Back to Journals » Drug Design, Development and Therapy » Volume 17
Deciphering the Mechanism of Xijiao Dihuang Decoction in Treating Psoriasis by Network Pharmacology and Experimental Validation
Authors Guo Y , Gan H, Xu S, Zeng G, Xiao L, Ding Z, Zhu J, Xiong X, Fu Z
Received 19 May 2023
Accepted for publication 6 September 2023
Published 12 September 2023 Volume 2023:17 Pages 2805—2819
DOI https://doi.org/10.2147/DDDT.S417954
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Yicheng Guo,1 Huiqun Gan,1 Shigui Xu,1 Guosheng Zeng,2 Lili Xiao,2 Zhijun Ding,2 Jie Zhu,3 Xinglong Xiong,3 Zhiyuan Fu1
1Department of Pharmacy, Dermatology Hospital of Jiangxi Province, Nanchang, People’s Republic of China; 2Jiangxi Provincial Clinical Research Center for Skin Diseases, Nanchang, People’s Republic of China; 3Candidate Branch of National Clinical Research Center for Skin Diseases, Nanchang, People’s Republic of China
Correspondence: Zhiyuan Fu, Department of Pharmacy, Dermatology Hospital of Jiangxi Province, 388, Yinbing Road, Nanchang, Jiangxi, People’s Republic of China, Tel +86 13970827820, Email [email protected]
Purpose: This study aims to confirm the efficacy of Xijiao Dihuang decoction (XJDHT), a classic prescription, in treating psoriasis and to explore the potential therapeutic mechanism.
Methods: For pharmacodynamic analysis, a mouse model of imiquimod cream (IMQ)-induced psoriasis was constructed. Active ingredients and genes of XJDHT, as well as psoriasis-related targets, were obtained from public databases. Intersecting genes (IGEs) of XJDHT and psoriasis were collected by Venn Diagram. A protein–protein interaction (PPI) network of IGEs is constructed through the STRING database. The Molecular Complex Detection (MCODE) and Cytohubba plug-ins of Cytoscape software were used to identified hub genes. In addition, we conducted enrichment analysis of IGEs using the R package clusterProfiler. Hub genes were validated via external GEO databases. The influence of XJDHT on Hub gene expression was examined by qPCR and ELISA, and molecular docking was used to evaluate the binding efficacy between active ingredients and hub genes.
Results: The results revealed that XJDHT possesses 92 potential genes for psoriasis, and 8 Hub genes were screened. Enrichment analysis suggested that XJDHT ameliorate psoriasis through multiple pathways, including AGE-RAGE, HIF-1, IL-17 and TNF signaling pathway. Validation data confirmed the differential expression of IL6, VEGFA, TNF, MMP9, STAT3, and TLR4. Molecular docking revealed a strong affinity between active ingredients and Hub genes. The efficacy of XJDHT in improving psoriatic lesions in model mice was demonstrated by PASI score and HE staining, potentially attributed to the down-regulation of VEGFA, MMP9, STAT3, TNF, and IL-17A, as evidenced by ELISA and qPCR.
Conclusion: This study employed network pharmacology and in vitro experiments to identify the potential mechanisms underlying the therapeutic effects of XJDHT on psoriasis, providing a new theoretical basis for its clinical application in the treatment of psoriasis.
Keywords: psoriasis, Xijiao Dihuang decoction, network pharmacology, molecular docking, angiogenesis
Introduction
Psoriasis, a prevalent chronic inflammatory skin disease, is characterized by inflammation, hyperproliferation of the epidermis, abnormal epidermal maturation, and vascular changes. It afflicts approximately 125 million individuals worldwide.1–3 Patients with psoriasis may also suffer from metabolic diseases, such as dyslipidemia and hypertension, as well as an increased risk of cardiovascular disease and cancer.4–7 Despite extensive research, the pathogenesis of psoriasis remains elusive and may be driven by immune regulation and environmental factors. This presents significant challenges for effective treating, and currently, there are no curative approaches available. While glucocorticoids, immunosuppressants, and tretinoin have demonstrated specific efficacy, their adverse reactions and recurrence during treatment should be taken seriously. For instance, long-term use of methotrexate (MTX) can cause nausea, leukopenia, and elevated liver transaminases.8
With its systematic treatment methods and more than 2000 years of clinical experience, Traditional Chinese medicine (TCM) is widely used in the prevention and treatment of diseases.9 Meanwhile, TCM has a rich history and extensive experience in treating psoriasis. Xiao-yin-fang is a formula composed of 5 herbs, which has been shown to inhibit γδT17 cell polarization and play a role in improving the symptoms of psoriasis-like skin lesions.10 Radix Rehmanniae Recens is the most frequently used herb in the clinical treatment of psoriasis and treats psoriasis by reducing oxidative stress and activating the AMPK pathway.11,12 Indigo exerts psoriasis intervention by regulating cell proliferation, differentiation, and inflammatory response.13 Nevertheless, TCM, characterized by the use of compound formulations (Fu-Fang), is multicomponent and multitarget agents, making it difficult to complete pharmacological and toxicological studies. In this context, Hopkins proposed a method of “network pharmacology” based on the idea that multiple active ingredients act on multiple targets.14 Currently, with the development of high-throughput detection technology, network pharmacology provides a more efficient and convenient means for the systematic study of TCM prescriptions and complex diseases. Tao15 employed a network pharmacology approach to investigate the mechanism of action of Huashi Baidu Formula on COVID-19, and outcomes demonstrated that active ingredients of HSBDF had therapeutic benefits via regulating MAPK signaling route, TNF signaling pathway and PI3K-Akt signaling pathway through ACE2. Research on the mechanism of TCM treatment of psoriasis through network pharmacology has also been widely reported. By using network pharmacological analysis, researchers discovered that Jueyin granules upregulated VDR expression levels in IMQ-induced psoriasis models, which may be its mechanism for ameliorating psoriasis.16
Xijiao Dihuang decoction (XJDHT), a classic TCM formula of Beiji Qianjin Yaofang, consists of four herbs: Bubalus bubalis Linnaeus., Rehmannia glutinosa Libosch, Paeonia lactiflora PalL. and Paeonia suffruticosa Andr., which has the effect of clearing heat and removing toxins, cooling blood and dispersing blood stasis.17 While main clinical manifestation of psoriasis is blood heat and blood stasis, which coincides with the main therapeutic effect of XJDHT. However, the exact efficacy of XJDHT in the treatment of psoriasis has yet to be reported, and its underlying mechanism remains elusive.
In this research, we performed an IMQ-induced psoriasis-like mice model to evaluate the ameliorative effect of XJDHT on psoriasis. Subsequently, a network pharmacology method was employed to explore the pharmacological mechanism and validate by bioinformatics. Finally, the analysis was reconfirmed by molecular biology experiments.
Materials and Methods
Materials and Reagent
Shuiniujiao (Bubalus bubalis Linnaeus., 30g, Cat: 220803), Shengdihaung (Rehmannia glutinosa Libosch., 24g, Cat: 220616), Chishao (Paeonia lactiflora PalL, 12g, Cat: 220522) and Mudanpi (Paeonia suffruticosa Andr., 9g, Cat: 220911) were obtained from the Department of Pharmacy, Dermatology Hospital of Jiangxi Province. Imiquimod cream was purchased from Mingxin Pharmaceutical Co., LTD., (IMQ, Cat. no. 40210904, Sichuan, China); Methotrexate tablets (MTX, Cat. no. 210801, Xinyi Pharmaceutical Co., LTD., Shanghai, China); Hematoxylin dye liquor (Cat. no. ZLI-9610, Zhongshan Jinqiao Biotechnology Co., LTD., Beijing, China); Eosin dye liquor (Cat. no. G1100) and 50 TAE buffer (Cat. no. T1060) were purchased from Solaibao Technology Co., LTD. (Beijing, China); Mouse IL-17A (Cat. no. MM-0759M2), TNF-α (Cat. no. MM-0132M2) Enzyme-linked immunosorbent assay (ELISA) kit were purchased from Jiangsu Enzyme Free Industry Co., LTD. (Nanjing, China); Trizon Reagent (Cat. no. CW0580S) and ultrapure RNA extraction kit (Cat. no. CW0581M) were purchased from CWBIO (Beijing, China); HiScript II Q RT SuperMix for qPCR (+ gDNA wiper) and ChamQ Universal SYBR qPCR Master Mix were obtained from Novizan Biotechnology Co., LTD. (Nanjing, China); The mRNA primers were synthesized by General Biotech Co., LTD. (Chuzhou, Anhui).
Preparation of XJDHT Decoction Mixture
The XJDHT mixture was soaked in 10 volumes of water for 0.5 h and then extracted by reflux for 2 h. The aqueous extract was filtered, and the residue was refluxed again with 8 volumes of water for 1 h. The combined filtrates obtained were concentrated to a residue in a vacuum evaporator, yielding an extract concentration of 2.5 g/mL. The extract was then stored at −80 °C.
Animals
Twenty male BALB/C mice were obtained from Zhonghong Boyuan Biotechnology Co., Ltd (Cat. no. SYXK 2020-0001). Mice weighing 20–25 g were all housed in specific pathogen-free (SPF) facility. All animal experimental procedures followed the guidelines in the Laboratory Animals Manual of the NIH Guide for the Care and Use of Animals. This study was approved by the Dermatology Hospital of Jiangxi Province Animal Ethics Committee (NO.KY2021-02-01).
Construction of Psoriasis-Like Animals Model and XJDHT Administration
To construct a psoriasis-like mouse model, 50mg of 5% IMQ was applied topically to the shaved area (3×2.5cm) on the back of mice. The control group received a similar daily dose of vehicle Vaseline cream.
The BALB/C mice were randomly divided into four groups (n=5): control group (CON) and Model group (Model), mice only received distilled water orally daily; MTX-treated group (MTX), mice received 1 mg/kg of MTX orally daily; XJDHT group (XJDHT), mice were administered at 25.0 g/kg of XJDHT orally each day. On the final day animals were euthanized and the shaved back skin was immediately excised (Figure 1A).
Psoriasis Area and Severity Index (PASI) and Histopathological Examination
The severity of skin lesions in mice was assessed daily using the PASI scoring system and photographs were taken,18 PASI score items included erythema, scale and thickness of skin lesions, and the score range was 0 to 4:0, None; 1, Mild; 2, Moderate; 3, Severe; 4, Extremely severe. All the skin specimens were pre-fixed in 10% formalin, subsequently embedded in paraffin, sectioned, and stained with hematoxylin and eosin, then examined by microscope (BX43, Olympus, Tokyo, Japan). Image J software was used to calculate the average epidermal thickness at the lesion skin.
Collection of Compounds and Prediction of Putative Targets in XJDHT
The TCMSP database (https://old.tcmsp-e.com/tcmsp.php),19 PubChem database (https://pubchem.ncbi.nlm.nih.gov), STITCH database (http://stitch.embl.de)20 and Swiss Target Prediction database (http://www.swisstargetprediction.ch/)21 were used to identify active ingredient and potential targets of XJDHT.
Collection of Psoriasis-Associated Genes
Genes associated with psoriasis were obtained by searching keyword “psoriasis” in GeneCards (https://www.genecards.org/) and DisGeNET database (https://www.disgenet.org/).
Then, a Venny tool from the online data analysis website (http://bioinformatics.psb.ugent.be/webtools/Venn/) was used to collect the intersection genes (IGEs) of XJDHT and psoriasis.
Protein–Protein Interaction (PPI) Network
The PPI network of IGEs was constructed using the STRING online database (http://string-db.org) with a confidence score of 0.7, which provided the basis for the functional study of the proteome.22 Results and network data were imported into Cytoscape (version 3.7) for visualization. For hub gene identification of IGEs, the Molecular Complex Detection (MCODE) plug-in was used to identify the most important subclusters of strongly interacting nodes. In addition, the plug-in “CytoHubba” enabled us to predict the top 15 significant genes according to the maximal clique centrality (MCC) algorithm. Finally, genes of MCODE significant module and the significant genes predicted by CytoHubba were intersected to obtain the hub genes of XJDHT in the treatment of psoriasis.
GO and KEGG Enrichment Analyses
KEGG (Kyoto encyclopedia of genes and genomes) pathway and GO (Gene ontology) term enrichment were acquired using clusterProfiler (v3.6.3) R package.23 The screening criteria was P-adjust <0.01.
Expression of Hub Genes in Validation Dataset
The validating dataset GSE13355 (180 samples)24 was obtained from Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/), which was used to analyze the differential expression of hub genes. A total of 58 psoriasis lesions tissue samples (PP) and 58 non-lesions tissue samples (PN) within the database were selected for analysis.
Ingredient-Gene Molecular Docking
Molecular docking was used to verify the binding ability of the active ingredients and hub genes. The 3D structure of ingredients was downloaded from PubChem database and then imported into Chemdraw 3D. The MM2 module is used for energy minimization to obtain the lowest energy dominant conformation as a ligand. The protein structures were downloaded from UniProt database (https://www.uniprot.org/) as receptors, visualized separately using PYMOL, dehydrated, hydrogenated and charge calculated by Mgtools (version 1.5.6). Ligand and receptor were saved separately as pdbqt files. Subsequently, molecular docking was performed using Autodock vina (version 1.5.6), and the higher scoring conformations were visualized using PYMOL and Discovery studio.
Quantitative PCR
This experiment was conducted with reference to protocols and guidelines in the published literature. Total RNA was obtained from the skin tissue using TRIzol and purified using an Ultrapure RNA extraction kit. RNA was reverse transcribed to cDNA using the HiScript II Q RT SuperMix for qPCR, qPCR was performed with ChamQ Universal SYBR qPCR Master Mix. Sequences of the primers are presented in Table 1. β-actin was used to housekeep the expression levels of gene and the relative expression levels of hub genes were calculated using the 2−ΔΔCt method.
 |
Table 1 q-PCR Primer Sequences |
ELISA
The concentrations of TNF-ɑ and IL-17A were detected by ELISA. Use the corresponding ELISA kit, and testing was performed according to the specifications of kit manufacturer.
Results
Effects of XJDHT on Skin Lesion Status and PASI Score
During the experiment, the characteristics and health of the mice, such as food and water consumption and breathing patterns, were normal. The model mice began exhibiting erythema, scaling, and thickness 2 days after receiving IMQ treatment as compared to the control group, while XJDHT significantly improved these psoriatic skin lesions symptoms (Figure 1B). Additionally, XJDHT reduced the PASI score compared to the model group, and the outcomes were comparable to the MTX group (Figure 1C).
Effect of XJDHT on Histomorphology
HE staining showed that the epidermal layer of the skin in the control group was thin, and the cells in each layer were normal without lesions. The back skin of the model group showed the characteristic pathological changes of psoriasis, such as parakeratosis and hyperkeratosis, spinous layer hypertrophy, epidermal process elongation, and inflammatory cell infiltration. However, the pathological alterations were improved to varying degrees by the intervention of XJDHT and MTX (Figure 1D). In addition, the skin thickness was significantly reduced in the XJDHT group compared to model group (Figure 1E).
Targets Screening of XJDHT and Psoriasis
The active ingredients of XJDHT were screened from the TCMSP database with criteria oral bioavailability (OB) ≧30% and drug like (DL) ≧0.18, and the constructs of active components were converted to SDF and SMILES formats through the PubChem database. Then, the SDF and SMILES files were imported into the Swiss Target Prediction database with “probability” >0 and into the STITCH database with “score” >0.4, and the species was set to “Homo sapiens”. Combining the results of the above two databases, 280 XJDHT-related genes were obtained. Meanwhile, 1631 psoriasis-related genes were collected through GeneCards and DisGeNET databases. Finally, integrating the genes of XJDHT and psoriasis, 92 intersecting genes (IGEs) were considered as targets of XJDHT for the treatment of psoriasis (Figure 2A, Supplementary Table 1).
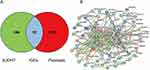 |
Figure 2 Analysis of the potential genes of XJDHT for the treatment of psoriasis. (A) Venn diagram of 92 intersecting genes. (B) Construction of a PPI network of IGEs. |
PPI Network Construction and Hub Gene Screening
A PPI network of IGEs was constructed through the STRING database, and the proteins that lack structure or interact with no other genes were eliminated. The PPI network consisted of 76 nodes and 363 edges (Figure 2B), and then interaction information of the PPI network imported to Cytoscape for visualization. The top 3 significant clusters (cluster 1, cluster 2 and cluster 3) were identified by subcluster analysis using the MCODE (Figure 3A–C). Additional, top 15 significant genes were identified by MCC algorithm of Cytoscape plug-in cytohubba (Figure 3D). Finally, based on the above data, 8 genes were identified as hub genes for XJDHT to treat psoriasis (Figure 3E, Table 2).
 |
Table 2 Information of the 8 Hub Genes |
Enrichment Analysis of IGEs
Functional enrichment analysis can further clarify the biological function of IGEs involved in the treatment of psoriasis with XJDHT. We enriched 1074 GO terms including 1007 biological processes (BP), 30 cell components (CC) and 37 molecular functions (MF) (Supplementary Table 2). The most significant terms of BP, CC and MF were visualized by histogram (Figure 4A). The BP terms demonstrated that IGEs were mostly involved in response to lipopolysaccharide, response to molecule of bacterial origin, response to nutrient levels, leukocyte cell–cell adhesion, etc. Additionally, the KEGG pathway enrichment analysis screened 82 significant entries, which suggested IGEs were mostly involved in AGE-RAGE signaling pathway in diabetic complications, HIF-1 signaling pathway, IL-17 signaling pathway and TNF signaling pathway, etc. (Supplementary Table 3). The top 10 terms pathways were presented as bubble charts (Figure 4B). Further, the top 10 pathways, ingredients, IGEs and Hub genes were selected to construct a “herb-ingredient-IGEs-pathway” network of XJDHT for psoriasis treatment by Cytoscape software (Figure 5). Rehmaionoside A, Rehmaionoside B, Albiflorin, Paeonoside, and Oxypaeoniflorin were considered as the key action ingredients with the highest degree values in the network (Table 3).
 |
Table 3 The Information About XJDHT Key Active Ingredients |
 |
Figure 4 GO and KEGG pathway enrichment analyses of IGEs. (A) GO term enrichment analysis. (B) KEGG pathway enrichment analysis. |
Differential Expression of the 8 Hub Genes
The limma package of R software was used to perform differential analysis on the validating GSE13355 dataset with |log FC|>1, P<0.05 as the filter conditions, and draw the volcano and heat map of the differential gene (Figure 6A and B). The results of differential expression analysis showed that compared with PN tissue, the expression levels of IL6, VEGFA, TNF, MMP9 and STAT3 were significantly increased in PP tissue (P<0.05), and the expression level of TLR4 decreased. Nevertheless, there were no significant differences in ALB and EGFR expression (P>0.05; Figure 6C).
Molecular Docking
We selected key action ingredients as ligand molecules, and IL6 (1ALU), VEGFA (1MKK), TLR4 (2Z62), TNF (2AZ5), MMP9 (6ESM) and STAT3 (6NUQ) as receptor proteins for molecular docking, then calculated binding energy and evaluated binding activity. Their binding energies to each other are shown in Figure 7A. The binding energy between TNF and Oxypaeoniflorin was −7.8 kcal/mol, indicating a very strong ability to bind (Figure 7B). The amino acid residues LEU187, ALA189, ALA191, HIS226, GLN227, VAL223, HIS230, HLS236 and TYR248 of MMP9 form hydrogen bonds with Oxypaeoniflorin with good affinity, and the binding energy is −8.7 kcal/mol (Figure 7C). The binding energy of STAT 3 and Albiflorin is −6.5 kcal/mol, and the interaction force is mainly on the hydrogen bonds (Figure 7D). The binding energy of VEGFA and Oxypaeoniflorin is −7.9 kcal/mol, and forms hydrogen bonds with amino acid residues TYR21, SER24, TYR25, CYS26, ALA104, LEU66, SER50, CYS60 and ASN62, respectively. In addition, hydrophobic functional groups in the active ingredient can form hydrophobic interactions with amino acid residues TYR25, ALA104, LEU66, and ASN62 (Figure 7E).
XJDHT Regulated the Expression of Hub Genes
The regulation of XJDHT on the expression of IL6, TNF-α, VEGFA, MMP9 and STAT3 was further clarified by animal experiments. As shown in Figure 8A, MMP9, STAS3 and VEGFA were elevated in model group compared with the control, while XJDHT and MTX reversed these tendencies. Meanwhile, compared with the control group, IL-6 and TNF were increased in model group, while relative to model rats, IL-6 and TNF were decreased by XJDHT as well as MTX group (Figure 8B). Angiogenesis is a significant aspect of psoriasis, and VEGFA is a crucial component of angiogenesis.25 We therefore sliced the dorsal skin of each mice group at the conclusion of the experiment to investigate the distribution of blood vessels. According to the findings, the vascular tortuosity and amount of neovascularization at the lesions in the IMQ model group significantly increased as compared to the control group; however, in the MTX and XJDHT groups, these alterations were reversed (Figure 8C).
Discussion
XJDHT is a classic Chinese medicine formula remedy with numerous clinical applications, including a notable improvement in sepsis, allergic purpura and psoriasis. In this work, the beneficial impact of XJDHT on the psoriasis-like lesion model mice has been confirmed. We investigated the active compounds, potential genes, and pathways of XJDHT for the treatment of psoriasis by network pharmacology theory and bioinformatics analytic methods. Through molecular docking and in vivo experiments, we validated our findings and gained insight into the molecular mechanisms of XJDHT in treating psoriasis. Overall, this work contributed to initially clarify the molecular mechanism of XJDHT for the treatment of psoriasis.
Through network analysis and molecular docking, 5 main active components of XJDHT, Rehmaionoside A, RehmaionosideB, Albiflorin, Paeonoside, Oxypaeoniflorin, were selected. Previous reports suggest that these five ingredients have significant pharmacological properties including alleviation of depression, inflammation, and sepsis.26–29 Therefore, we hypothesize that these 5 compounds may have an essential function in the treatment of psoriasis based on the interference with inflammation. This is similar to previous findings that XJDHT can reduce erlotinib-induced dermatitis by inhibiting the increase of inflammatory cytokines and chemokines.30
Through constructing a PPI network, we performed topological analysis of 92 IGEs and screened 8 hub genes by Cytohubba and MCODE plugins. We subsequently examined the expression level of hub genes GEO validation set, IL6, MMP9, STAT3, TNF and VEGFA were up-regulated in lesion samples, while TLR4 was down-regulated. Molecular docking is an important technique for drug discovery based on structural analysis of small molecules and proteins. VEGFA, STAT3, MMP9, and TNF were effectively bind to the active compounds (binding energy <-5) evaluate by molecular docking.
VEGFA is an essential factor in promoting angiogenesis, which is one of the pathological symptoms of psoriasis. Previous research has demonstrated that inhibiting VEGFA expression greatly improves immune infiltration, epidermal thickness, and angiogenesis in psoriasis.31,32 It has been found that XJDHT can affect neovascularization exerting neuroprotective effects against acute ischemic stroke.33 Our experiments also corroborate this idea that XJDHT reverses the increase in the number of neovascularization and vascular tortuosity in psoriasis models. MMP-9 is a proteolytic enzyme involved in wound healing, inflammatory responses, and cell mobilization. Jiaoling Chen reported that MMP9 influenced skin VEC (vessels within epithelial circle) vascular permeability and cytokine release via ERK=1/2 and MAPK signaling, whereas MMP9 suppression effectively reduced psoriatic-like symptoms in model mice.34 Besides, MMP-9 involve in angiogenesis of psoriasis by release VEGF, and stimulate the release of VEGF,35 suggesting that it has the potential to indirectly influence the angiogenesis of psoriasis. STAT3, a crucial regulator of the inflammatory response, has been linked to the pathological development of most diseases, an increasing number of studies have discovered that it is also intimately connected to the progression of psoriasis.36,37 By repeatedly phosphorylating STAT3, the researchers found that mice acquired psoriatic skin lesions; however, inhibition of STAT3 phosphorylation and activation revealed significant improvement in inflammatory skin lesions.38 The disease process of psoriasis is aggravated by STAT3 phosphorylation, which disrupts γδT cells and encourages the generation of IL-17 in the skin.39 Additionally, the amount of IL-21 activated by STAT3 promotes keratinocyte proliferation, which is related to the severity of psoriasis.40 TNF-α is an inflammatory cytokine with a proven role in the pathogenesis of psoriasis, and TNF-α inhibitors adalimumab, infliximab and etanercept are utilized extensively in the clinical treatment of psoriasis.41 More importantly, Hub genes were up-regulated in the skin tissues of psoriasis model mice during the in vivo experiments, while the XJDHT and MTX groups were able to reverse this upregulation, proving the validity of this study’s findings.
There are limitations in this study: firstly, the study only carried out in vivo experiments and not in vitro experimental studies, which could not fully reflect the pathologic changes in psoriasis. In addition, changes in the protein expression level of hun gene were not detected in this study. We will investigate the ameliorative effect of XJDHT on psoriasis at the cellular level in subsequent studies and examine the changes in the expression levels of the relevant proteins with various techniques.
Conclusions
Comprehensive network pharmacological analysis, molecular docking, and in vitro experiments indicate that the probable mechanism of XJDHT for the treatment of psoriasis involves the regulation of Hub genes VEGFA, MMP9, TNF, STAT3, and IL-17 signaling pathways, which influence the development of psoriasis angiogenesis and inflammation. These findings provide a theoretical foundation for the therapeutic application of XJDHT in the treatment of psoriasis.
Abbreviations
XJDHT, Xijiao Dihuang decoction; IMQ, Imiquimod; IGEs, Intersecting genes; PPI, Protein–protein interaction; MCODE, Molecular Complex Detection; KEGG, Kyoto Encyclopedia of Genes and Genomes; GO, Gene Ontology; GEO, Gene Expression Omnibus; MTX, methotrexate; TCM, traditional Chinese medicine; SPF, specific pathogen-free; PASI, Psoriasis area and severity index; MCC, maximal clique centrality; BP, biological process; CC, cellular component; MF, molecular function.
Institutional Review Board Statement
Animal care and experimentation were carried out strictly in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health, and the animal study was approved by the IRB of the Dermatology Hospital of Jiangxi Province (NO.KY2021-02-01). This paper is based on a study involving publicly available data, which is exempt from ethical review. It was approved by the IRB of the Dermatology Hospital of Jiangxi Province.
Acknowledgments
This study was supported by the Science and Technology program for Traditional Chinese Medicine of Jiangxi Province (2021A331); Science and Technology plan of Jiangxi province Health Commission (202311339); Scientific research project of Jiangxi Clinical Medical Research Center for Dermatology (20212BCG74003).
Disclosure
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Bai F, Li GG, Liu Q, Niu X, Li R, Ma H. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019. doi:10.1155/2019/2546161
2. Costa S, Marini O, Bevilacqua D, et al. Role of MyD88 signaling in the imiquimod‐induced mouse model of psoriasis: focus on innate myeloid cells. J Leukoc Biol. 2017;102(3). doi:10.1189/jlb.3ma0217-054rr
3. Gong X, Wang W. Profiles of innate immune cell infiltration and related core genes in psoriasis. Biomed Res Int. 2021;2021. doi:10.1155/2021/6656622
4. Ikeda K, Morizane S, Akagi T, et al. Obesity and dyslipidemia synergistically exacerbate psoriatic skin inflammation. Int J Mol Sci. 2022;23(8). doi:10.3390/ijms23084312
5. De Brandt E, Hillary T. Comorbid psoriasis and metabolic syndrome: clinical implications and optimal management. Psoriasis. 2022;12:113–126. doi:10.2147/PTT.S293107
6. Shapiro J, Cohen AD, Weitzman D, Tal R, David M. Psoriasis and cardiovascular risk factors: a case-control study on inpatients comparing psoriasis to dermatitis. J Am Acad Dermatol. 2012;66(2):252–258. doi:10.1016/j.jaad.2010.11.046
7. Vaengebjerg S, Skov L, Egeberg A, Loft ND. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156(4):421–429. doi:10.1001/jamadermatol.2020.0024
8. Van Lint JA, Bakker T, Ten Klooster PM, van Puijenbroek EP, Vonkeman HE, Jessurun NT. Neuropsychiatric adverse drug reactions associated with low dose methotrexate in rheumatoid arthritis patients. Expert Opin Drug Saf. 2022;21(3):417–423. doi:10.1080/14740338.2022.2003328
9. Yang S-P, Yue J-M. Discovery of structurally diverse and bioactive compounds from plant resources in China. Acta Pharmacol Sin. 2012;33(9):1147–1158. doi:10.1038/aps.2012.105
10. Zhang X, Li X, Chen Y, et al. Xiao-Yin-Fang Therapy alleviates psoriasis-like skin inflammation through suppressing γδT17 cell polarization. Front Pharmacol. 2021;12:629513. doi:10.3389/fphar.2021.629513
11. Yang L, Feng X, Li Y, Zhang S, Ying Y. Therapeutic efficacy of catalpol against apoptosis in cardiomyocytes derived from human induced pluripotent stem cells. AMB Express. 2020;10(1):56. doi:10.1186/s13568-020-00986-9
12. Wang Z-H, Zhan-Sheng H. Catalpol inhibits migration and induces apoptosis in gastric cancer cells and in athymic nude mice. Biomed Pharmacother. 2018;103:1708–1719. doi:10.1016/j.biopha.2018.03.094
13. Zhang Q, Xie J, Li G, et al. Psoriasis treatment using Indigo Naturalis: progress and strategy. J Ethnopharmacol. 2022;297:115522. doi:10.1016/j.jep.2022.115522
14. Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682–690. doi:10.1038/nchembio.118
15. Tao Q, Du J, Li X, et al. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Dev Ind Pharm. 2020;46(8):1345–1353. doi:10.1080/03639045.2020.1788070
16. Kuai L, Song J-K, Zhang R-X, et al. Uncovering the mechanism of Jueyin granules in the treatment of psoriasis using network pharmacology. J Ethnopharmacol. 2020;262:113214. doi:10.1016/j.jep.2020.113214
17. Wei F, Song Y, Gong A, et al. Investigating the Molecular Mechanism of Xijiao Dihuang Decoction for the Treatment of SLE based on network pharmacology and molecular docking analysis. Biomed Res Int. 2022;2022:5882346. doi:10.1155/2022/5882346
18. Goon PKC, Farooqui UA, Koopmans I, Skellett AM, Levell NJ. Assessment of a 3-Dimensional Computerised psoriasis area and severity index (PASI) Tool for calculating and documenting PASI scores. J Eur Acad Dermatol Venereol. 2017;31(8):e352–e353. doi:10.1111/jdv.14154
19. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. doi:10.1186/1758-2946-6-13
20. Kuhn M, Szklarczyk D, Pletscher-Frankild S, et al. STITCH 4: integration of protein-chemical interactions with user data. Nucleic Acids Res. 2014;42(Database issue):D401–D407. doi:10.1093/nar/gkt1207
21. Gfeller D, Grosdidier A, Wirth M, Daina A, Michielin O, Zoete V. SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014;42(Web Server issue):W32–W38. doi:10.1093/nar/gku293
22. Scott DE, Bayly AR, Abell C, Skidmore J. Small molecules, big targets: drug discovery faces the protein-protein interaction challenge. Nat Rev Drug Discov. 2016;15(8):533–550. doi:10.1038/nrd.2016.29
23. Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi:10.1089/omi.2011.0118
24. Nair RP, Duffin KC, Helms C, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41(2):199–204. doi:10.1038/ng.311
25. Lee HJ, Hong YJ, Kim M. Angiogenesis in Chronic Inflammatory Skin Disorders. Int J Mol Sci. 2021;22(21). doi:10.3390/ijms222112035
26. Zhao Z-X, Fu J, S-r M, et al. Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics. 2018;8(21):5945–5959. doi:10.7150/thno.28068
27. Guohua F, Tieyuan Z, Rui W, Juan X. Oxypaeoniflorin prevents acute lung injury induced by lipopolysaccharide through the PTEN/AKT pathway in a Sirt1-dependent manner. Oxid Med Cell Longev. 2021;2021:6878026. doi:10.1155/2021/6878026
28. Zhang H, Wang J, Lang W, et al. Albiflorin ameliorates inflammation and oxidative stress by regulating the NF-κB/NLRP3 pathway in Methotrexate-induced enteritis. Int Immunopharmacol. 2022;109:108824. doi:10.1016/j.intimp.2022.108824
29. Li G, Seo C-S, Lee K-S, et al. Protective constituents against sepsis in mice from the root cortex of Paeonia suffruticosa. Arch Pharm Res. 2004;27(11):1123–1126. doi:10.1007/BF02975116
30. Zheng Y, Zhao Q, Lin J, et al. Xijiao Dihuang decoction relieves the erlotinib-induced dermatitis. Exp Cell Res. 2023;423(2):113437. doi:10.1016/j.yexcr.2022.113437
31. Zablotna M, Sobjanek M, Nedoszytko B, et al. Association of psoriasis with the VEGF gene polymorphism in the northern Polish population. J Eur Acad Dermatol Venereol. 2013;27(3):319–323. doi:10.1111/j.1468-3083.2011.04393.x
32. Jung K, Lee D, Lim HS, et al. Double anti-angiogenic and anti-inflammatory protein Valpha targeting VEGF-A and TNF-alpha in retinopathy and psoriasis. J Biol Chem. 2011;286(16):14410–14418. doi:10.1074/jbc.M111.228130
33. Fei X, Zhang X, Wang Q, et al. Xijiao Dihuang Decoction Alleviates Ischemic Brain Injury in MCAO rats by regulating inflammation, neurogenesis, and angiogenesis. Evid Based Complement Alternat Med. 2018;2018:5945128. doi:10.1155/2018/5945128
34. Chen J, Zhu Z, Li Q, et al. Neutrophils enhance cutaneous vascular dilation and permeability to aggravate psoriasis by releasing matrix metallopeptidase 9. J Invest Dermatol. 2021;141(4):787–799. doi:10.1016/j.jid.2020.07.028
35. Cai H, Ma Y, Jiang L, et al. Hypoxia response element-regulated MMP-9 promotes neurological recovery via glial scar degradation and angiogenesis in delayed stroke. Mol Ther. 2017;25(6):1448–1459. doi:10.1016/j.ymthe.2017.03.020
36. Bian G, Wang L, Xie Q, et al. DGT, a novel heterocyclic diterpenoid, effectively suppresses psoriasis via inhibition of STAT3 phosphorylation. Br J Pharmacol. 2021;178(3):636–653. doi:10.1111/bph.15306
37. Chen R, Zhai Y-Y, Sun L, et al. Alantolactone-loaded chitosan/hyaluronic acid nanoparticles suppress psoriasis by deactivating STAT3 pathway and restricting immune cell recruitment. Asian J Pharm Sci. 2022;17(2):268–283. doi:10.1016/j.ajps.2022.02.003
38. Sano S, Chan KS, Carbajal S, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11(1):43–49. doi:10.1038/nm1162
39. Hartwig T, Pantelyushin S, Croxford AL, Kulig P, Becher B. Dermal IL-17-producing γδ T cells establish long-lived memory in the skin. Eur J Immunol. 2015;45(11):3022–3033. doi:10.1002/eji.201545883
40. Caruso R, Botti E, Sarra M, et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat Med. 2009;15(9):1013–1015. doi:10.1038/nm.1995
41. Campanati A, Paolinelli M, Diotallevi F, Martina E, Molinelli E, Offidani A. Pharmacodynamics OF TNF α inhibitors for the treatment of psoriasis. Expert Opin Drug Metab Toxicol. 2019;15(11):913–925. doi:10.1080/17425255.2019.1681969
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






