Back to Journals » Infection and Drug Resistance » Volume 16
Decreased Sphingosine Due to Down-Regulation of Acid Ceramidase Expression in Airway of Bronchiectasis Patients: A Potential Contributor to Pseudomonas aeruginosa Infection
Authors Qi Q , Xu J , Wang Y , Zhang J , Gao M, Li Y , Dong L
Received 17 February 2023
Accepted for publication 21 April 2023
Published 28 April 2023 Volume 2023:16 Pages 2573—2588
DOI https://doi.org/10.2147/IDR.S407335
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Qian Qi,1,2,* Jiawei Xu,1,2,* Yujiao Wang,3,* Jian Zhang,1 Mingxia Gao,1 Yu Li,4 Liang Dong1,2
1Department of Respiratory, the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong Institute of Respiratory Diseases, Jinan, Shandong Province, People’s Republic of China; 2Department of Respiratory, Shandong Provincial Qianfoshan Hospital, Shandong University, Jinan, Shandong Province, People’s Republic of China; 3Department of Clinical Laboratory Medicine, the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Jinan, Shandong Province, People’s Republic of China; 4Department of Respiratory, Qilu Hospital, Shandong University, Jinan, Shandong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liang Dong, Department of Respiratory, the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, #16766, Jingshi Road, Jinan, Shandong Province, 250014, People’s Republic of China, Tel +86 13505401207, Email [email protected]
Purpose: To assess the metabolites associated with Pseudomonas aeruginosa infection by analyzing the microbial diversity and metabolomics in lower respiratory tract of bronchiectasis patients and to explore the therapeutic approaches for Pseudomonas aeruginosa infection.
Methods: Bronchoalveolar lavage fluid samples from bronchiectasis patients and controls were analyzed by 16S rRNA and ITS sequencing, and metabolomic analysis was performed by liquid chromatography/mass spectrometry. A co-culture model of air-liquid interface cultured human bronchial epithelial cell with Pseudomonas aeruginosa was constructed to verify the correlation between sphingosine metabolism, acid ceramidase expression, and Pseudomonas aeruginosa infection.
Results: After screening, 54 bronchiectasis patients and 12 healthy controls were included. Sphingosine levels in bronchoalveolar lavage fluid were positively correlated with lower respiratory tract microbial diversity and negatively correlated with the abundance of Pseudomonas spp. Moreover, sphingosine levels in bronchoalveolar lavage fluid and acid ceramidase expression levels in lung tissue specimens were significantly lower in bronchiectasis patients than in healthy controls. Sphingosine levels and acid ceramidase expression levels were also significantly lower in bronchiectasis patients with positive Pseudomonas aeruginosa cultures than in bronchiectasis patients without Pseudomonas aeruginosa infection. Acid ceramidase expression in air-liquid interface cultured human bronchial epithelial cell had significantly increased after 6 h of Pseudomonas aeruginosa infection, while it had decreased significantly after 24 h of infection. In vitro experiments showed that sphingosine had a bactericidal effect on Pseudomonas aeruginosa by directly disrupting its cell wall and cell membrane. Furthermore, adherence of Pseudomonas aeruginosa on bronchial epithelial cells was significantly reduced after sphingosine supplementation.
Conclusion: Down-regulation of acid ceramidase expression in airway epithelial cells of bronchiectasis patients leads to insufficient metabolism of sphingosine, which has a bactericidal effect, and consequently weakens the clearance of Pseudomonas aeruginosa; thus, a vicious circle is formed. Exogenous supplementation with sphingosine aids bronchial epithelial cells in resisting Pseudomonas aeruginosa infection.
Keywords: metabolomics, bronchiectasis, sphingosine, Pseudomonas aeruginosa, acid ceramidase
Introduction
Bronchiectasis is a chronic purulent disease of the airways causing permanent dilatation of the bronchi and bronchioles; this predisposes the patient to opportunistic infections, the most common being Pseudomonas aeruginosa (P. aeruginosa).1 When the microbial diversity of sputum in bronchiectasis patients decreases, P. aeruginosa infection predominates, increasing the risk of disease severity and death.2,3 Impaired airway flora homeostasis and damaged airway epithelium might increase the susceptibility to P. aeruginosa infection for bronchiectasis patients.4 Likewise, figuring out the interaction between respiratory flora and host metabolites contributes to an explanation of the pathogenesis of respiratory diseases and leads to a breakthrough in treatment.5 Analysis of respiratory microbial diversity, macrogenomics, and metabolomics in cystic fibrosis patients found unique combinations of bacterial genera and metabolites in different disease states and indicated that three additional exacerbations and treatments culminated in a new airway microbiome and metabolome.6 However, the correlation between lower respiratory microbial diversity, P. aeruginosa infection, and host metabolites has not been thoroughly explained in bronchiectasis patients.
Sphingosine, a natural sphingolipid produced by ceramide metabolism by the action of ceramidase, is present in healthy airways and has bactericidal activity against many pathogens, including P. aeruginosa.7 Moreover, reduced levels of sphingosine in the airway increase the risk of bacterial infections. Sphingosine was also significantly reduced in bronchial epithelial cells in a post-burn mouse model, increasing susceptibility to lung infections.8 A sepsis mouse model showed exacerbated pulmonary infections due to significantly reduced sphingosine levels in the respiratory epithelium.9 Normal bronchial epithelial cells upregulate sphingosine levels after P. aeruginosa infection; whereas, these cells do not increase sphingosine levels after P. aeruginosa infection in patients with cystic fibrosis.10 A dysregulated sphingosine 1-phosphate signaling pathway is seen in both bronchiectasis and persistent bacterial bronchitis.11 Moreover, sphingosine 1-phosphate stabilized the endothelial barrier function by binding to S1P1 receptors on endothelial cells.12 Sphingosine was dramatically decreased in alveolar macrophages of cystic fibrosis mice and humans, while the correction of sphingosine levels prevented the acute and chronic pulmonary infections in cystic fibrosis mice with P. aeruginosa.13 The relevance of sphingosine metabolism to P. aeruginosa infection in adult bronchiectasis patients remains unexplored, and it is necessary to assess whether imbalances in sphingosine metabolism are present in such patients.
Herein, we analyzed the bronchoalveolar lavage fluid (BALF) from bronchiectasis patients and normal healthy controls by 16S rRNA and ITS sequencing, conducted metabolomics analysis by liquid chromatography coupled with mass spectrometry (LC-MS), and identified metabolites significantly correlated with microbial diversity and Pseudomonas spp. abundance in the lower respiratory tract of bronchiectasis patients to explore the correlation between sphingosine metabolism and P. aeruginosa infection. We also constructed a co-culture model of air-liquid interface cultured differentiated human bronchial epithelial cells with P. aeruginosa to verify the correlation between sphingosine metabolism, acid ceramidase expression, and P. aeruginosa infection.
Methods
Study Subjects
This study was conducted in accordance with the Declaration of Helsinki. Bronchiectasis patients who underwent bronchoscopy from March 2021 to March 2022 in two hospitals were included. Inclusion criteria: (i) chest CT met the diagnostic criteria for bronchiectasis,14 (ii) aged 18–80 years, (iii) idiopathic or post-infection bronchiectasis, and (iv) no history of smoking. Exclusion criteria: (i) previous diagnosis of chronic obstructive pulmonary disease or asthma; (ii) immunodeficiency, primary ciliary dyskinesia syndrome, airway obstruction, allergic bronchopulmonary aspergillosis, or connective tissue disease; (iii) history of smoking; (iv) lung cancer or other malignancies; (v) ICU admission for mechanical ventilation; (vi) severe cardiac, hepatic or renal insufficiency; (vii) glucocorticoid use in the last 4 weeks; and (viii) BALF extracted DNA failed by PCR amplification.
Patients without bronchiectasis on chest CT who underwent bronchoscopy at two hospitals during the same period were gender- and age-matched and included as the control group. Inclusion criteria: (i) underwent electronic bronchoscopy for unilateral peripheral pulmonary nodules (nodule diameter ≤3 cm) or (ii) underwent electronic bronchoscopy for hemoptysis (no significant abnormality detected by chest CT and pulmonary artery computed tomography arteriography). Exclusion criteria: (i) history of smoking; (ii) other abnormalities on chest CT, including pneumonia, interstitial pneumonia, multiple pulmonary nodules, or pleural effusion; (iii) lung cancer with lymph node metastasis or obstructive pneumonia; (iv) ICU admission for mechanical ventilation; (v) severe cardiopulmonary vascular disease; and (vi) connective tissue disease.
This study was approved by the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital ethics committee and Qilu Hospital of Shandong University ethics committee, and all study participants provided informed consent.
Subgroups of Bronchiectasis
Clinical characteristics of bronchiectasis patients were analyzed, including etiology,1,15 chest CT score,15 bronchiectasis severity index (BSI),15 blood test, sputum and/or BALF cultures and pulmonary function tests (see Supplementary Material). Bronchiectasis patients were divided into subgroups according to different grouping criteria: neutrophilic bronchiectasis (BALF neutrophils ≥60% of total cells) and non-neutrophilic bronchiectasis (BALF neutrophils <60% of total cells);16 acute exacerbation and stable bronchiectasis;15 severe bronchiectasis (BSI score ≥9) vs mild to moderate bronchiectasis (BSI score 0–8);15 frequent-acute-exacerbation group (acute exacerbations ≥3 times/year) and less-acute-exacerbation group (acute exacerbations <3 times/year); P. aeruginosa culture-positive group and P. aeruginosa culture-negative group; P. aeruginosa chronic colonization group and no P. aeruginosa chronic colonization group.17
BALF Specimen Collection and Processing
For bronchiectasis patients, the lavage sites were the segments and subsegments of the bronchiectasis lesions determined per chest CT; for the control group, the dorsal and basal segments of the inferior lobes on the healthy side were lavaged. The specimen was taken during bronchoscopy, and sterile saline lavage was performed several times to retrieve BALF, with a total recycling volume of 25 to 30 mL, which was divided into two parts (about BALF specimen processing, see Supplementary Material). Hematoxylin and eosin (HE) staining was done for cell sorting and counting.18
Microbial Diversity by PCR Amplification of 16S rRNA and ITS
DNA extraction of BALF were described in Supplemental Material. The bacterial 16S rRNA gene V3 using 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) -V4 variable region was amplified by PCR; ITS1F (5’-CTTGGTCATTTAGAGGAAGTAA-3’) and ITS2R (5’-GCTGCGTTCTTCATCGATGC-3’) were used for PCR amplification of the ITS1 variable region of the fungal ITS gene. Illumina Miseq sequencing and bioinformatics analysis were performed (see Supplementary Material).
Metabolomics of BALF by LC-MS
The sample processing were described in Supplemental Material. LC-MS analysis was performed using an ultra-performance liquid chromatography-tandem time-of-flight mass spectrometry UPLC-TripleTOF system from AB SCIEX. Next, database retrieval and differential metabolite analyses were performed (see Supplementary Material).
Co-Culture Model of Air-Liquid Interface Cultured Human Bronchial Epithelial Cell with P. aeruginosa
Air-liquid interface cultured differentiated human bronchial epithelial cell were performed (see Supplementary Material).19 Calu-3 (Cat#CL-0054) cells were purchased from Procell. The human bronchial epithelial cell lines BEAS-2B and 16HBE are challenging to culture in the air-liquid interface cultured; whereas, the calu-3 cell line can be cultured in the air-liquid interface for >14 days.20 P. aeruginosa PAO1 strain (ATCC) was resuscitated and incubated overnight in a shaker, and the logarithmic growth phase was diluted to different colony forming unit (CFU)s with LB broth and added to the upper chamber of the transwell.
ELISA, Immunofluorescence, and Western Blot
P. aeruginosa infected air-liquid interface cultured differentiated human primary bronchial epithelial cells and cell supernatants were subjected to enzyme-linked immunosorbent assay (ELISA), including acid ceramidase (ZCI Bio, ZC-56014), IL-1β (liankebio, EK101B-96), IL-6 (liankebio, EK106/2-96), IL-8 (liankebio, EK108-96), and TNF-α (liankebio, EK182-96). Immunofluorescence of acid ceramidase (ProteinTech, 11274-1-AP) and Western blot (acid ceramidase, AbCam, ab282276) compared the effects of different CFUs of P. aeruginosa infections on the expression levels of acid ceramidase in bronchial epithelial cells, and laser co-focused microscope photographs were obtained.
Immunohistochemistry of Lung Tissue Specimens
Patients who underwent lobectomy for bronchiectasis, including bronchiectasis with recurrent refractory hemoptysis and limited bronchiectasis with chronic abscess formation, and were admitted to two hospitals from January 2021 to August 2022 were included; lung tissue specimens from bronchiectasis lesion sites were obtained. Inclusion criteria: CT of the chest met the diagnostic criteria of bronchiectasis and HE staining of tissue sections was confirmed by pathologists to be consistent with bronchiectasis. Exclusion criteria: pulmonary malignancy on pathologic examination and a history of smoking.
The control group included patients who were admitted to the thoracic surgery departments of both hospitals during the same period, were age- and gender-matched with the bronchiectasis group, and underwent lobectomy for isolated peripheral pulmonary nodules (≤3 cm in diameter); specimens were collected beyond 5 cm from the lung lesion. Inclusion criteria: surgical specimens with pathology of carcinoma in situ or microinvasive carcinoma, cancerous nodules of ≤3 cm maximum diameter, and absence of carcinoma at the cut edge, and no history of smoking. Immunohistochemistry for acid ceramidase (ab282276, AbCam, Britain) was performed.21
In vitro Bactericidal Effect of Sphingosine on P. Aeruginosa
P. aeruginosa PAO1 bacteria solution was resuspended in airway surface liquid buffer,22 and 5 mL of bacteria solution (0.5×10^5 CFU) was added to each tube. Different concentrations of sphingosine (Selleck, CAS: 123–78-4) were added to the tubes of six groups of bacteria. All centrifuge tubes were incubated in a shaker and sampled after 1 h. Next, 0.2 mL/dish was coated on trypticase soy broth (TSB) plates and incubated overnight in an incubator at 37 °C, and colonies were counted in CFU (see Supplementary Material).
Effect of Sphingosine on the Ultrastructure of P. Aeruginosa
The P. aeruginosa PAO1 bacterial solution was adjusted to 10^6 CFU/mL and was divided into two groups (group 1: 100 μL airway surface liquid buffer was added; group 2: 100 μL 51.2 mg/mL sphingosine was added to make the final concentration 51.2 μg/mL). All conical flasks were placed into a shaker for 1 h. The bacterial suspension was then centrifuged and fixed by 3% glutaraldehyde. Scanning electron microscope (model Inspect, FEI, USA) and transmission electron microscope (JEM-1400FLASH, JAPAN) were used to ascertain the changes in the microstructure of P. aeruginosa (see Supplementary Material).
Inhibition of P. aeruginosa Adhesion on Bronchial Epithelial Cells by Sphingosine Supplementation
For the air-liquid interface cultured culture of calu-3 cell line, 100 ul of P. aeruginosa solution (0.5×10^5 CFU) was added to the upper chamber of the transwell. Different concentrations of sphingosine were added to the upper and lower chambers of the transwell. The chambers were incubated at 37 °C in a CO2 incubator for 24 h, the upper chamber was washed twice with PBS, and the number of P. aeruginosa adhering to the surface of bronchial epithelial cells was counted by immunofluorescence with P. aeruginosa antibody (ThermoFisher, PA1-73116).
Statistical Analysis
Normally distributed measures were described by 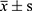 ; two-group and multi-group comparisons were performed using t-test and one-way ANOVA, respectively; non-normally distributed measurement data were expressed as median (interquartile spacing) and non-parametric tests (Kruskal−Wallis rank sum test) were used for two-group and multi-group comparisons; categorical variable data were described by rate or composition ratio and χ2 tests were used for two-group and multi-group comparisons. Partial least squares discriminant analysis (PLS-DA) and analysis of similarities (ANOSIM) were used to compare microbial community structure. Canonical correspondence analysis (CCA) and Spearman correlation analysis were used to compare the correlation between the flora and metabolites and clinical indicators. SPSS 25.0 software was used for statistical analysis. P<0.05 was considered statistically significant.
; two-group and multi-group comparisons were performed using t-test and one-way ANOVA, respectively; non-normally distributed measurement data were expressed as median (interquartile spacing) and non-parametric tests (Kruskal−Wallis rank sum test) were used for two-group and multi-group comparisons; categorical variable data were described by rate or composition ratio and χ2 tests were used for two-group and multi-group comparisons. Partial least squares discriminant analysis (PLS-DA) and analysis of similarities (ANOSIM) were used to compare microbial community structure. Canonical correspondence analysis (CCA) and Spearman correlation analysis were used to compare the correlation between the flora and metabolites and clinical indicators. SPSS 25.0 software was used for statistical analysis. P<0.05 was considered statistically significant.
Results
Comparison of Basic Information Between Bronchiectasis and Control
After screening, 54 bronchiectasis patients and 12 healthy controls were included (Figure S1 flow chart), among which 7 bronchiectasis patients and 5 healthy controls were excluded because their BALF-extracted DNAs were not amplified by PCR. Finally, data from 47 bronchiectasis patients and 7 healthy controls were analyzed. Of the 47 bronchiectasis patients (mean age, 59.2±11.8 years), 24 (51.1%) were females; of the 7 healthy controls (mean age, 55.6±8.8 years), 3 (42.9%) were females. There was no difference in the age and gender composition between the bronchiectasis and control groups (t=0.787, P=0.435; x2=0.435; and t=0.435, x2=0.165, P=0.685).
Clinical Characteristics of Subgroups of Bronchiectasis Patients
Clinical characteristics of bronchiectasis patients were shown in Table 1 and Supplementary Table 1. The proportions of P. aeruginosa culture positivity and obstructive ventilatory dysfunction were significantly higher in patients with neutrophilic bronchiectasis than in those with non-neutrophilic bronchiectasis (both P < 0.05; Table 1).
 |
Table 1 Comparison of Bronchiectasis Patients in BALF Neutrophils ≥ 60% and < 60% Groups |
Factors Influencing Microbial Diversity and Flora in Bronchiectasis
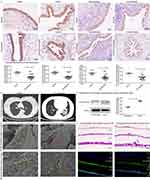
BALF bacterial diversity was not significantly different between bronchiectasis patients and controls (Figure 1A); however, the flora composition was significantly different (Figure 1B). For bronchiectasis patients with BALF neutrophil percentage ≥ 60%, be in acute exacerbation duration, BSI score ≥ 9, ≥3 acute exacerbations/year, positive P. aeruginosa culture, and chronic colonization of P. aeruginosa, their lower respiratory tract microbial diversity was less, and the flora composition was significantly altered (Figure 1A and B, Figure S2A and B). The number of years with a history of bronchiectasis, number of acute exacerbations, BSI score, and BALF neutrophil percentage were significantly and positively correlated with the abundance of Pseudomonas spp. (Figure 1C and D).
BALF Sphingosine Were Lower in Bronchiectasis Than in Controls
Sphingolipid metabolic pathways were significantly different between the bronchiectasis and control groups (Figure S3A), and BALF sphingosine levels were significantly lower in bronchiectasis patients than in controls, especially in stable bronchiectasis or chronic colonization with P. aeruginosa (Figure 2A and B, Figure S3B). Sphingosine levels were significantly lower in neutrophilic bronchiectasis patients than in those with non-neutrophilic bronchiectasis (Figure S3B) and in patients with P. aeruginosa culture-positive bronchiectasis than in those with culture-negative bronchiectasis (Figure 2B).
Positive Correlation of Sphingosine with Microbial Diversity and Negative Correlation with the Abundance of Pseudomonas Spp
In bronchiectasis patients, BALF sphingosine was positively correlated with microbial diversity (Figure 2C). There was a negative correlation between the abnormal ratio of Proteobacteria phylum and Firmicutes phylum and sphingosine metabolism (Figure S3C), and a significant negative correlation between sphingosine and P. aeruginosa abundance (Figure 2D).
Co-Culture Model of Air-Liquid Interface Cultured Bronchial Epithelial Cell with P. aeruginosa
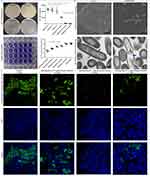
Using Air-liquid interface cultured culture, human primary bronchial epithelial cells can be differentiated into pseudostratified ciliated columnar epithelium with ciliated and secretory cell characteristics, which are also seen in the calu-3 cell line. These cells form ideal models for studying P. aeruginosa infection of human bronchial epithelial cells (Figure 3).
Up-Regulation Followed by Down-Regulation of Acid Ceramidase in Normal Bronchial Epithelial Cells Infected with P. aeruginosa
P. aeruginosa PAO1 infection of air liquid interface cultured human bronchial epithelial cells disrupted cilia and intercellular tight junctions, most severely in the high bacterial group (0.5×10^5 CFU) (Figure 4A and B). After P. aeruginosa PAO1 infection for 6 h, acid ceramidase expression in bronchial epithelial cells was significantly up-regulated (Figure 4C and D). Contrarily, acid ceramidase levels in bronchial epithelial cells decreased significantly 24 h after P. aeruginosa PAO1 infection (Figure 4E).
Decreased Ceramidase Expression Level in Airway Epithelial Cells in Bronchiectasis
Immunohistochemistry was performed on lung tissue specimens from 23 bronchiectasis patients and 11 gender- and age-matched controls. The results indicated that the acid ceramidase levels in airway epithelial cells of bronchi, bronchioles, and terminal bronchioles in bronchiectasis patients were significantly lower than those in controls (Figure 5A and B). The acid ceramidase expression in primary bronchial epithelial cells of bronchiectasis patients was lower than that of controls (Figure 5C). Acid ceramidase expression in airway epithelial cells from P. aeruginosa culture-positive bronchiectasis patients was significantly lower than those from culture-negative bronchiectasis patients (Figure 5D and E).
In vitro Experiments Confirmed the Bactericidal Effect of Sphingosine Against P. aeruginosa
Sphingosine was bactericidal against P. aeruginosa at three concentrations of 512 μg/mL, 51.2 μg/mL, and 5.12 μg/mL compared with the control at an initial level of 0.5×10^5 CFU, indicating that this was concentration-dependent with about 50% of the bacteria dying after 1 h at low concentration (5.12 μg/mL), at least 90% of the bacteria dying after 1 h at a medium concentration (51.2 μg/mL), and all bacteria dying after 1 h at high concentration of sphingosine (512 μg/mL) (Figure 6A).
Ultrastructural Effects of Sphingosine on P. Aeruginosa
Scanning electron microscopy showed that a 1 h application of sphingosine (51.2 μg/mL) resulted in a large number of P. aeruginosa dying. Transmission electron microscopy showed that the cell wall of P. aeruginosa in the sphingosine-treated group was fractured; the local cell wall was lysed and blurred; the cell membrane edge was blurred, thinned, or even disappeared; and the cytoplasmic contents were lost (Figure 6B). Presumably, sphingosine disrupts the cell wall and cell membrane of P. aeruginosa, causing its rapid rupture and death.
Sphingosine Supplementation Significantly Reduced P. aeruginosa Adherence to Bronchial Epithelial Cells
After culturing the calu-3 cell line in air-liquid interface cultured for >14 days, adding a medium concentration of sphingosine (51.2 μg/mL) to the upper or lower chambers of the transwell significantly reduced the P. aeruginosa adhering to the surface of bronchial epithelial cells for 24 h (Figure 6C).
Discussion
By comparing the microbial diversity and metabolomics of the lower respiratory tract between bronchiectasis patients and controls and between different subgroups of bronchiectasis, we found that sphingosine levels in the airways were positively correlated with the microbial diversity of the lower respiratory tract and negatively correlated with the abundance of Pseudomonas spp.; furthermore, low sphingosine levels in the airway of bronchiectasis patients may be related to the down-regulation of acid ceramidase. A co-culture model of bronchial epithelial cells with P. aeruginosa revealed that persistent P. aeruginosa infection led to a significant downregulation of acid ceramidase expression, in turn leading to a decreased sphingosine levels and a weakened airway epithelium against P. aeruginosa infection, thus forming a vicious cycle. Additionally, sphingosine had a bactericidal effect on P. aeruginosa, and its supplementation significantly reduced the number of P. aeruginosa adhered to bronchial epithelial cells.
Microbial diversity analysis of the lower respiratory tract in bronchiectasis patients is important for understanding the pathogenic distribution, subtype, disease severity, and stratification of at-risk patients.2,23 Microbial diversity analysis of sputum specimens from 281 bronchiectasis patients revealed that a decrease in microbial diversity with a predominance of Pseudomonas spp. was significantly correlated with disease severity, frequency of exacerbations, and increased risk of death.24 We also found that bronchitis in bronchiectasis patients with Pseudomonas spp. predominance and reduced microbial diversity were predominantly neutrophilic, had high BSI scores with numerous acute exacerbations. Moreover, sphingosine levels were found to be positively correlated with microbial diversity and negatively correlated with the abundance of Pseudomonas spp. Reduced sphingosine levels significantly increase the susceptibility of pathogenic bacteria,25,26 and minimal sphingosine levels were seen in a P. aeruginosa-infected cystic fibrosis lung model.27 We also found that sphingosine levels in the airways of bronchiectasis patients were significantly lower than those of controls, and this decrease was even more significant in patients with P. aeruginosa-infected bronchiectasis, suggesting that the decreased sphingosine levels weaken airway epithelial cell resistance to pathogenic bacteria, leading to their susceptibility to P. aeruginosa infection.
The correlation between abnormal sphingosine metabolism and P. aeruginosa infection necessitates the exploration of the factors contributing to abnormal sphingosine metabolism. Ceramidase hydrolyzes ceramide to sphingosine, and sphingosine is phosphorylated to sphingosine 1-phosphate by sphingosine kinase; factors affecting these enzymatic activities may cause abnormal sphingosine metabolism.28 Cystic fibrosis patients have reduced airway epithelial sphingosine due to excessive accumulation of β1 integrin in airway epithelial cells, which downregulates acid ceramidase expression.22 We found significantly downregulated acid ceramidase expression in bronchi, bronchioles, and terminal bronchiole epithelial cells in bronchiectasis patients than in controls; similarly, acid ceramidase levels in airway epithelial cells were significantly lower in bronchiectasis patients with P. aeruginosa infection than in those without P. aeruginosa infection. Thus, we hypothesized that reduced sphingosine levels can be associated with down-regulated acid ceramidase expression. Additionally, we found that the acid ceramidase expression levels of bronchial epithelial cells increased significantly after P. aeruginosa infection in a co-culture model of human bronchial epithelial cells differentiated in the Air-liquid interface cultured and P. aeruginosa; however, with >24 h of P. aeruginosa infection, acid ceramidase levels in bronchial epithelial cells were significantly downregulated. This may be caused by severe cellular damage due to the massive proliferation of P. aeruginosa.
P. aeruginosa infection with down-regulation of acid ceramide expression and insufficient sphingosine makes bronchiectasis patients more susceptible to P. aeruginosa infection, forming a vicious circle. We verified the in vitro bactericidal activity of sphingosine against P. aeruginosa, and > 90% killed by 1 h of drug action. Assuming that sphingosine is important in this vicious cycle, its supplementation can break this cycle. One study found a four-fold reduction in sphingosine levels in the airways of a burn mouse model with severe P. aeruginosa infection 24 h after burn injury and a significant reduction in the bacterial load of P. aeruginosa after administration of inhaled sphingosine.8 Our results are consistent with the finding that exogenous supplementation with sphingosine significantly reduces the number of P. aeruginosa adhering to the surface of bronchial epithelial cells. The mechanism of sphingosine’s bactericidal effect on P. aeruginosa is becoming unravelled. Bactericidal mechanism of sphingosine was shown associated with making pores on bacterial membrane following binding of sphingosine’s positively charged NH3+ group to bacterial membrane’s negatively charged cardiolipin.29 Sphingosine induces outer membrane permeation, disrupts membrane potential, and leads to intracellular acidification in bacteria.9 By observing sphingosine’s ultrastructural effects on P. aeruginosa, we found that sphingosine exerted its bactericidal effect through direct disruption of the bacterial cell wall and membrane. Therefore, exogenous sphingosine may be an effective antibacterial drug.26
Macrophages and endothelial cells also play important roles in cystic fibrosis, bronchiectasis and other lung inflammatory diseases, and the role of sphingosine has been addressed in macrophages and endothelial cells. Sphingosine kinase-1 and sphingosine kinase-2 convert sphingosine into sphingosine-1-phosphate and modulate inflammatory responses in macrophages.30 The generation of sphingosine-1-phosphate by sphingosine kinase-2 in CD11b+ macrophages suppressed alveolar macrophage-STING signaling and led to the resolution of lung injury.31 An analog of sphingosine-1-phosphate protected human lung endothelial cell from barrier disruption induced by methicillin-resistant Staphylococcus aureus infection.32 In this study, abnormal metabolism of sphingosine was found in the BALF samples of patients with bronchiectasis, which may be related to the down-regulation of acid ceramidase expression in bronchial epithelial cells. The levels of sphingosine and the enzyme ceramidase in endothelial cells and alveolar macrophage in patients with bronchiectasis need to be further explored.
This study identified and validated a correlation between reduced sphingosine in the airways and P. aeruginosa infection in bronchiectasis patients, which may be beneficial for treating P. aeruginosa infection; however, there are some limitations to our study. First, the selection of BALF samples resulted in a small sample size, which may be biased, and further studies with larger sample sizes are needed for validating our results. Second, we only explored bacterial and fungal diversity of the lower respiratory tract and did not explore viral diversity. Third, there are no animal models to verify the causal relationship between P. aeruginosa infection, down-regulated acid ceramidase expression, and altered sphingosine metabolism in the airways. Fourth, we explored the sphingosine bactericidal effect on plank-tonic bacteria, while further exploration is needed to determine whether it can interfere with Quorum sensing system and disrupt biofilms.
Conclusions
Sphingosine was insufficient in bronchiectasis patients. Acid ceramidase expression is down-regulated in the airway epithelial cells of bronchiectasis patients, resulting in a vicious cycle of insufficient metabolism of bactericidal sphingosine, which in turn weakens P. aeruginosa clearance. Exogenous sphingosine supplementation aids bronchial epithelial cells in resisting P. aeruginosa infection.
Abbreviations
P. Aeruginosa, Pseudomonas aeruginosa; BALF, bronchoalveolar lavage fluid; LC-MS, liquid chromatography coupled with mass spectrometry; BSI, bronchiectasis severity index; HE, hematoxylin and eosin; CFU, colony forming unit; ELISA, enzyme-linked immunosorbent assay; TSB, trypticase soy broth; PLS-DA, partial least squares discriminant analysis; ANOSIM, analysis of similarities; CCA, canonical correspondence analysis.
Data Sharing Statement
All data generated or analysed during this study are included in this published article and its Supplementary Material.
Ethics Approval and Informed Consent
This study was approved by the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital ethics committee [2021 (S402)] and Qilu Hospital of Shandong University ethics committee [2019 (147)], and all study participants provided informed consent.
Author Contributions
QQ, XJW and WYJ acquired and analyzed the data, and drafted the work. ZJ, GMX and LY provided specimens for bronchoscopy of patients and were contributors in writing the manuscript. DL designed the work and substantively revised it. All authors reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. All authors agree to take responsibility and are accountable for the contents of the article. All authors approved the final manuscript and agreed on the journal to which the article will be submitted.
Funding
This work was supported by grants from National Natural Science Foundation of China (82100056), Natural Science Foundation of Shandong Province (ZR2021QH170), Natural Science Foundation of Shandong Province (ZR2022QH235) and Natural Science Foundation of Shandong Province (ZR2022QH078).
Disclosure
The authors declare that they have no competing interests.
References
1. Qi Q, Wang W, Li T, Zhang Y, Li Y. Aetiology and clinical characteristics of patients with bronchiectasis in a Chinese Han population: a prospective study. Respirol Carlton Vic. 2015;20(6):917–924. doi:10.1111/resp.12574
2. Tiew PY, Jaggi TK, Chan LLY, Chotirmall SH. The airway microbiome in COPD, bronchiectasis and bronchiectasis-COPD overlap. Clin Respir J. 2021;15(2):123–133. doi:10.1111/crj.13294
3. Rada B. Interactions between Neutrophils and Pseudomonas aeruginosa in Cystic Fibrosis. Pathog Basel Switz. 2017;6(1):E10. doi:10.3390/pathogens6010010
4. Chai YH, Xu JF. How does Pseudomonas aeruginosa affect the progression of bronchiectasis? Clin Microbiol Infect. 2020;26(3):313–318. doi:10.1016/j.cmi.2019.07.010
5. Zanella D, Liden T, York J, Franchina FA, Focant JF, Schug KA. Exploiting targeted and untargeted approaches for the analysis of bacterial metabolites under altered growth conditions. Anal Bioanal Chem. 2021;413(21):5321–5332. doi:10.1007/s00216-021-03505-2
6. Hahn A, Whiteson K, Davis TJ, et al. Longitudinal associations of the cystic fibrosis airway microbiome and volatile metabolites: a Case Study. Front Cell Infect Microbiol. 2020;10:174. doi:10.3389/fcimb.2020.00174
7. Carstens H, Kalka K, Verhaegh R, et al. Antimicrobial effects of inhaled sphingosine against Pseudomonas aeruginosa in isolated ventilated and perfused pig lungs. PLoS One. 2022;17(7):e0271620. doi:10.1371/journal.pone.0271620
8. Rice TC, Seitz AP, Edwards MJ, Gulbins E, Caldwell CC. Frontline Science: sphingosine rescues burn-injured mice from pulmonary Pseudomonas aeruginosa infection. J Leukoc Biol. 2016;100(6):1233–1237. doi:10.1189/jlb.3HI0416-197R
9. Beckmann N, Pugh AM, Auteri NJ, Edwards MJ, Gulbins E, Caldwell CC. Therapeutic Inhaled Sphingosine for Treating Lung Infection in a Mouse Model of Critical Illness. Cell Physiol Biochem. 2020;54(5):1054–1067.
10. Gardner AI, Haq IJ, Simpson AJ, et al. Recombinant acid ceramidase reduces inflammation and infection in cystic fibrosis. Am J Respir Crit Care Med. 2020;202(8):1133–1145. doi:10.1164/rccm.202001-0180OC
11. Hodge S, Macowan M, Liu H, et al. Sphingosine signaling dysfunction in airway cells as a potential contributor to progression from protracted bacterial bronchitis to bronchiectasis in children. Pediatr Pulmonol. 2020;55(6):1414–1423. doi:10.1002/ppul.24728
12. Tibboel J, Reiss I, de Jongste JC, Post M. Sphingolipids in lung growth and repair. Chest. 2014;145(1):120–128. doi:10.1378/chest.13-0967
13. Becker KA, Riethmüller J, Seitz AP, et al. Sphingolipids as targets for inhalation treatment of cystic fibrosis. Adv Drug Deliv Rev. 2018;133:66–75. doi:10.1016/j.addr.2018.04.015
14. Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet Lond Engl. 2018;392(10150):880–890. doi:10.1016/S0140-6736(18)31767-7
15. Martinez-Garcia MA, Maiz L, Olveira C, et al. Spanish guidelines on the evaluation and diagnosis of bronchiectasis in adults. Arch Bronconeumol. 2018;54(2):79–87. doi:10.1016/j.arbr.2017.07.013
16. Esther CR, Turkovic L, Rosenow T, et al. Metabolomic biomarkers predictive of early structural lung disease in cystic fibrosis. Eur Respir J. 2016;48(6):1612–1621. doi:10.1183/13993003.00524-2016
17. Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189(5):576–585. doi:10.1164/rccm.201309-1575OC
18. Yuan J, Liu R, Ma Y, Zhang Z, Xie Z. Curcumin Attenuates Airway Inflammation and Airway Remolding by Inhibiting NF-κB Signaling and COX-2 in Cigarette Smoke-Induced COPD Mice. Inflammation. 2018;41(5):1804–1814. doi:10.1007/s10753-018-0823-6
19. Yeo AJ, Henningham A, Fantino E, et al. Increased susceptibility of airway epithelial cells from ataxia-telangiectasia to S. pneumoniae infection due to oxidative damage and impaired innate immunity. Sci Rep. 2019;9(1):2627. doi:10.1038/s41598-019-38901-3
20. Kreft ME, Jerman UD, Lasic E, et al. The characterization of the human cell line Calu-3 under different culture conditions and its use as an optimized in vitro model to investigate bronchial epithelial function. Eur J Pharm Sci. 2015;69:1–9. doi:10.1016/j.ejps.2014.12.017
21. Frija-Masson J, Martin C, Regard L, et al. Bacteria-driven peribronchial lymphoid neogenesis in bronchiectasis and cystic fibrosis. Eur Respir J. 2017;49(4):1601873. doi:10.1183/13993003.01873-2016
22. Grassmé H, Henry B, Ziobro R, et al. β1-integrin accumulates in cystic fibrosis luminal airway epithelial membranes and decreases sphingosine, promoting bacterial infections. Cell Host Microbe. 2017;21(6):707–718.e8. doi:10.1016/j.chom.2017.05.001
23. Huang JT, Cant E, Keir HR, et al. Endotyping Chronic Obstructive Pulmonary Disease, Bronchiectasis, and the ‘Chronic Obstructive Pulmonary Disease-Bronchiectasis Association’. Am J Respir Crit Care Med. 2022;206(4):417–426. doi:10.1164/rccm.202108-1943OC
24. Dicker AJ, Lonergan M, Keir HR, et al. The sputum microbiome and clinical outcomes in patients with bronchiectasis: a prospective observational study. Lancet Respir Med. 2021;9(8):885–896. doi:10.1016/S2213-2600(20)30557-9
25. Wu Y, Liu Y, Gulbins E, Grassme H. The anti-infectious role of sphingosine in microbial diseases. Cells. 2021;10(5):1105. doi:10.3390/cells10051105
26. Martin GE, Boudreau RM, Couch C, et al. Sphingosine’s role in epithelial host defense: a natural antimicrobial and novel therapeutic. Biochimie. 2017;141:91–96. doi:10.1016/j.biochi.2017.03.014
27. Pewzner-Jung Y, Tavakoli Tabazavareh S, Grassme H, et al. Sphingoid long chain bases prevent lung infection by Pseudomonas aeruginosa. EMBO Mol Med. 2014;6:1205–1214. doi:10.15252/emmm.201404075
28. Hadas Y, Vincek AS, Youssef E, et al. Altering sphingolipid metabolism attenuates cell death and inflammatory response after myocardial infarction. Circulation. 2020;141(11):916–930. doi:10.1161/CIRCULATIONAHA.119.041882
29. Verhaegh R, Becker KA, Edwards MJ, Gulbins E. Sphingosine kills bacteria by binding to cardiolipin. J Biol Chem. 2020;295(22):7686–7696. doi:10.1074/jbc.RA119.012325
30. Pyne NJ, Adams DR, Pyne S. Sphingosine Kinase 2 in Autoimmune/Inflammatory Disease and the Development of Sphingosine Kinase 2 Inhibitors. Trends Pharmacol Sci. 2017;38(7):581–591. doi:10.1016/j.tips.2017.04.003
31. Joshi JC, Joshi B, Rochford I, et al. SPHK2-Generated S1P in CD11b+ Macrophages Blocks STING to Suppress the Inflammatory Function of Alveolar Macrophages. Cell Rep. 2020;30(12):4096–4109.e5. doi:10.1016/j.celrep.2020.02.112
32. Wang L, Letsiou E, Wang H, et al. MRSA-induced endothelial permeability and acute lung injury are attenuated by FTY720 S-phosphonate. Am J Physiol Lung Cell Mol Physiol. 2022;322(1):L149–L161. doi:10.1152/ajplung.00100.2021
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.