Back to Journals » Cancer Management and Research » Volume 10
Decreased expression of TRPV1 in renal cell carcinoma: association with tumor Fuhrman grades and histopathological subtypes
Authors Wu YY, Liu XY, Zhuo DX, Huang HB, Zhang FB, Liao SF
Received 24 February 2018
Accepted for publication 20 April 2018
Published 22 June 2018 Volume 2018:10 Pages 1647—1655
DOI https://doi.org/10.2147/CMAR.S166390
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leylah Drusbosky
Yong-Yang Wu,1 Xin-Yu Liu,2 De-Xiang Zhuo,1 Huai-Bin Huang,1 Fa-Biao Zhang,1 Shang-Fan Liao1
1Department of Urology, Affiliated Sanming First Hospital, Fujian Medical University, Sanming, Fujian 365100, China; 2State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University and Collaborative Innovation Center for Biotherapy, Chengdu, Sichuan 610041, China
Purpose: The aim of this study was to investigate whether the expression of the ligand-gated Ca2+ channel transient receptor potential vanilloid type-1 (TRPV1) in primary human renal cell carcinoma (RCC) is associated with clinicopathological features.
Patients and methods: Fresh and frozen primary tumor and normal peritumoral kidney tissues from 127 patients diagnosed with RCC were analyzed for TRPV1 expression by quantitative reverse transcription polymerase chain reaction (RT-PCR), Western blotting and immunohistochemistry.
Results: Quantitative RT-PCR revealed that TRPV1 was decreased 3.20-fold in RCC tissue vs normal peritumoral kidney tissue (p=0.012). Significantly different TRPV1 mRNA expression was detected in RCC tissues of different Fuhrman grades and histopathological subtypes (F=4.282, p=0.015 and F=5.205, p=0.014, respectively). Decreased TRPV1 expression was correlated with RCC histopathological subtype (R=-0.554, p=0.003) and Fuhrman grade (R=−0.525, p=0.006). Western blot analysis of TRPV1 protein expression showed similar results. Immunohistochemical analysis showed strong expression of TRPV1 in kidney tubules but demonstrated weak or no immunostaining in RCC tissues.
Conclusion: TRPV1 expression was decreased in RCC, which was significantly associated with tumor Fuhrman grades and histopathological subtypes. It seems to suggest that TRPV1 expression may be a valuable tool to predict the extent of RCC progression.
Keywords: renal cell carcinoma, TRPV1, Fuhrman grade, histopathological subtype, prognostic factor
Introduction
Renal cell carcinoma (RCC) is the most common form of kidney cancer with an estimated 63,990 new cases diagnosed and 14,400 deaths in 2017 in the USA.1 Calcium is an important regulator of several cellular processes; many of which are deregulated in cancer cells, such as proliferation, differentiation and apoptosis.2,3 Alteration of the expression of specific calcium-permeable ion channels is related to cancer development in various cancers.4–6 Transient receptor potential vanilloid type-1 (TRPV1), a ligand-gated Ca2+-permeable ion channel, is involved in the important process of Ca2+ transport and then maintains the intro-cellular calcium ([Ca2+]i) level in cells.7 TRPV1 was detected in prostate, breast and bladder cancer8–10 and associated with cancer prognosis and histopathological differentiation.11,12 These findings showed that TRPV1 might be an important factor in cancer development and progression.
Capsaicin, as an agonist of TRPV1, has been shown to inhibit in vivo and in vitro cancer growth and to induce apoptosis of different cancer cells.13,14 The capsaicin-induced antitumor effects are mainly mediated through the TRPV1 channel by increasing intracytoplasmic Ca2+.15,16 A striking increase in carcinogenesis of TRPV1-/- mice was observed in carcinogenesis experiments,17 while capsaicin could not induce tumor apoptosis with TRPV1 knockout.18 All these findings indicated that TRPV1 was involved in malignant transformation of certain types of human carcinoma.
TRPV1 is mainly expressed in primary sensory neurons and also can be present in nonneuronal cells, such as urinary tract cells.10,12 With respect to the kidney, TRPV1 was also detected19 in and contributed to the pathogenesis of ischemia/reperfusion-induced acute kidney injury.20,21 However, little is known about the relationship of TRPV1 and kidney disease. Specifically, no report has been published on possible alterations of TRPV1 expression in human RCC.
Therefore, the aim of this study was to investigate the alteration of TRPV1 expression in the primary tumors of RCCs and normal peritumoral kidney tissues and then to define the correlation between its expression level and various clinicopathological features of RCC. We confirmed that decreased expression of TRPV1 was detected in RCCs and that TRPV1 expression was significantly associated with tumor Fuhrman grades and histopathological subtypes.
Patients and methods
Patients
A total of 58 women and 69 men with a mean ± SD age of 59.93±13.56 years (range 47–83 years, median 59 years) diagnosed with RCC at the Department of Urology, Affiliated Sanming First Hospital, Fujian Medical University, between 2007 and 2010, were included in this study. The mean ages of the women and men were 60.25±10.62 and 59.79± 14.88 years, respectively. Table 1 lists the patients’ clinical and pathological features.
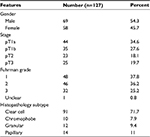  | Table 1 Clinical and pathological features of 127 study patients |
Histopathological diagnosis was established according to the World Health Organization (WHO) guidelines (2004). Cases were selected according to tissue availability and were not stratified for any known preoperative or pathological prognostic factors. A pathologist divided the tissue blocks into malignant RCCs and normal peritumoral kidney tissues immediately after nephrectomy. One part was frozen at -80°C directly for mRNA and protein isolation, while another part was fixed in Tissue-Tek® O.C.T.™ (Tissue-Tek, Sakura, Tokyo, Japan) and then stored at -80°C for immunohistochemical (IHC) analysis.
Ethical approval
Ethical and legal approval was obtained from the ethics committee of the Sanming First Hospital affiliated to Fujian Medical University prior to the commencement of the study. Written informed consent was obtained from all patients.
Hematoxylin–eosin (HE) staining
HE staining was performed for staging of all the specimens. Two blinded independent pathologists assessed the HE staining. When the pathologists disagreed about tumor stage and grade, they reviewed the slides in a non-blinded way.
Quantitative reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from normal peritumoral kidney tissues and RCC samples with an RNeasy® Mini Kit and RNase-free DNase set (Qiagen NV, Venlo, the Netherlands), and approximately 0.5 μg of extracted RNA was reverse transcribed using a First Strand cDNA Synthesis Kit for RT-PCR (Hoffman-La Roche Ltd., Basel, Switzerland) with random primers.
Real-time RT-PCR assays were performed with a SmartCycler® System (Cepheid, Sunnyvale, CA, USA) using SYBR® Green-I as the fluorogenic dye (Molecular Probes, Eugene, OR, USA). Two microliters of the complementary DNA were added into a 25 μL reaction system of Ex-taq® RT-PCR version (TaKaRa, Otsu, Japan) with 0.2 μM of each pair of gene-specific primers and then subjected to 45 polymerase chain reaction (PCR) cycles. The primer sequences of TRPV1 (GenBank® no. NM-080705.3) were as follows: forward, 5′-TCA ACA AGA TCG CAC AGG AG-3′; reverse, 5′-GCC TGA AAC TCT GCT TGA CC-3′.
The thermal program was 5 seconds at 95°C for denaturation and 20 seconds at 60°C for annealing and elongation. The mRNA expression level was normalized as the ratio to β-actin expression (forward, 5′-GGA CTT CGA GCA AGA GAT GG-3′; reverse, 5′-AGC ACT GTG TTG GCG TAC AG-3′) in each sample. Amplified PCR products were electrophoresed on 2.5% agarose gel for verification.
Western blot analysis
Proteins were extracted from frozen tissues using TRIzol® reagent (Thermo Fisher Scientific, Waltham, MA, USA). Protein (100 μg) was separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and electrotransferred onto a nitrocellulose transfer membrane using the ECL® System (Amersham Biosciences, Buckinghamshire, UK). After blocking with 3% bovine serum albumin (BSA) in PBS, the membranes were incubated with primary antibody. The following primary antibodies were used: anti-TRPV1 (N-15) sc-12500 (1:200; Santa Cruz Biotechnology Inc., Dallas, TX, USA), anti-β-actin (1:30,000; Sigma-Aldrich Co., St Louis, MO, USA). After washing with PBS (0.1%) Tween-20, filters were probed with streptavidin–horseradish peroxidase (HRP)-labeled secondary antibody (Dako; Dako Denmark A/S, Glostrup, Denmark). Immunoreactivity was detected by an enhanced chemiluminescence system (Amersham Biosciences). Films were scanned, and the optical density of the bands was measured with ImageJ software (National Institutes of Health, Bethesda, MD, USA). Data are representative of three independent experiments.
IHC staining
Frozen tissues embedded in optimum cutting temperature compound (Tissue-Tek) were sectioned into 8 μm slices. Three to five sections of each sample per patient (normal peritumoral kidney tissue and RCCs) were examined. Each slice was mounted onto an aminopropyl-triethoxysilane-coated glass slide. After air-drying, all slides were fixed in acetone for 10 minutes at 4°C and washed with Tris-buffered saline supplemented with 0.3% Triton X-100. The slides were then treated with 3% hydrogen peroxidase (Dako, S2001; Dako Denmark A/S) for 15 minutes to block endogenous peroxidase and then treated with the blocking reagent Dako X0909 to block nonspecific protein binding. Then, slides were incubated for 90 minutes at room temperature or overnight at 4°C with primary commercial antibodies (goat polyclonal antibody to human TRPV1, N-15, sc-12500; Santa Cruz Biotechnology Inc.; the optimal dilution 1:50). For the negative control, the primary antibody was replaced by antibody dilution buffer (Dako, S-3022). After washing with Tris-buffer solution, slides were incubated with a streptavidin–HRP-labeled secondary antibody (anti-rabbit antibody from Dako K 1390; anti-goat antibody from Dako P 0499 for 1:200 dilution; anti-rat antibody from Thermo PA1-28779 (Thermo Fisher Scientific) for 1:500 dilution) for 60 minutes at room temperature and treated with the substrate solution, 3,3’-diaminobenzidine (DAB; Dako K1390), for a color reaction. After 4 minutes of counterstaining with hematoxylin, the slides were viewed by a light microscope.
Statistical analyses
All values are shown as mean ± standard error of the mean (SEM). Statistical analysis was performed using SPSS, version 20.0 (IBM Corporation, Armonk, NY, USA). Paired sample t-tests were applied to assess the significance of the expression of mRNA between the normal peritumoral kidney tissues and RCC tissues, and one-way Hochberg’s analysis of variance (ANOVA) was applied to assess the TPRV1 mRNA expression differences between normal peritumoral kidney tissues and RCC tissues among age, gender, pT stage, Fuhrman grade and histopathological subtype groups, separately. Spearman’s correlation analysis was used for statistical analyses of the correlations between the TRPV1 mRNA expression in renal cell cancer and clinicopathological parameters. All p-values were two tailed, and those <0.05 was considered statistically significant.
Results
HE staining
HE staining was performed for all specimens and all tumors, which were reviewed by a pathologist for histopathological subtype and nucleolar grade features (Table 1).
TRPV1 mRNA quantification
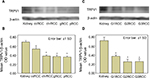
RT-PCR revealed TRPV1 gene expression in normal peritumoral kidney tissues and RCC tissues (Figure 1A). However, TRPV1 mRNA was decreased 3.20-fold in RCC vs normal peritumoral kidney tissues. The differences were statistically significant (p=0.012).
Different TRPV1 mRNA expression among histopathological subtype and Fuhrman grades of RCC
We analyzed differences in quantified TRPV1 mRNA expression by patients’ age (≥65 years vs <65 years), gender, pT stage, Fuhrman grade and histopathological subtype using one-way ANOVA of all tissues. No significant difference of TRPV1 mRNA expression between normal peritumoral kidney tissues and RCC tissues was found among the age, gender and pT stage groups. However, we detected significant differences in TRPV1 expression among histopathological subtype and Fuhrman grades of RCC (F=5.205, p=0.014 and F=4.282, p=0.015, respectively; Figure 2A and B).
In detail, no significant difference was detected in clear cell RCC vs normal peritumoral kidney tissues (t=1.537, p=0.144), but significant differences were detected in chromophobe, granular and papillary RCC samples vs normal peritumoral kidney tissues (t=3.393, 5.228, 12.518; p=0.043, 0.020, 0.006, respectively). When RCC Fuhrman grade was assessed, no significant difference was detected in grade 1 RCC (G1RCC) vs normal peritumoral kidney tissues (t=0.32, p=0.759), but significant differences were detected in grade 2 RCC (G2RCC) and grade 3 RCC (G3RCC) vs normal peritumoral kidney tissues (t=6.172, 4.98; p=0.010, 0.038, respectively).
TRPV1 protein semiquantification in normal peritumoral kidney tissues and RCC tissues
Western blot analyses showed TRPV1 protein in normal peritumoral kidney tissues and RCC extracts. In parallel with our RT-PCR findings, peritumoral tissues had much higher TRPV1 expression levels than RCC tissues (Figure 1B and C). Different TRPV1 expression levels were detected among histopathological subtype and Fuhrman grades of RCC (Figure 3A and C). After normalization to β-actin, densitometric measurements revealed that peritumoral tissues expressed significantly higher TRPV1 protein levels than different RCC histopathological subtypes (p=0.078, 0.009, 0.005 and 0.003; Figure 3B) and RCC Fuhrman grades (p=0.012, 0.001 and 0.001; Figure 3D).
TRPV1 immunostaining in normal peritumoral kidney tissues and RCC tissues
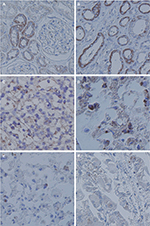
IHC analysis revealed TRPV1 expression in malignant and nonmalignant renal epithelium (Figures 4 and 5). Generally, positive TRPV1 staining was absent in glomeruli but strongly present in proximal tubules, while weak to moderate staining was detected in collecting ducts (CDs) and distal tubules (DTs; Figure 4A and B). The intracytoplasmic staining was focal or diffuse.
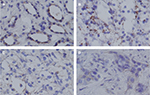
Similar to the mRNA results, relatively weak TRPV1 immunostaining was observed much more frequently in the RCC tissues, and differences were significant compared with those in normal peritumoral kidney tissues. Granular and papillary RCC samples showed a similar pattern of immunostaining with negative to weak TRPV1 staining (Figure 4E and F), while in chromophobe RCC, weak staining was detected (Figure 4D). However, the immunostaining intensity of clear cell RCC was significantly stronger and showed diffusely moderate positive staining (Figure 4C). TRPV1 staining was mainly found in the cytoplasm of the epithelium and appeared focal or diffuse.
For Fuhrman grade, gradually decreased TRPV1 staining was seen in parallel with higher Fuhrman grade of clear cell RCC. In G1RCC specimens, the labeling intensity was similar or slightly lower than in normal peritumoral kidney tissues (Figure 5A) as focal and granular staining (Figure 5B). However, in the grade 2 (Figure 5C) and grade 3 cases (Figure 5D), the labeling intensity was markedly reduced and appeared as weak or negative staining. No staining was observed in controls as well as in both normal peritumoral kidney tissues and RCC samples when the primary antibody was replaced using antibody dilution buffer.
TRPV1 correlation with clinicopathological parameters
We examined the statistical relationship between the quantified expression level of TRPV1 mRNA in RCC specimens and the histopathological grading and clinical staging of patients using Spearman correlation analysis in the 127 patients. No significant correlation was detected between RCC TRPV1 mRNA expression levels and patients’ age, gender and pT stage. However, decreased RCC TRPV1 expression was correlated with histopathological subtypes (R=-0.554, p=0.003) and Fuhrman grades (R=-0.525, p=0.006).
Discussion
In the current study, we confirmed that the normal peritumoral kidney tissue expressed TRPV1 mRNA and protein, but the mRNA and protein levels were markedly decreased in RCC. Furthermore, expression of this receptor decreased progressively as the Fuhrman grade increased. Indeed, while TRPV1 expression was slightly reduced in grade 1 cases, the receptor was rarely detected or completely absent in G2RCC and G3RCC samples. The implications of these findings are twofold: first, these data confirm that TRPV1 cannot be considered a “specific neuronal sensory receptor” only; second, the gradual loss of expression in higher-grade RCC suggests that the receptor expression might be correlated with cell differentiation.
Previously, TRPV1 was described as a Ca2+-permeable ion channel activated by heat and capsaicin and engaged in tactile, thermal and taste sensing or osmolality regulation. Malignant cell transformation is often accompanied by changes in ion channel expression, and among these channels, several members of the transient receptor potential (TRP) family, Ca2+ and Na+-permeable channels, show altered expression in malignant tumors.6,12 Recently, altered expression of TRPV1 has been found to be associated with prostate cancer and breast and bladder cancers.8,9,12 Capsaicin, the major TRPV1 agonist, has been shown to inhibit in vivo and in vitro cancer growth and progression and to induce apoptosis of different cancer cells.7 All these findings suggest that TRPV1 may be involved in tumor development and progression.
The kidneys are profusely innervated organs in which renal adrenergic neurons supply all the segments of renal vasculature, such as renal tubules.22 TRPV1, as a neuronal sensory receptor, is widely distributed in the kidney.23 In this study, we also confirmed that TRPV1 is strongly expressed in the tubules but is absent in glomeruli. TRPV1 was located in the intracytoplasmic regions and mainly confined to granular structures. A similar location has been found in the transitional cells of the human bladder, and TRPV1 was located in the endoplasmic reticulum (ER) and mitochondria and acted as a modulator of [Ca2+]i.12
Some evidence suggests that the effects of capsaicin against some cancerous cells may be via convergence of mitochondrial pathways.7 Capsaicin leads to the dissipation of transmembrane mitochondrial potential and then induces large increases in [Ca2+]i via TRPV1.2 Decreased TRPV1 expression in the ER or mitochondria will lead to [Ca2+]i reduction. The role of Ca2+ in the overall cancer-related cell signaling pathways is uncontested. Altered Ca2+ homeostasis increases proliferation and induces differentiation or apoptosis.24,25 The increased [Ca2+]i and mitochondrial Ca2+ could activate mitochondrial membrane permeabilization and lead to apoptosis and necrosis. In contrast, decreased Ca2+ content in the ER lumen is associated with resistance to apoptosis.26,27 Thus, decreased TRPV1 expression could possibly induce disordered Ca2+ transport and change the [Ca2+]i level to enhance the proliferation or resist apoptosis in RCC. In addition, TRPV1 could lead to increased or decreased production of reactive oxygen species, which are known to be involved in intrinsic apoptosis, although this is possibly a less important pathway in proliferation or apoptosis.18 However, how TRPV1 affects RCC carcinogenesis is still unclear and should be further verified in the follow-up study.
We found a progressive loss of TRPV1 expression as RCC Fuhrman grade increased, and labeling was absent in the less differentiated cells. Consistent with the IHC data, Western blot and RT-PCR analyses confirmed the presence of TRPV1 in the kidney and demonstrated its progressive disappearance in the RCC samples as the Fuhrman grade increased. Moreover, decreased TRPV1 expression was correlated with Fuhrman grade. Thus, decreased TRPV1 expression may lead to active proliferation and dedifferentiation of cells.
Different TRPV1 expression levels among the different RCC histopathological subtypes were detected and verified by Western blot and RT-PCR analyses. In clear cell RCCs, TRPV1 staining was diffuse and moderate in the cytoplasm, but the average immunostaining intensity was significantly lower in granular, chromophobe and papillary RCCs. Interestingly, we also observed that TRPV1 was mainly located in distal nephrons and absent in glomeruli.
RCC is believed to arise from the precursor cells of different specialized epitheliums located along the length of the nephron. Ordinarily, clear cell and papillary RCCs arise from the epithelium of the proximal tubule epithelium. In contrast, chromophobe, CD and medullary RCC subtypes are believed to arise from the distal nephrons.28,29 We presume that the selective expression of TRPV1 observed in normal renal tubules might also be present in the various origins of RCC and lead to different expression in the different RCC histopathological subtypes.
Decreased TRPV1 expression was observed in the RCC tissues, which was correlated with RCC Fuhrman grade. Different expression levels were detected among the different histopathological subtypes of RCC that arise from different origins. These findings likely suggest that decreased TRPV1 expression may be associated with cell differentiation and acts as a valuable tool to predict the extent of RCC progression. Thus, further studies are necessary to identify the mechanisms regulating TRPV1 expression in RCC and its relationship to cell proliferation and differentiation.
Conclusion
TRPV1 expression was decreased in RCC, which was significantly associated with tumor histopathological subtypes and Fuhrman grades. It seems to suggest that TRPV1 expression may be a valuable tool to predict the extent of RCC progression.
Acknowledgment
This study was funded by the National Natural Science Foundation of China (No. 81372734), the Training Foundation of Fujian Provincial Health Family Planning Commission for the Young and Middle-aged Core Talent (2014-ZQN-ZD-30), the Natural Science Foundation of Fujian Province (No. 2014J01440) and the Special Fund of Fujian Medical University for Scientific and Technological Development (No. FZS13035Y).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Monteith G, McAndrew D, Faddy H, Roberts-Thomson S. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer. 2007;7(7):519–530. | ||
Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11(8):609–618. | ||
Vanden Abeele F, Shuba Y, Roudbaraki M, et al. Store-operated Ca2+ channels in prostate cancer epithelial cells: function, regulation, and role in carcinogenesis. Cell Calcium. 2003;33(5–6):357–373. | ||
de Jong PR, Takahashi N, Harris AR, et al. Ion channel TRPV1-dependent activation of PTP1B suppresses EGFR-associated intestinal tumorigenesis. J Clin Invest. 2014;124(9):3793–3806. | ||
Wu Y, Miyamoto T, Li K, et al. Decreased expression of the epithelial Ca2+ channel TRPV5 and TRPV6 in human renal cell carcinoma associated with vitamin D receptor. J Urol. 2011;186(6):2419–2425. | ||
Bley K, Boorman G, Mohammad B, McKenzie D, Babbar S. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol Pathol. 2012;40(6):847–873. | ||
Morelli MB, Amantini C, Nabissi M, et al. Cross-talk between alpha1D-adrenoceptors and transient receptor potential vanilloid type 1 triggers prostate cancer cell proliferation. BMC Cancer. 2014;14:921. | ||
Wu TT, Peters AA, Tan PT, Roberts-Thomson SJ, Monteith GR. Consequences of activating the calcium-permeable ion channel TRPV1 in breast cancer cells with regulated TRPV1 expression. Cell Calcium. 2014;56(2):59–67. | ||
Kalogris C, Caprodossi S, Amantini C, et al. Expression of transient receptor potential vanilloid-1 (TRPV1) in urothelial cancers of human bladder: relation to clinicopathological and molecular parameters. Histopathology. 2010;57(5):744–752. | ||
Miao X, Liu G, Xu X, et al. High expression of vanilloid receptor-1 is associated with better prognosis of patients with hepatocellular carcinoma. Cancer Genet Cytogenet. 2008;186(1):25–32. | ||
Lazzeri M, Vannucchi M, Spinelli M, et al. Transient receptor potential vanilloid type 1 (TRPV1) expression changes from normal urothelium to transitional cell carcinoma of human bladder. Eur Urol. 2005;48(4):691–698. | ||
Mori A, Lehmann S, O’Kelly J, et al. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006;66(6):3222–3229. | ||
Ito K, Nakazato T, Yamato K, et al. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004;64(3):1071–1078. | ||
Amantini C, Ballarini P, Caprodossi S, et al. Triggering of transient receptor potential vanilloid type 1 (TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of urothelial cancer cells in an ATM-dependent manner. Carcinogenesis. 2009;30(8):1320–1329. | ||
Caprodossi S, Amantini C, Nabissi M, et al. Capsaicin (CPS) promotes a more aggressive gene expression phenotype and invasiveness in null-TRPV1 urothelial cancer cells. Carcinogenesis. 2011;32(5):686–694. | ||
Bode A, Cho Y, Zheng D, et al. Transient receptor potential type vanilloid 1 suppresses skin carcinogenesis. Cancer Res. 2009;69(3):905–913. | ||
Hwang M, Bode A, Byun S, et al. Cocarcinogenic effect of capsaicin involves activation of EGFR signaling but not TRPV1. Cancer Res. 2010;70(17):6859–6869. | ||
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. | ||
Chen L, Markó L, Kaßmann M, Zhu Y, Wu K, Gollasch M. Role of TRPV1 channels in ischemia/reperfusion-induced acute kidney injury. PLoS One. 2014;9(10):e109842. | ||
Tsagogiorgas C, Wedel J, Hottenrott M, et al. N-octanoyl-dopamine is an agonist at the capsaicin receptor TRPV1 and mitigates ischemia-induced acute kidney injury in rat. PLoS One. 2012;7(8):e43525. | ||
Barajas L, Liu L, Powers K. Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can J Physiol Pharmacol. 1992;70(5):735–749. | ||
Ditting T, Freisinger W, Siegel K, et al. Tonic postganglionic sympathetic inhibition induced by afferent renal nerves? Hypertension. 2012;59(2):467–476. | ||
Vanden Abeele F, Skryma R, Shuba Y, et al. Bcl-2-dependent modulation of Ca(2+) homeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 2002;1(2):169–179. | ||
Vanoverberghe K, Vanden Abeele F, Mariot P, et al. Ca2+ homeostasis and apoptotic resistance of neuroendocrine-differentiated prostate cancer cells. Cell Death Differ. 2004;11(3):321–330. | ||
Rizzuto R, Pinton P, Ferrari D, et al. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22(53):8619–8627. | ||
Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13(8):1409–1418. | ||
Orsola A, Trias I, Raventos CX, Espanol I, Cecchini L, Orsola I. Renal collecting (Bellini) duct carcinoma displays similar characteristics to upper tract urothelial cell carcinoma. Urology. 2005;65(1):49–54. | ||
Polascik TJ, Bostwick DG, Cairns P. Molecular genetics and histopathologic features of adult distal nephron tumors. Urology. 2002;60(6):941–946. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.