Back to Journals » Cancer Management and Research » Volume 11
Definitive radiotherapy or chemoradiotherapy for distal rectal cancer with early stage of cT1-2N0
Authors Peng HH, Liao ZW, Lin XD, Qiu XS, You KY
Received 13 December 2018
Accepted for publication 30 April 2019
Published 10 June 2019 Volume 2019:11 Pages 5221—5229
DOI https://doi.org/10.2147/CMAR.S198113
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Hai-Hua Peng,1,* Zhi-Wei Liao,1,* Xiao-Dan Lin,1 Xing-Sheng Qiu,2 Kai-Yun You2
1Department of Radiation Oncology, Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou 510075, People’s Republic of China; 2Department of Radiation Oncology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510120, People’s Republic of China
*These authors contributed equally to this work
Objectives: Patients with early-stage distal rectal cancer, if treated with radical surgery, usually suffer a poor quality of life. Definitive radiotherapy or chemoradiotherapy may be another treatment option for them. The aim of this study was to evaluate the role of definitive external beam radiotherapy or chemoradiotherapy in treating distal rectal cancer with stage cT1-2N0.
Methods: We performed a retrospective study of 231 distal rectal cancer patients who were staged as cT1-2N0 from March 2002 to March 2015. All patients were treated by definitive radiotherapy or chemoradiotherapy. Overall survival (OS), progression-free survival (PFS), and short-term efficacy were analyzed. Multivariate analysis was performed to explore clinical factors significantly associated with PFS, local recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS) for the whole group.
Results: For the whole group, 135 patients (58.4%) achieved clinical complete response (cCR). The 5-year OS, PFS, and LRFS were 86.19%, 83.30%, and 92.50%, respectively. Patients with cCR acquired better survival than those with non-cCR. In multivariable analysis, it revealed that clinical stage, carcinoembryonic antigen (CEA level) and concurrent chemotherapy were independent predictors of PFS.
Conclusion: Definitive radiotherapy or chemoradiotherapy may be feasible in some early-stage distal rectal cancer regarding its favorable efficacy.
Keywords: rectal cancer, definitive radiotherapy, chemotherapy, early stage
Introduction
Although surgery serves an extremely important role in the clinical management of rectal cancer, radiotherapy as adjuvant or neo-adjuvant treatment is becoming increasingly common in the comprehensive treatment of locally advanced rectal cancer.1–4 In early-stage rectal cancer, even though surgery remains the optimal treatment method, a small group of patients who are not suitable for surgical resection persist. For example, they may be medically unfit for surgery due to a comorbidity or some of these patients may be reluctant to undergo colostomy for tumors located in the lower rectum.5
Definitive radiotherapy for rectal cancer has been previously reported.6–15 However, patients enrolled in those studies were usually unselected, and the details of the clinical tumor stage were unclear. The reported 5-year overall survival (OS) for patients undergoing definitive external beam radiation therapy in most of these studies was <50%,9,14,15 but it is still unclear if patients with early-stage cancer achieved better clinical outcomes than the average OS reported for total patients. As we have described, some patients with low rectal tumors demand a sphincter-sparing treatment course. Whether there is a subset of early-stage, low rectal cancers that could be treated by radiotherapy remains unknown. It would be quite a value to patients to identify a new treatment method that would avoid restorative anastomosis for early-stage, low rectal cancer patients. Toward this aim, our current study evaluates the effectiveness of definite external beam radiation therapy in treating early-stage, distal rectal cancer and explores clinical factors that predict clinical outcome.
Materials and methods
Ethics statement
This research was approved by the Ethics Committee of Affiliated Cancer Hospital & Institute of Guangzhou Medical University. The written informed consent was obtained from every patient included in the study. This study was conducted in accordance with the Declaration of Helsinki.
Patients
We underwent a retrospective review of rectal cancer patients who underwent treatment at the Affiliated Cancer Hospital & Institute of Guangzhou Medical University from March 2002 to March 2015. The selection criteria in this study were as follows: 1) patients with rectal adenocarcinoma located in the distal rectum (tumors located <7 cm from the anal verge) with a clinical stage of cT1-2N0M0, 2) patients who underwent definitive radiotherapy or chemoradiotherapy, 3) no evidence of distant metastases during the treatment, 4) no concurrent malignancy or prior history of radiotherapy to the pelvis. After carefully reviewing the available medical records, 231 cases were included. All patients refused surgery or were medically unfit for surgery.
Evaluation
Before treatment, patients underwent staging workup that included chest radiography, abdominal ultrasound and CT with the aim of ruling out possible metastatic disease. Sigmoidoscopy or colonoscopy was performed to accurately measure the distance from the tumor to the anal verge. Endorectal ultrasound was performed on patients to accurately determine the T stage. Pelvic computed tomography (CT) or magnetic resonance imaging (MRI) was also administered to patients to detect possible lymph node metastasis in the pelvis. Other examinations, such as serum CEA, complete blood count and liver function tests, were also performed.
Treatment
The employed radiotherapy technique was based on a three-dimensional conformal radiotherapy treatment planning system or intensity-modulated radiation therapy (IMRT). In contouring the relevant radiation area on the planning computer, the principles were as follows: the clinical target volume (CTV) included primary rectal tumor, perirectal tissues, pre-sacral lymph nodes, internal iliac lymph nodes and obturator lymph nodes. The superior border of the CTV was the bottom of the L5, and the inferior border was 2–3 cm distal to the tumor. The anterior border was the posterior margin of the bladder or uterus, and the posterior border was the anterior margin of the sacrum. The planning target volume (PTV) was typically defined as the CTV plus 5 mm (P-CTV).
Definitive radiotherapy consisted of two phases. In the first phase, the prescription dose was administered to the whole pelvis (P-CTV). The most frequently prescribed schedule was 46 Gy in 23 fractions over 4 weeks. Some patients were administered 50 Gy in 25 fractions over 5 weeks. For those who received IMRT, the prescription does was typically as follows: P-GTV: 50 Gy in 25 fractions, P-CTV: 46 Gy in 25 fractions. After the first phase, an additional 10–18 Gy divided in 5–9 fractions was boosted into the primary tumor in a second phase. The selection of the second phase radiation dose was based on the tumor response to (chemo)radiotherapy during the first phase and the physician’s preference. Furthermore, the prescription dose for each patient was tailored with the consideration of limited dose for the small intestine and colon. One hundred twenty patients received concurrent chemotherapy. The selection of concurrent chemotherapy was according to the advice given by the physician, who usually made the treatment decision based on the tumor stage, age, tumor grade, performance status and patient’s consent. The regimens for concurrent chemotherapy were FOLFOX6 (oxaliplatin 85 mg/m2 D1+ leucovorin 400 mg/m2 D1+5-FU 400 mg/m2 IV D1, then 2,400 mg/m2 CIV 46–48 hrs, 2 weeks per cycle), XELOX (oxaliplatin 130 mg/m2 D1+ capecitabine 1,000 mg/m2 BID PO D1–14, 3 weeks per cycle) and single agent of Xeloda (capecitabine 1,250 mg/m2 BID PO D1–14, 3 weeks per cycle). The median cycle of concurrent chemotherapy was 3 (2–4).
Assessment of treatment toxicity
In the present study, the treatment toxicity was graded by using Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. Any grade ≥3 toxicity was defined as severe adverse events. And the details of adverse events for each patient were recorded in our database.
Follow-up
The first evaluation was at 3 months after the completion of radiotherapy; subsequent follow-ups occurred every 2 months for the first 2 years and every 6 months thereafter. Important evaluations included complete blood count, biochemical routine, CEA and physical examination during each visit. Chest radiography, CT or MRI scanning of the abdomen and pelvis and colonoscopy were conducted every 6 months. The follow-up for each patient was clearly recorded in the database.
Study endpoints
The endpoints for our study was short-term response of the primary tumor or local control rate. The secondary endpoints were progression-free survival (PFS), OS, local recurrence-free survival (LRFS) and distant metastasis-free survival (DMFS). Treatment-related toxicities were graded according to the Radiation Therapy Oncology Group (RTOG).
Statistical analysis
All statistical analyses were performed using SPSS software, version 19.0. Categorical variables were analyzed using the Chi-square test or Fisher’s exact test. Continuous variables were analyzed with the Student’s t-test or the Mann–Whitney U test. Univariate and multivariate analyses of clinical complete response (cCR) for the whole group was performed using binary logistic regression. And in the multivariate analyses of cCR, all the possible clinical factors were included. The Kaplan–Meier method was used to compare PFS and OS rates. Multivariate analysis of PFS, LRFS and DMFS was performed with Cox proportional hazards regression, and the Cox-proportional hazards model was performed using a forward conditional selection of variables. p<0.05 was considered to be statistically significant.
Results
Clinical characteristic
There were 231 patients who met the criteria and were included in this study. Of them, there were 129 males and 102 females. The median level of pretreatment hemoglobin was 129 g/L and the median age was 56 years old. There were 118 patients who presented with elevated CEA levels, while the levels of CEA in the other 113 patients were normal. Well, moderately and poorly differentiated tumors accounted for 8.2% (19 patients), 74.5% (172 patients) and 17.3% (40 patients), respectively. The clinical stage of T1 and T2 tumors was found in 75 (32.5%) patients and 156 (67.5%) patients, respectively. There were 107 patients who received a prescription dose at least 60 Gy, and the other 124 patients received a dose less than 60 Gy. Concurrent chemotherapy was administered in 120 patients and the regimens of chemotherapy consisted of FOLFOX6, XELOX and Xeloda (Table 1).
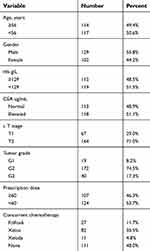 | Table 1 Patient demographics, baseline tumor characteristics |
Short-term efficacy
The short-term response was evaluated 3 months after the completion of definitive radiotherapy. cCR was defined as no gross tumor on digital rectal examination, endoscopy or pelvic MRI. Biopsy was not routinely performed after (chemo)radiotherapy. For the whole group, 135 patients (58.4%) achieved cCR, 84 patients achieved partial response (PR) and 12 patients showed no response to definite radiotherapy or chemoradiotherapy. No patients had tumor progression. Further subgroup analysis based on clinical T stage was performed. In patients with T1N0, the rates of CR, PR and SD were 76.1%, 23.9% and 0%, respectively. However, for patients with T2N0, only 51.2% of patients achieved CR, with PR in 41.5% and SD in 7.3% (Table 2). In multivariate analysis, we found that clinical T stage, concurrent chemotherapy and radiation dose were independent predictors of cCR rate. Other variables, such as gender, Hb, CEA and tumor grade, were not significantly associated with cCR (Table 3).
 | Table 2 Short-term efficacy |
 | Table 3 Univariate and multivariate analyses of cCR for the whole group |
Survival analysis
During long-term follow-up, 31 patients died, and the 3-year and 5-year OS were 93.90% and 86.19%, respectively, for all patients (Figure 1). There were 35 patients who had tumor progression. Among them, local progression occurred in 10 patients, distant failure occurred in 19 patients and 6 patients developed both local and distant failure. The 3-year and 5-year PFS were 90.03% and 83.30%, respectively (Figure 2). Furthermore, patients who achieved cCR had improved survival over those without cCR (Table 4, Figures 3 and 4). Multivariate analysis revealed that clinical stage, CEA level and concurrent chemotherapy were independent predictors of PFS (Table 5).
 | Table 4 Long-term survival |
 | Table 5 Univariate and multivariate analysis of PFS for the whole group |
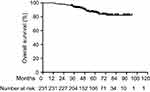 | Figure 1 Overall survival (OS) for the whole group. |
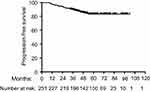 | Figure 2 Progression-free survival (PFS) for the whole group. |
 | Figure 3 The comparison of overall survival (OS) between clinical complete response (cCR) and non-cCR patients. Patients with cCR acquired better OS than those with non cCR (p=0.007). |
Local and regional control
During follow-up, there were 16 patients who recurred or progressed locally. Of them, 6 patients had internal iliac lymph node recurrence, and the other 10 patients recurred or progressed at the primary tumor site. The 5-year LRFS was 92.50%. Multivariate analysis was performed to further explore the clinical factors that predict LRFS. We found that age, gender, Hb, tumor grade and clinical stage were not significant predictors of LRFS (Table 6). However, radiation dose, CEA and concurrent chemotherapy were significantly correlated with LRFS. Patients who received prescription dose more than 60 Gy or underwent concurrent chemotherapy achieved better local control than those who did not. Additionally, patients with a normal level of CEA also showed improved local control than those with elevated CEA.
 | Table 6 Univariate and multivariate analysis of LRFS for the whole group |
Distant failure
In all, 31 patients suffered distant failure. Among them, 12 patients developed only liver metastasis and 10 patients developed only lung metastasis. There were 3 patients who developed both liver and lung metastasis. Bone metastasis was observed in 4 patients, and 2 patients’ tumors metastasized to the paraaortic lymph nodes. The 5-year DMFS was 87.40%. Using multivariate analysis, clinical stage and CEA were identified as significant prognostic factors associated with DMFS. There was no significant association between distant relapse and other factors, such as age, gender, Hb, tumor grade or prescription dose (Table 7).
 | Table 7 Univariate and multivariate analysis of DMFS the whole group |
Toxicity of treatment
During the treatment, the most common acute toxicity types were diarrhea, neutropenia and radiodermatitis. Besides, the major types of severe adverse events (grade ≥3) observed were also diarrhea (17.3%), neutropenia (5.6%) and radiodermatitis (5.2%). As for the late toxicity, anal stenosis was found in 55 patients and 6 patients were graded as Grade 3. Anal ulcer occurred in 47 patients and 2 patients were with bad condition (Grade 4). Additionally, 52 patients were with chronic anal/rectal bleeding with different degrees (Table 8). All the patients with severe adverse events were managed with further drug therapy or surgery with the aim to eliminate or alleviate the uncomfortable symptoms caused by the treatment. No patents died of severe adverse events.
 | Table 8 Treatment toxicity |
Discussion
Our current study was focused on early-stage rectal cancer treated by definitive radiotherapy or chemoradiotherapy. The goal of this study was to explore the efficacy of definitive radiotherapy in early-stage rectal cancer and to determine the clinical factors associated with outcome. Our data showed favorable short-term response to definitive radiotherapy with an overall cCR rate of 58.4% and a 76.1% 3-month cCR rate in patients whose cancer was stage T1. Previous studies reported cCR rates varying between 29% and 56%.6–9,14 The possible reason for the difference between our cCR rate and that of prior studies is that patients with more advanced-stage tumors were enrolled in prior studies. In contrast, the patients included in our study were all diagnosed with early-stage tumors. We further performed multivariate analyses, which revealed that clinical T stage, concurrent chemotherapy and radiation dose were each independent predictors of cCR. A published report showed that tumor fixation was the only significant prognostic factor for cCR.9 The cCR rates for mobile, partially fixed and fixed tumors were 49%, 22%, and 9%, respectively. Actually, tumor fixation usually indicates T stage, with a fixed tumor often presenting with an advanced T stage, thus leading to a poorer cCR rate. We also found that concurrent chemotherapy or higher radiation dose corresponded with a higher cCR rate, which was not reported in other studies.9,14,15 Perhaps more studies are needed to address this discrepancy.
The use of definitive external beam radiotherapy for rectal cancer has been previously reported. In the study by Brierley et al, 229 rectal cancer patients were treated by radical external radiation therapy, with prescription doses ranging from 40 Gy in 10 fractions administered as a split course over 6 weeks to 60 Gy in 30 fractions over 6 weeks. The overall 5-year actuarial survival rate was only 27%, with local recurrence found to be the main cause of treatment failure. Age and tumor fixation were significant predictors of survival, indicating that patients with older age or fixed tumors achieved a shorter survival.15 However, risk factors associated with local control and distant metastasis were not analyzed. A subsequent study performed by Wang et al further enrolled more patients at the same institution. Their study showed that tumor fixation and annular tumor were poor prognostic factors for local relapse in patients who achieved initial complete response. Additionally, patients with high pre-treatment CEA levels and unresectable tumors were more likely to suffer distant failures.9 However, there were some flaws concerning these two studies. First, the two studies did not present clear clinical stage evaluations before treatment. Although the OS was poor for the whole group, the OS for the subgroup with early stage may have been favorable. Furthermore, the main factor analyzed in their studies was tumor fixation, which is difficult to accurately evaluate as it is a subjective measure obtained during physical examination. For unselected rectal cancer patients treated by definitive radiation, although the 5-year OS is still poor for the whole group, chemo-radiation or radiotherapy alone do offer safe alternatives with acceptable efficacy in patients who are considered medically inoperable or refuse to undergo surgery.6
Our data showed that the 5-year OS and PFS for early-stage rectal cancer treated by definitive radiotherapy were 86.19% and 83.30%, respectively. Multivariate analysis showed that clinical stage and CEA level were independent predictors of PFS, which is consistent with previous studies.9 Further analysis showed that a higher radiation dose resulted in better local control, which was not observed in other studies.9,14,15 The local control rate was quite favorable in our study, which may be due to the early stage of the included patients, high radiation dose and high percentage of cCR. We also found that patients who achieved cCR attained better outcomes than those without cCR. A similar conclusion was also reported by Habr-Gama et al16. In their study, 265 patients with resectable tumors of the distal rectum were treated with chemoradiation with concurrent 5-fluorouracil-based chemotherapy. Seventy-one patients (27%) achieved cCR. Conversely, 22 patients (8%) were scored as having an incomplete clinical response and underwent resection with a final pathologic stage of ypT0N0 (pCR). When comparing the cCR group to the pCR group, the 5-year OS was significantly higher in the cCR group (100% vs 88%; p=0.01), and the 5-year disease-free survival was similar at 92% versus 83% (p=0.09), respectively. This study was further updated in 2006 with similar findings.17 Due to these favorable results, a wait-and-see policy was suggested for cCR patients when surgery was considered to be necessary after chemoradiation.18,19
Our results showed that some patients achieved cCR after definitive radiotherapy or chemoradiotherapy, while some patients only achieved PR or SD. Specific molecular mechanisms must regulate the tumor response to chemoradiotherapy. For example, Astakhova et al found that chymotrypsin-like activity of proteasomes (CTLA) was threefold higher in untreated rectal cancer patients compared to normal tissue. However, expression of total proteasomes and immunoproteasomes was 1.4–3.3 times reduced in patients who received neoadjuvant chemoradiation therapy compared to untreated patients. The authors hypothesized that CTLA in rectal carcinoma may be regulating the expression of immune subunits. Chemoradiation therapy suppresses the expression of immune subunits in the tumor and thereby downregulates proteasomal CTLA. Thus, the expression of immune subunits and proteasomal CTLA may serve as potential markers to predict the effectiveness of neoadjuvant chemoradiation in patients with rectal cancer.20 In another study performed by Rau et al, results showed that patients with decreased p21 expression following chemoradiotherapy achieved better disease-free survival (p=0.03) and patients with increased proliferative activity, as measured by increased post-therapy Ki-67 expression, also had better disease-free survival (p<0.005). They concluded that dynamic induction of p21/WAF1/CIP1 was associated with a lower proliferative activity but an ultimately worse treatment outcome following neoadjuvant chemoradiotherapy, and the induction of p21 represented a novel resistance mechanism in rectal cancer undergoing preoperative chemoradiotherapy.21
In clinical practice, organ preservation is important for maintaining quality of life. Radical resection for distal rectal cancer may result in a permanent stoma or coloanal anastomosis with associated bowel dysfunction. Along with this, sexual and urinary dysfunction, difficulties with wound-healing, infection and anastomotic leaks are also common in patients who receive surgery.5 To the best of our knowledge, this current study is the first large-scale study to explore the efficacy of definitive radiotherapy or chemoradiation for distal rectal cancer, and we have demonstrated that definitive radiation for early-stage rectal cancer is feasible with favorable OS and organ preservation. With respect to treatment toxicity, the incidence of severe adverse events (grade ≥3) was found to be higher in our study compared to that reported by Wang et al.9 Possible explanations for this difference include the higher radiation dose given and the concurrent chemotherapy used in our study. However, all the patients tolerated the treatment toxicity with or without supportive medication. Of course, our study is not without limitations. First, it is a retrospective analysis. Second, some eligible patients were lost to follow-up, which may have created a selection bias.
In conclusion, definitive radiotherapy or chemoradiotherapy is feasible for early-stage distal rectal cancer. However, further, randomized controlled clinical trials are in need to more directly assess this treatment approach.
Ethics approval and consent to participate
This research was approved by the Ethics Committee of Affiliated Cancer Hospital & Institute of Guangzhou Medical University, and the written informed consent was obtained from patients included in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saurer RB, Becker H, Hohenberger W. Preoperative versus postoperative chemo-radiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi:10.1056/NEJMoa040694
2. Folkesson JB, Birgisson H, Pahlman L. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–5650. doi:10.1200/JCO.2005.08.144
3. Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi:10.1056/NEJMoa060829
4. Gérard J, Conroy T, Bonnetain F, et al. Preoperative radiatiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi:10.1200/JCO.2006.06.7629
5. Higgins KA, Willett CG, Czito BG. Nonoperative management of rectal cancer: current perspectives. Clin Colorectal Cancer. 2010;9(2):83–88. doi:10.3816/CCC.2010.n.011
6. Lim L, Chao M, Shapiro J, et al. Long-term outcomes of patients with localized rectal cancer treated with chemoradiation or radiotherapy alone because of medical inoperability or patient refusal. Dis Colon Rectum. 2007;50:2032–2039. doi:10.1007/s10350-007-9062-x
7. Rades D, Kuhn H, Schultze J, et al. Prognostic factors affecting locally recurrent rectal cancer and clinical signifi cance of hemoglobin. Int J Radiat Oncol Biol Phys. 2008;70:1087–1093. doi:10.1016/j.ijrobp.2007.07.2364
8. Taylor RE, Kerr GR, Arnott SJ. External beam radiotherapy for rectal adenocarcinoma. Br J Surg. 1987;74:455–459. doi:10.1002/bjs.1800740606
9. Wang Y, Cummings B, Catton P, et al. Primary radical external beam radiotherapy of rectal adenocarcinoma: long term outcome of 271 patients. Radiother Oncol. 2005;77:126–132. doi:10.1016/j.radonc.2005.09.001
10. Overgaard M, Bertelsen K, Dalmark M, et al. A randomized feasibility study evaluating the effect of radiotherapy alone or combined with 5-fluorouracil in the treatment of locally recurrent or inoperable colorectal carcinoma. Acta Oncol. 1993;32:547–553. doi:10.3109/02841869309096116
11. Wong CS, Cummings BJ, Keane TJ, et al. Results of external beam irradiation for rectal carcinomas locally recurrent after local excision or electrocoagulation. Radiother Oncol. 1991;22:145–148. doi:10.1016/0167-8140(91)90012-6
12. Lybeert ML, Martijn H, de Neve W, et al. Radiotherapy for locoregional relapses of rectal carcinoma after initial radical surgery: definite but limited infl uence on relapse-free survival and survival. Int J Radiat Oncol Biol Phys. 1992;24:241–246.
13. Frykholm GJ, Påhlman L, Glimelius B. Treatment of local recurrences of rectal carcinoma. Radiother Oncol. 1995;34:185–194. doi:10.1016/0167-8140(95)01519-M
14. Sprawka A, Pietrzak L, Garmol D, et al. Definitive radical external beam radiotherapy for rectal cancer: evaluation of local effectiveness and risk of late small bowel damage. Acta Oncol. 2013;52(4):816–823. doi:10.3109/0284186X.2012.707786
15. Brierley JD, Cummings BJ, Wong CS, et al. Adenocarcinoma of the rectum treated by radical external radiation therapy. Int J Radiat Oncol Biol Phys. 1995;31(2):255–259.
16. Habr-Gama AP, Perez RO, Nadalin W. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy. Ann Surg. 2004;240:711–718.
17. Habr-Gama A. Assessment and management of the complete clinical response of rectal cancer to chemoradiotherapy. Colorectal Dis. 2006;8(suppl 3):21–24. doi:10.1111/j.1463-1318.2006.01066.x
18. Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cance. J Clin Oncol. 2011;29(35):4633–4640. doi:10.1200/JCO.2011.37.7176
19. Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919–927. doi:10.1016/S1470-2045(15)00120-5
20. Astakhova TM, Ivanova EV, Rodoman GV, et al. Effect of neoadjuvant chemoradiation therapy on proteasome pool in rectal cancer. Bull Exp Biol Med. 2017;164(2):191–194. doi:10.1007/s10517-017-3955-z
21. Rau B, Sturm I, Lage H, et al. expression profile of p21WAF1/CIP1 and Ki-67 predicts survival in rectal carcinoma treated with preoperative radiochemotherapy. J Clin Oncol. 2003;21(18):3391–3401. doi:10.1200/JCO.2003.07.077
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

