Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Decline in Working Memory in Stable Schizophrenia May Be Related to Attentional Impairment: Mediating Effects of Negative Symptoms, a Cross-Sectional Study
Authors Du N, Meng X, Li J, Shi L, Zhang X
Received 3 November 2023
Accepted for publication 14 January 2024
Published 23 January 2024 Volume 2024:20 Pages 149—158
DOI https://doi.org/10.2147/NDT.S447965
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Nan Du,1,2 Xiaojing Meng,1,2 Jingwei Li,2 Li Shi,2 Xulai Zhang1,2
1Affiliated Psychological Hospital of Anhui Medical University, Hefei Fourth People’s Hospital, Hefei, 230022, People’s Republic of China; 2Anhui Clinical Center for Mental and Psychological Diseases, Hefei Fourth People’s Hospital, Hefei, 230022, People’s Republic of China
Correspondence: Xulai Zhang, Hefei Fourth People’s Hospital, 316 Mei shan Road, Hefei, Anhui, 230032, People’s Republic of China, Email [email protected]
Background: Schizophrenia (SCZ) is a severe mental illness, Cognitive deficits and negative symptoms (NS) are prevalent in individuals with SCZ and are crucial indicators of functional recovery. It is well known that cognitive symptoms and negative symptoms are interrelated and that negative symptoms can affect the ability to take cognitive tests. However, the specific relationship between attention, working memory (WM), and NS in stable SCZ remains unclear. This study aims to explore these associations and provide valuable insights for the subsequent treatment of SCZ.
Methods: We conducted a comprehensive assessment of 145 patients with stable SCZ using the Chinese Brief Neurocognitive Suite of Tests (C-BCT) and the Positive and Negative Symptom Scale (PANSS).
Results: Patients with abnormal cognition exhibited significantly higher PANSS total scores, cognitive symptom scores, and NS than those with normal cognition (P< 0.05). Pearson’s correlation analysis revealed significant positive correlations between digital breadth(DB) and continuous operation(CO) (r=0.389, P< 0.001), as well as a significant negative correlation between DB and NS (r=− 0.291, P< 0.001). Moreover, CO showed a negative correlation with NS (r=− 0.173, P< 0.05). However, no significant correlations were found between the digital breadth-anterograde score and CO or NS (r=0.148, P> 0.05; r=− 0.068, P> 0.05). Notably, NS were identified as a mediator in the relationship between attention and WM (effect size=0.024).
Conclusion: Our findings highlight significant associations between WM, attention, and NS in individuals with stable SCZ. Moreover, attention not only directly impacts WM but also indirectly influences it through NS. Addressing cognitive deficits and NS in the treatment of SCZ may lead to improved overall outcomes for affected individuals.
Keywords: stable schizophrenia, working memory, attention, negative symptom, intermediary effect
Introduction
Schizophrenia (SCZ), one of the schizophrenia spectrum disorders, is a severe mental disorder of undetermined etiology and cognitive-emotional dysregulation, with the poorest prognosis of all mental disorders,1 even affecting the patient’s whole life, and the burden of disease for SCZ is one of the highest of all disorders.2 Cognitive deficits are prominent and treatment-resistant symptoms of SCZ and serve as prominent indices of patient functioning and treatment outcomes.3 Numerous studies have demonstrated measurable cognitive deficits in individuals with SCZ, including impairments in working memory (WM), attention, information processing speed, and executive functioning.4 Notably, in severe mental illness, WM performance has emerged as the most robust predictor of functional outcomes.5,6 Moreover, attention and WM are closely intertwined,7,8 and individual differences in these abilities are dependent on their interrelated variations.9 Lapses in WM performance have been associated with differences in individuals’ attention10, wherein low-ability individuals exhibit more frequent mind-wandering, particularly during cognitively demanding tasks, and this phenomenon demonstrates synchronized behavior.7
The negative symptoms (NS) of SCZ, such as emotional flattening, social withdrawal, lack of pleasure, apathy, and reduced emotional expression, significantly impair social functioning.1 The underlying mechanisms are complex and may involve reduced gray matter volume in the prefrontal, medial, and superior temporal lobes.11 Dysregulation of multiple neurotransmitters, especially dopamine release, plays a pivotal role in these pathologic changes.12 Furthermore, these alterations are closely associated with impairments in executive function, attention, and WM.13 Previous research has demonstrated that decrement in motor and cognitive speed plays a significant role in both the verbal WM impairment observed in patients and the associations between verbal WM impairment and clinical symptoms and is associated with NS.14 Another study showed that clinical symptoms, mainly NS, mediate the influence of neurocognition and social cognition on functional outcome of SCZ.15 Interestingly, we observed that medication showed limited effectiveness in alleviating negative and cognitive symptoms. Thus, it is imperative to investigate the relationship between these factors to better manage symptom progression.
The main objective of this study was to explore the relationship between WM, attention, and NS using a mediation model. We hypothesized that attention influences WM, and that NS may mediate the association between attention and WM. The inclusion of a mediation model will help elucidate the intricate interactions among these cognitive and symptomatic aspects of SCZ, paving the way for more targeted and effective interventions.
Materials and Methods
Subjects
In this study, 145 patients with stable SCZ who were hospitalised at Anhui Mental Health Centre from June 2022 to June 2023 were selected for this study; participants were recruited during their hospitalisation and all participants and guardians gave informed consent.
To be eligible for the study, patients needed to meet the following criteria: Meets DSM-V SCZ diagnostic criteria (schizophrenic patients only); Symptomatic stabilizers: clinically stable for ≥3 months, with P1, P2, P3, and P6 items ≤3 on the PANSS scale, and taking regular antipsychotic single-agent therapy and remaining stable for ≥3 months; be between 18 and 60 years old; Obtain informed consent, agree to take cognitive function tests, be able to understand and read Chinese, understand the test program, be able to understand the voice introduction, and be able to operate a simple iPad.
The size of the study sample was estimated using the G*Power 3.1.9 program. The power analysis was conducted using an alpha of 0.05, a power (1-β) of 0.80, and a medium effect size of 0.50 for a two-tailed test. The desired total sample size for detecting differences between two groups was 128. A total of 180 inpatients were screened, of whom 35 were excluded for the following reasons: Alcohol or substance dependence in the past six months (n =7); Significant clinical neurologic disorders; head trauma (loss of consciousness for more than an hour), Parkinson’s disease, dementia (n =14); Pregnant or breastfeeding women (n =6); Presence of a family history of mental retardation (n =1); Combined other mental disorders (n =2); Their level of visual or auditory functioning affects the test (n =5).(shown in Figure 1). Finally, a total of 145 individuals were included in this study.
 |
Figure 1 Flow chart. |
We collected general clinical data on the day of admission from SCZ patients who met the inclusion criteria, which included data on gender, age, height, weight, body mass index (BMI), duration of illness, and years of education, and calculated chlorpromazine equivalent.16,17 We used a 5-factor model of the PANSS scale to assess clinical symptoms, including cognitive factors (P2, N5, N7, G10, G11), arousal factors (P4, P7, G4, G8, G14), depression factors (G2, G3, G6), negativity factors (N1-N7) and positivity factors (P1-P7). Rated by 2 consistency-trained psychiatric clinicians, the higher the symptom score, the more severe the symptom. Cognitive functions of SCZ patients were assessed using the C-BCT. C-BCT is a localized cognitive assessment tool for SCZ adapted for China.18 The target patients are those who have cognitive impairment and those who have been treated with medication to observe the effects of the treatment. A total of four tests are included, including the connectivity test, symbol encoding, continuous operation, and digital breadth. The patients were tested for information processing speed, executive function, attention, and WM. Each measure shows its T score, deficit, degree of impairment, and percentile (%) in the Chinese test population as a means of assessing the patient’s cognitive function. In addition to the four tests, there is a total score T-score, deficit, degree of impairment, and percentile (%) in the Chinese test population. This is a composite score of several domains, also including attention and working memory.
We grouped them according to the degree of impairment in the total score, classifying those with relatively normal impairment into the cognitively normal group (not up to the level of the healthy population) and the others into the cognitively abnormal group.
The study was reviewed and approved by the Medical Ethics Committee of the Affiliated Psychological Hospital of Anhui Medical University (Ethics No. HSY-IRB-PJ-JZB-001), and the participants clearly understood the purpose of the study and signed an informed consent form.
Research Design
This study used a cross-sectional design to investigate the relationship between WM and attention in patients with stable SCZ and to analyse their relationship with NS. We categorised them into cognitively normal (CN) and cognitively abnormal (N-CN) groups by the impairment scores in the total score component of the results of the Chinese Brief Neurocognitive Test (C-BCT). We compared whether the two groups differed in general demographic information and scores on each item of the PANSS scale; analysed whether WM, attention and NS were correlated; and performed mediation analyses.
Data Analysis
Statistical analyses were performed using SPSS 26.0, first checking whether the measured data were characterized by a normal distribution as well as consistency of variance. Normal distribution was represented by (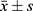 ) and skewed distribution was represented by M (P25, P75). General data such as age, height, weight, body mass (BMI), duration of disease, years of education, and chlorpromazine equivalents were analyzed using the independent samples t-test, while gender was studied using the chi-square test. Comparisons of 2 groups of measured data that conformed to a normal distribution were performed using 2 independent samples t-test; Pearson’s correlation analysis was used in this study to explore the correlation between the variables; the difference was considered statistically significant at P<0.05. For the mediation analysis, attention (Continuous Operation, CO) was set as the independent variable and WM (Digital Breadth, DB) as the dependent variable. The mediating effect of the model was explored using the coefficient of variation test.
) and skewed distribution was represented by M (P25, P75). General data such as age, height, weight, body mass (BMI), duration of disease, years of education, and chlorpromazine equivalents were analyzed using the independent samples t-test, while gender was studied using the chi-square test. Comparisons of 2 groups of measured data that conformed to a normal distribution were performed using 2 independent samples t-test; Pearson’s correlation analysis was used in this study to explore the correlation between the variables; the difference was considered statistically significant at P<0.05. For the mediation analysis, attention (Continuous Operation, CO) was set as the independent variable and WM (Digital Breadth, DB) as the dependent variable. The mediating effect of the model was explored using the coefficient of variation test.
Results
General Demographic Information and Analysis of Variance
A total of 145 patients with stable SCZ were included in this study. Based on the results of the C-BCT, 68 patients exhibited normal cognition(NC), while 77 patients had abnormal cognition (N-NC). Importantly, there were no significant differences in sex, age, body mass index (BMI), illness duration, years of education, or chlorpromazine equivalents between the two groups (P>0.05). However, the PANSS total score, NS scores, and cognitive symptom scores showed statistically significant differences between the groups (P<0.05). In addition, we compared the cognitive scores and negative symptom scores of men and women separately, and the results showed that there were no significant differences, except for a significant difference in the negative symptom scores (P<0.05). For detailed information, refer to Table 1.
 |
Table 1 Comparison of General Demographic Information and PANSS Scores of CN and N-CN |
A Study of Relevance
The associations between WM and attention and NS in schizophrenic patients are summarized in Table 2. WM can be represented by DB, digital breadth-retrograde score (DB-RS), digital breadth-anterograde score (DB-AS), and attention can be represented by CO. According to Pearson correlation analysis, DB became significantly positively correlated with CO (r=0.389, p<0.001) and negatively correlated with NS (r=−0.291, p<0.001). CO was found to be negatively correlated with NS (r=−0.173, p<0.05). However, DB-AS were not significantly correlated with CO and NS (r=0.148, p>0.05; r=−0.068, p>0.05).
 |
Table 2 Correlations Between WM and Attention and NS in Patients with SCZ |
NS Mediate the Relationship Between WM and Attention Associations
Based on the correlation analyses described above, mediating effects were examined using the SPSS macro program PROCESS developed by Hayes.19 A mediation model based on NS was constructed by creating a structural equation with CO as the independent variable in the model 4 condition (Table 3 and Table 4). In Step 1, regression analyses revealed an estimated effect size of 0.229 (P < 0.05) for CO on DB. In Step 2, the regression coefficients were standardized, and the estimated effect size of CO on DB was 0.205 (P < 0.05). A difference-in-difference coefficient test indicated that NS mediated the association between CO and DB, with an effect size of 0.024 (Figure 2). Thus, it appears that CO scores not only directly influence DB scores but also have an indirect effect through NS.
 |
Table 3 Mediating Effects of NS on the Relationship Between CO and DB |
 |
Table 4 Total, Direct, and Indirect Effects of NS on the Relationship Between CO and DB |
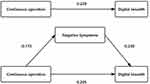 |
Figure 2 Mediator model of NS in patients with SCZ. |
Discussion
The primary objective of this study was to explore the intricate relationship between attention, WM, and NS in stable SCZ. Our findings revealed a strong association between attention, as assessed by the C-BCT, and WM in patients with stable SCZ. Moreover, we observed that NS, as evaluated using the PANSS scale, were also closely related to and mediated the association between attention and WM. This suggests that impaired attention directly or indirectly contributes to a decline in WM through NS. These significant findings offer a valuable theoretical basis for advancing the treatment and prevention of cognitive impairment in SCZ.
Historically, the treatment of SCZ has primarily focused on managing hallucinations and delusions, while negative and cognitive symptoms have proven more challenging to address than positive symptoms.20,21 However, numerous previous studies have underscored that negative and cognitive symptoms serve as the most robust predictors of functional outcomes in SCZ.22–24 Consequently, targeted improvement of patients’ negative symptoms may lead to concurrent enhancements in cognitive impairment symptoms. Our study corroborates these previous findings, as we observed that patients with SCZ exhibiting notable cognitive abnormalities had higher PANSS scale scores and elevated NS scores than those with normal cognition. This finding aligns with previous research, indicating that patients with higher levels of NS exhibit reduced engagement and face greater difficulty in completing cognitive tests. In addition, we briefly compared the cognitive and negative symptoms of men and women due to gender differences in the clinical manifestations of schizophrenia patients, and the results showed that the cognitive and negative symptoms were milder in women compared to men, which may be related to oestrogen,25 and that the cognitive symptoms of women also differed according to the time of month and the reproductive period, which remains to be further investigated.26 Overall, these results further support the association between NS and cognitive impairment in individuals with SCZ.27,28
A significant negative correlation has been reported between NS and prior cognitive functioning, and this correlation has been associated with poorer daily functioning29–32. However, there is limited research exploring how NS specifically impact cognitive functioning subfields.33 In light of this, our study aimed to examine the relationship between NS, attention, and WM. We found that NS were negatively correlated with attention and WM, while attention and WM showed a positive correlation. These findings suggest that targeting NS may lead to improvements in attention and WM, thereby benefiting overall cognition. Neurobiological evidence further supports the connection between NS and executive/attentional dysfunction in SCZ, involving biochemical, structural, and functional abnormalities in the frontal cortex and related subcortical circuits.34–36 Additionally, significant negative correlations were observed between intrinsic hippocampal activity in SCZ, a broad range of cognitive measures, and its association with NS.37 NS and cognitions in schizophrenia have always been a challenge to treat. Considering that medications often show limited effectiveness in treating NS and cognitive deficits in SCZ, which substantially impact patients’ quality of life.38 There are also no pharmacological therapies currently approved specifically for the treatment of negative symptoms,39 and advances in conceptualisation and evaluation have not yet been fully reflected in treatment studies,40 many of which have found that cognitive remediation can produce meaningful benefits in terms of cognition and function.41,42
The present study aimed to construct a mediation model using attention (CO) and WM(DB), wherein NS scores play a mediating role. Specifically, we found that attention indirectly or directly influences WM by affecting NS. As argued by Brazo,35 primary and secondary NS may be linked to varying degrees of executive/attentional dysfunction. In our study, we explored this relationship and aimed to contribute to future cognitive improvements by addressing NS. Lam43 suggested that social cognition mediates the relationship between neurocognition and symptoms, and neurocognitive deficits predispose individuals with SCZ to more severe psychiatric symptoms through impairments in social cognition. However, in our study, we focused on subdividing cognitive functioning into attention and WM rather than social cognition. This implies that attention and working memory deficits may adversely affect social cognition and thus social functioning.44 Combined neurological and social cognitive treatments may reveal synergistic effects and be integral to creating and sustaining change in the domain of functional outcomes.6 To achieve adequate functional outcomes, basic attention and working memory need to be restored first.
Several limitations of the present study are worth noting. Firstly, it is important to emphasise that the mediation analyses of attention, working memory and neurological symptoms were conducted based on cross-sectional data and did not follow up the enrolled patients, thus making it impossible to address the question of which came first. Nevertheless, this study represents a crucial initial step toward longitudinal and more comprehensive research. Second, the present study serves as a preliminary investigation and did not delve into subdivisions of cognitive functions, such as neurocognitive and social cognition, verbal and spatial WM, and even sub-programs of NS. Future studies should explore these aspects in greater depth. In addition, the measurement tool used in this study was the C-BCT, which may have introduced biases in the measurement process, such as subjects’ familiarity with the Chinese zodiac sign being variable as the stimulus material was turned into an animal, which is an inherent limitation that should be addressed in future studies. Moreover, cognitive functioning in SCZ is influenced by various complex factors, and in this study, we focused solely on the relationship between attention and WM. To gain a comprehensive understanding of the effects of cognition and symptoms in SCZ, as well as depression and other disorders, further exploration of the mechanisms and influencing factors of cognitive decline and symptom onset is warranted. Such research will provide essential theoretical support for identifying potent drug targets and developing effective behavioral interventions in the future.
Conclusion
Based on our investigation of the relationship between cognitive functioning and NS, we found that both attention deficits and NS significantly contribute to WM decline. In addition, noteworthy studies have shown that in patients with SCZ, attention deficits affect NS, thereby directly or indirectly exacerbating the decline in WM. This suggests that effectively addressing NS could lead to more comprehensive treatment of cognitive deficits. For example, aerobic exercise45,46 and cognitive remediation, among others, hold promise for treating both cognitive deficits and NS in SCZ, making them a potentially valuable therapeutic intervention. However, this is based on a cross-sectional study and also lays the groundwork for future longitudinal studies.
Abbreviations
SCZ, Schizophrenia; WM, working memory; NS, negative symptoms; PANSS, the Positive and Negative Symptom Scale; CN, cognitively normal group; N-CN, cognitively abnormal group; C-BCT, the Chinese Brief Neurocognitive Compact Test; DB-AS, Digital breadth-anterograde score; DB-RS, Digital breadth-retrograde score; DB, Digital breadth; CO, Continuous operation.
Data Sharing Statement
The data that support the findings of this study are available from Hefei Fourth People’ Hospital but restrictions apply to the availability of those data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Hefei Fourth People’ Hospital. To obtain the data in this study, the researchers may be contacted at [email protected].
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of The Anhui Mental Health Centre. Informed consent was obtained from all the subjects. The trial registration number was HSY-IRB-PJ-JZB-001. All procedures carried out in studies conformed to the 1964 Helsinki Declaration and its subsequent amendments or similar ethical standards.
Acknowledgments
We would like to thank the support of Hefei Fourth People’s Hospital.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Funding this work was supported by the Scientific and technological research project of Anhui Provincial Science and Technology Department (201904a07020009).
Disclosure
All authors declare no conflict of interest.
References
1. Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. 2022;399(10323):473–486. doi:10.1016/S0140-6736(21)01730-X
2. Collaborators GBDMD. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–150. doi:10.1016/S2215-0366(21)00395-3
3. Bora E, Yucel M, Pantelis C. Cognitive impairment in schizophrenia and affective psychoses: implications for DSM-V criteria and beyond. Schizophr Bull. 2010;36(1):36–42.
4. Guo JY, Ragland JD, Carter CS. Memory and cognition in schizophrenia. Mol Psychiatry. 2019;24(5):633–642.
5. Mahmood Z, Burton CZ, Vella L, Twamley EW. Neuropsychological predictors of performance-based measures of functional capacity and social skills in individuals with severe mental illness. J Psychiatr Res. 2018;102:201–206. doi:10.1016/j.jpsychires.2018.04.011
6. Halverson TF, Orleans-Pobee M, Merritt C, Sheeran P, Fett AK, Penn DL. Pathways to functional outcomes in schizophrenia spectrum disorders: meta-analysis of social cognitive and neurocognitive predictors. Neurosci Biobehav Rev. 2019;105:212–219. doi:10.1016/j.neubiorev.2019.07.020
7. deBettencourt MT, Keene PA, Awh E, Vogel EK. Real-time triggering reveals concurrent lapses of attention and working memory. Nat Hum Behav. 2019;3(8):808–816. doi:10.1038/s41562-019-0606-6
8. Oberauer K. Working memory and attention - a conceptual analysis and review. J Cogn. 2019;2(1):36. doi:10.5334/joc.58
9. Adam KC, Mance I, Fukuda K, Vogel EK. The contribution of attentional lapses to individual differences in visual working memory capacity. J Cogn Neurosci. 2015;27(8):1601–1616. doi:10.1162/jocn_a_00811
10. Mrazek MD, Smallwood J, Franklin MS, Chin JM, Baird B, Schooler JW. The role of mind-wandering in measurements of general aptitude. J Exp Psychol Gen. 2012;141(4):788–798. doi:10.1037/a0027968
11. Karlsgodt KH, Sun D, Cannon TD. Structural and functional brain abnormalities in schizophrenia. Curr Dir Psychol Sci. 2010;19(4):226–231. doi:10.1177/0963721410377601
12. Chuhma N, Mingote S, Kalmbach A, Yetnikoff L, Rayport S. Heterogeneity in dopamine neuron synaptic actions across the striatum and its relevance for schizophrenia. Biol Psychiatry. 2017;81(1):43–51. doi:10.1016/j.biopsych.2016.07.002
13. Luvsannyam E, Jain MS, Pormento MKL, et al. Neurobiology of Schizophrenia: a comprehensive review. Cureus. 2022;14(4):1–7.
14. Brebion G, Stephan-Otto C, Huerta-Ramos E, et al. Decreased processing speed might account for working memory span deficit in schizophrenia, and might mediate the associations between working memory span and clinical symptoms. Eur Psychiatry. 2014;29(8):473–478. doi:10.1016/j.eurpsy.2014.02.009
15. Lin CH, Huang CL, Chang YC, et al. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr Res. 2013;146(1–3):231–237. doi:10.1016/j.schres.2013.02.009
16. Leucht S, Samara M, Heres S, Davis JM. Dose Equivalents for Antipsychotic Drugs: the DDD Method. Schizophr Bull. 2016;42(Suppl 1):S90–S94. doi:10.1093/schbul/sbv167
17. Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: comparing apples and oranges! Indian J Psychiatry. 2013;55(2):207–208. doi:10.4103/0019-5545.111475
18. Ye S, Xie M, Yu X, et al. The Chinese Brief Cognitive Test: normative data stratified by gender, age and education. Front Psychiatry. 2022;13:933642. doi:10.3389/fpsyt.2022.933642
19. Hayes AF, Rockwood NJ. Regression-based statistical mediation and moderation analysis in clinical research: observations, recommendations, and implementation. Behav Res Ther. 2017;98:39–57.
20. Fakra E, Belzeaux R, Azorin JM, Adida M. Symptômes négatifs, émotions et cognition dans la schizophrénie Negative symptoms, emotion and cognition in schizophrenia [Negative symptoms, emotion and cognition in schizophrenia]. Encephale. 2015;41(6 Suppl 1):6S18–6S21. French. doi:10.1016/S0013-7006(16)30005-7
21. Kokurcan A. Comparison of clinical characteristics between the patients with schizophrenia on clozapine treatment with those taking combination of long-acting injectable and oral antipsychotics. Noro Psikiyatr Ars. 2019;56(3):219–223. doi:10.29399/npa.23548
22. Charernboon T. Interplay among positive and negative symptoms, neurocognition, social cognition, and functioning in clinically stable patients with schizophrenia: a network analysis. F1000Res. 2021;10:1258. doi:10.12688/f1000research.74385.3
23. Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573–588. doi:10.1016/j.neubiorev.2010.07.001
24. Ucok A, Direk N, Kaya H, et al. Relationship of negative symptom severity with cognitive symptoms and functioning in subjects at ultra-high risk for psychosis. Early Interv Psychiatry. 2021;15(4):966–974. doi:10.1111/eip.13042
25. Riecher-Rossler A. Sex and gender differences in mental disorders. Lancet Psychiatry. 2017;4(1):8–9. doi:10.1016/S2215-0366(16)30348-0
26. Rubin LH, Carter CS, Drogos LL, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Effects of sex, menstrual cycle phase, and endogenous hormones on cognition in schizophrenia. Schizophr Res. 2015;166(1–3):269–275. doi:10.1016/j.schres.2015.04.039
27. Addington J, Addington D, Maticka-Tyndale E. Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr Res. 1991;5(2):123–134. doi:10.1016/0920-9964(91)90039-T
28. Zouraraki C, Tsaousis I, Karamaouna P, et al. Associations of differential schizotypal dimensions with executive working memory: a moderated-mediation analysis. Compr Psychiatry. 2016;71:39–48. doi:10.1016/j.comppsych.2016.08.010
29. Gerritsen C, Maheandiran M, Lepock J, et al. Negative symptoms in the clinical high-risk state for psychosis: connection with cognition and primacy in impacting functioning. Early Interv Psychiatry. 2020;14(2):188–195. doi:10.1111/eip.12843
30. Garcia-Lopez M, Alonso-Sanchez M, Leal I, et al. The relationship between negative symptoms, social cognition, and social functioning in patients with first episode psychosis. J Psychiatr Res. 2022;155:171–179. doi:10.1016/j.jpsychires.2022.08.004
31. Lepage M, Bodnar M, Raucher-Chene D, et al. Neurocognitive functions in persistent negative symptoms following a first episode of psychosis. Eur Neuropsychopharmacol. 2021;47:86–97. doi:10.1016/j.euroneuro.2021.02.008
32. Kokurcan A, Guriz SO, Karadag H, Erdi F, Orsel S. Treatment strategies in management of schizophrenia patients with persistent symptoms in daily practice: a retrospective study. Int J Psychiatry Clin Pract. 2021;25(3):238–244. doi:10.1080/13651501.2021.1879157
33. Au-Yeung C, Penney D, Rae J, Carling H, Lassman L, Lepage M. The relationship between negative symptoms and MATRICS neurocognitive domains: a meta-analysis and systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2023;127:110833. doi:10.1016/j.pnpbp.2023.110833
34. Thoma P, Daum I. Neurokognitive Veränderungen und Negativsymptomatik bei schizophrenen Erkrankungen [Neurocognitive changes and negative symptoms in schizophrenia]. Fortschr Neurol Psychiatr. 2005;73(6):333–342. German. doi:10.1055/s-2004-830233
35. Brazo P, Delamillieure P, Morello R, Halbecq I, Marie RM, Dollfus S. Impairments of executive/attentional functions in schizophrenia with primary and secondary negative symptoms. Psychiatry Res. 2005;133(1):45–55. doi:10.1016/j.psychres.2004.10.001
36. Dibben CR, Rice C, Laws K, McKenna PJ. Is executive impairment associated with schizophrenic syndromes? A meta-analysis. Psychol Med. 2009;39(3):381–392. doi:10.1017/S0033291708003887
37. Tregellas JR, Smucny J, Harris JG, et al. Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am J Psychiatry. 2014;171(5):549–556. doi:10.1176/appi.ajp.2013.13070981
38. Ercan Dogu S, Kokurcan A, Orsel S. An occupation-based healthy nutrition and wellness program for individuals with schizophrenia. OTJR. 2023;43(4):626–636. doi:10.1177/15394492231153113
39. Marder SR, Umbricht D. Negative symptoms in schizophrenia: newly emerging measurements, pathways, and treatments. Schizophr Res. 2023;258:71–77. doi:10.1016/j.schres.2023.07.010
40. Galderisi S, Kaiser S, Bitter I, et al. EPA guidance on treatment of negative symptoms in schizophrenia. Eur Psychiatry. 2021;64(1):e21.
41. Vita A, Barlati S, Ceraso A, et al. Effectiveness, core elements, and moderators of response of cognitive remediation for schizophrenia: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2021;78(8):848–858. doi:10.1001/jamapsychiatry.2021.0620
42. Lejeune JA, Northrop A, Kurtz MM. A meta-analysis of cognitive remediation for schizophrenia: efficacy and the role of participant and treatment factors. Schizophr Bull. 2021;47(4):997–1006. doi:10.1093/schbul/sbab022
43. Lam BY, Raine A, Lee TM. The relationship between neurocognition and symptomatology in people with schizophrenia: social cognition as the mediator. BMC Psychiatry. 2014;14:138. doi:10.1186/1471-244X-14-138
44. Schmidt SJ, Mueller DR, Roder V. Social cognition as a mediator variable between neurocognition and functional outcome in schizophrenia: empirical review and new results by structural equation modeling. Schizophr Bull. 2011;37(Suppl 2):S41–S54.
45. Firth J, Stubbs B, Rosenbaum S, et al. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43(3):546–556. doi:10.1093/schbul/sbw115
46. Shimada T, Ito S, Makabe A, Yamanushi A, Takenaka A, Kobayashi M. Aerobic exercise and cognitive functioning in schizophrenia: a pilot randomized controlled trial. Psychiatry Res. 2019;282:112638. doi:10.1016/j.psychres.2019.112638
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
