H, Sun Y, Zhu MJ, Duan YR. A mPEG-PLGA-b-PLL copolymer carrier for adriamycin and siRNA delivery. Biomaterials. 2012;33(17):4403–4412. | ||
Wu XH, Ye L, Liu K, et al. Antibacterial properties of mesoporous copper-doped silica xerogels. Biomed Mater. 2009;4(4):045008. | ||
Yao QQ, Nooeaid P, Detsch R, et al. Bioactive glass (R)/chitosan-polycaprolactone bilayered composite scaffolds intended for osteochondral tissue engineering. J Biomed Mater Res A. 2014;102(2):4510–4518. | ||
Zhou PY, Cheng XS, Xia Y, et al. Organic/inorganic composite membranes based on poly(L-lactic-co-glycolic acid) and mesoporous silica for effective bone tissue engineering. ACS Appl Mater Interfaces. 2014;6(23):20895–20903. | ||
Fu Q, Saiz E, Rahaman MN, Tomsia AP. Bioactive glass scaffolds for bone tissue engineering: state of the art and future perspectives. Mater Sci Eng C Mater Biol Appl. 2011;31(7):1245–1256. | ||
Shruti S, Salinas AJ, Lusvardi G, Malavasi G, Menabue L, Vallet-Regi M. Mesoporous bioactive scaffolds prepared with cerium-, gallium- and zinc-containing glasses. Acta Biomater. 2013;9(1):4836–4844. | ||
Hoppe A, Guldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–2774. | ||
Wu X, Wang S. Regulating MC3T3-E1 cells on deformable poly(ε-caprolactone) honeycomb films prepared using a surfactant-free breath figure method in a water-miscible solvent. ACS Appl Mater Interfaces. 2012;4(9):4966–4975. | ||
Wu XH, Wang SF. Integration of photo-crosslinking and breath figures to fabricate biodegradable polymer substrates with tunable pores that regulate cellular behavior. Polymer. 2014;55(7):1756–1762. | ||
Zhu M, Zhang LX, He QJ, Zhao JJ, Guo LM, Shi JL. Mesoporous bioactive glass-coated poly(L-lactic acid) scaffolds: a sustained antibiotic drug release system for bone repairing. J Mater Chem A. 2011;21(4):1064–1072. | ||
Zhao S, Li YB, Li DX. Synthesis of CaO-SiO2-P2O5 mesoporous bioactive glasses with high P2O5 content by evaporation induced self assembly process. J Mater Sci Mater Med. 2011;22(2):201–208. | ||
Gunawidjaja PN, Izquierdo-Barba I, Mathew R, et al. Quantifying apatite formation and cation leaching from mesoporous bioactive glasses in vitro: a SEM, solid-state NMR and powder XRD study. J Mater Chem. 2012;22(15):7214–7223. | ||
Luo YX, Wu CT, Lode A, Gelinsky M. Hierarchical mesoporous bioactive glass/alginate composite scaffolds fabricated by three-dimensional plotting for bone tissue engineering. Biofabrication. 2013;5(1):234–239. | ||
Wu X, Wang S. Biomimetic calcium carbonate concentric microgrooves with tunable widths for promoting MC3T3-E1 cell functions. Adv Healthc Mater. 2013;2(2):326–333. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.