Back to Journals » OncoTargets and Therapy » Volume 14
Daam1 Overexpression Promotes Gastric Cancer Progression and Regulates ERK and AKT Signaling Pathways
Authors Zhang Y, Bai X, Zhang Y, Li Y
Received 19 April 2021
Accepted for publication 10 August 2021
Published 27 August 2021 Volume 2021:14 Pages 4609—4619
DOI https://doi.org/10.2147/OTT.S316157
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Yue Zhang, Xue Bai, Yi Zhang, Yan Li
Department of Gerontology and Geriatrics, Shengjing Hospital of China Medical University, Shenyang, People’s Republic of China
Correspondence: Yue Zhang
Department of Gerontology and Geriatrics, Shengjing Hospital of China Medical University, 36 Sanhao Road, Shenyang, 110004, People’s Republic of China
Email [email protected]
Objective: The dishevelled-associated activator of morphogenesis 1 (DAAM1) has been reported to be closely associated with human cancers. However, its involvement in human gastric cancer (GC) remains largely unexplored. This study aimed to investigate the clinical significance and biological roles of Daam1 in human GC.
Methods: Daam1 protein expression was examined in 124 cases of gastric adenocarcinomas using immunohistochemistry. Daam1 plasmid and siRNA transfection were carried out in SGC7901 and AGS cell lines. CCK-8, colony formation, Annexin V/PI, JC-1 staining, and Western blotting were used to explore the biological functions and potential underlying mechanisms of Daam1 in GC cells.
Results: Our results showed that Daam1 was overexpressed in GC specimens. A high Daam1 level was associated with tumor-node-metastasis (TNM) stage, T status, nodal metastasis, and poor patient survival. Analysis of the Oncomine dataset revealed upregulation of Daam1 mRNA in GC tissues. Western blot showed that Daam1 protein expression was higher in GC cell lines compared to the normal GES-1 cell line. CCK-8 and colony formation assays showed that ectopic Daam1 expression upregulated the cell growth rate and colony number in SGC-7901 cells, while Daam1 siRNA knockdown downregulated the growth rate and colony number in AGS cells. CCK-8 and Annexin V/PI apoptosis assays demonstrated that Daam1 overexpression decreased cisplatin sensitivity and downregulated cisplatin-induced apoptosis. JC1 staining showed that Daam1 overexpression upregulated, while Daam1 depletion downregulated mitochondrial membrane potential. Mechanistically, Daam1 overexpression downregulated p21 and upregulated p-ERK and p-AKT. The increased proliferation rate and decreased cisplatin sensitivity/apoptosis induced by ectopic Daam1 were reversed after treatment with AKT and ERK inhibitors.
Conclusion: Taken together, our results showed that Daam1 overexpression was associated with poor prognosis and promoted malignant activity via regulation of ERK and AKT pathways in GC cells, indicating Daam1 is a malignant biomarker and potential therapeutic target in GC.
Keywords: Daam1, ERK, AKT, apoptosis, gastric cancer
Introduction
Gastric cancer (GC) is one of the most common types of malignant cancer and the leading cause of cancer-related deaths.1 Despite introduction of more effective detection methods and therapeutic regimens, the prognosis for advanced gastric cancer remains poor.2 During GC treatment, the effect of traditional chemotherapeutic drugs is limited, probably due to the development of chemoresistance.3,4 Thus, identifying the mechanisms underlying the development of chemoresistance and developing novel therapeutic targets is of great importance.
Dishevelled-associated activator of morphogenesis 1 (DAAM1) is a formin protein that mediates cytoskeletal rearrangement via regulating Wnt/PCP signaling pathway.5 Daam1 is a regulator of filopodia formation and phagocytosis via regulation of actin filament network and related biological behaviors.6,7 DAAM1 can also stabilize epithelial junctions by restraining lateral membrane motility.8 Recent studies have found that Daam1 expression is dysregulated in various cancers. Daam1 overexpression correlates with metastasis and predicts poor prognosis in breast cancer.9 Daam1 promotes Wnt5a-induced invasion in breast cancer and glioblastoma cells.10,11 Daam1 also mediates cancer migration and invasion in ovarian cancer cells.12 However, the involvement of Daam1 in GC has not been elucidated. In this study, we examined the clinical significance of Daam1 in GC tissues and explored its biological roles in GC cell lines.
Methods and Materials
Patients and Specimens
This study was approved by the Institutional Review Boards of the Shengjing Hospital of China Medical University and was conducted following the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all patients. The paraffin-embedded GC specimens were obtained from the Pathology archives of the Shengjing Hospital of China Medical University, which contained specimens no longer required to be maintained.
Immunohistochemistry
Sections (4-μm thick) were firstly prepared by the Department of Pathology. Sections were deparaffinized in xylene to de-paraffinize, followed by treatment with graded ethanol to rehydrate. Endogenous peroxidase was blocked by H2O2 solution. Antigen retrieval was performed in citrate buffer (pH=6.0). After incubation with Daam1 antibody (1:400, Proteintech, USA), the sections were treated using the Elivision plus Polymer HRP IHC Kit (Maixin Biotech, Fuzhou, China). The sections were stained by DAB kit and counterstained with hematoxylin. Cytoplasmic and membrane staining was regarded as positive staining. The intensity of staining was scored as 0 negative/weak; 1 moderate; and 2 strong. The percentage of cells with positive expression was categorized as: 1 (<25%); 2 (25–50%); 3 (50–75%); and 4 (75–100%). The final scores were obtained by multiplying the intensity score times the percentage of positive expression. Samples with scores <4 were considered as low expression. Samples with scores between 4 and 8 were considered as high expression.
Cell Culture and Transfection
Human gastric cancer cell lines (AGS, SGC-7901, NCI-N87, BGC-823) and the human normal gastric mucosa cell line GES-1 were obtained from Shanghai Cell Bank, Chinese Academy of Sciences. All these cell lines were incubated in PRIM-1640 medium supplemented with 10% fetal bovine serum (Gibco, USA).
The Daam1 overexpression plasmid and the corresponding negative control plasmid (pCMV6) were obtained from Origene (Rockville, USA). Transfection was performed using Lipofectamine 3000 (Invitrogen, USA). Daam1 small interfering siRNA was purchased from Dharmacon (siRNA1 target sequence: GAGAUAAGUUUGUGUCUGU; siRNA2 target sequence: GUACGAAUGUUGGUUAAUG) (Horizon, Lafayette, CO, USA). siRNA transfection was performed using Dharmafect1.
Western Blotting
Total protein lysate was harvested using RIPA cell lysis buffer with protease inhibitors. Forty micrograms proteins were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, USA). The PVDF membranes were incubated with primary antibodies against Daam1 (1:1000, Proteintech, USA), p21, p-ERK, ERK, p-AKT, AKT (1:1000, Cell Signal Technology, USA), Actin (1:2000, Santa Cruz, USA). The PVDF membranes were washed with TBS-T buffer and incubated with anti-mouse/rabbit peroxidase-conjugated secondary antibodies. Protein visualization was performed using a chemiluminescence ECL detection kit (Thermo, USA) and Bio-Rad BioImaging system.
Quantitative RT-PCR
Total RNA was extracted using TRIzol reagent. After quantification, RNA was reverse transcribed using PrimeScript RT Master Mix Kit (Takara, Dalian, China). SYBR select master Mix Kit (Thermo, MA, USA) was used for qPCR, and the reaction was conducted as follows: 96°C for 3 min followed by 40 cycles at 96°C for 20 s and 62°C for 30 s. Relative expression of Daam1 was normalized to GAPDH and calculated according to 2−ΔΔct. The experiments were repeated in triplicate. The sequences of primers were as follows:
Daam1 for 5′-ACAGAGAAGCCATGTTTGCACT-3′, rev 5′-AGAATTCAGGCCAACTTGTAGCTC-3′; GADPH for 5′-GAAATCCCATCACCATCTTCCAG-3′, rev 5′-GAGCCCCAGCCTTCTCCAT-3′.
CCK-8 and Colony Formation Assays
For cell viability, cells were plated in 96-well plates (3000 cells/well). CCK-8 reagent (10 μL; Dojindo, Japan) was added into each well. After incubation for 2 hours, absorbance was examined using a microplate reader (wavelength: 450 nm). For colony formation capacity, cells were seeded in 6-cm plates (concentration: 1000 cells/plate) and cultured for about 2 weeks. The cells were then stained using Giemsa and colonies were counted under a microscope. The experiment was repeated three times.
Apoptosis Analysis
For apoptosis assay, cells were stained with 5 mg/mL propidium iodide (PI) together with Annexin V/FITC (BD Bioscience, USA). The cell solution was incubated in the dark for 15 minutes, and flow cytometry (ACEA, USA) was used to detect the fluorescence.
JC-1 Staining
Mitochondrial membrane potential was measured using JC-1 staining followed by flow cytometry. Cells were treated with JC-1 staining solution at a concentration of 5 μM (Abcam) for 30 minutes in the incubator. Then, stained cells were washed twice and examined for green/red fluorescence using a flow cytometer (ACEA, USA).
Statistical Analysis
Statistical analysis was carried out using the software package SPSS, version 16.0 (SPSS, Chicago, IL). A χ2 test was used to analyze the possible associations between Daam1 expression and clinicopathological parameters. Overall survival was visualized using Kaplan–Meier survival analysis and compared by Log rank test. Differences in gene expression, proliferation capacity, and apoptosis rate were assessed using the Student’s t-test. P < 0.05 was considered to indicate statistical significance.
Results
Daam1 is Overexpressed in Gastric Cancer
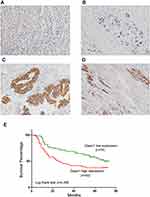
Daam1 protein levels were examined in 124 cases of GC samples and 16 cases of normal gastric mucosa by immunohistochemistry (IHC). Daam1 showed weak cytoplasmic staining in the normal gastric mucosa (Figure 1A), while 50 of the 124 (40.3%) GC cases showed elevated Daam1 staining (Figure 1B–D), which was mainly located in the cytoplasmic and membranous compartments of cancer cells. As shown in Table 1, high Daam1 levels were associated with higher T-N-M stage (p=0.0041), T status (p=0.0073), and positive nodal metastasis (p=0.0193). In addition, Kaplan–Meier analysis showed that a high Daam1 level was associated with poor patient prognosis (p=0.035, Figure 1E). Daam1 expression did not correlate with histological subtypes or relapse.
 |
Table 1 Correlation of DAAM1 Expression with Clinicopathological Characteristics in Gastric Cancer |
We also analyzed data from the Oncomine database. The Chen dataset showed that Daam1 level was higher in diffuse gastric adenocarcinoma compared with normal gastric mucosa (Figure 2A). Cho gastric dataset showed that Daam1 expression was higher in gastric mixed adenocarcinoma compared with gastric tissues (Figure 2B). DErrico gastric dataset of Oncomine showed that Daam1 expression in gastric intestinal adenocarcinoma was higher than in gastric mucosa (Figure 2C). Wang gastric dataset indicated that Daam1 was higher in GC tissues compared with gastric tissues (Figure 2D). The Cancer Gene Atlas (TCGA) data also indicated that patients with higher Daam1 levels tended to have shorter survival compared to those with lower Daam1 levels (p=0.0438, Figure 2E).
Daam1 Promotes GC Cell Proliferation
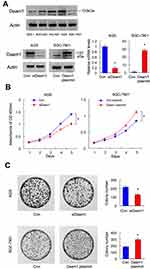
Next, we examined Daam1 protein levels in the normal gastric mucosa GES-1 cell line and in four cancer cell lines, including NCI-N87, AGS, SGC-7901 and BGC-823. As shown in Figure 3A, the normal GES-1 cell line expressed a relatively low level of Daam1. Endogenous expression of Daam1 was relatively higher in GC cell lines, especially in AGS cell line. We performed Daam1 plasmid transfection and siRNA knockdown in SGC-7901 and AGS cell lines, respectively. We screen Daam1 siRNA1 and 2 efficiency in AGS cell line. siRNA1 showed better knockdown efficiency, which was used in the following experiments (Supplementary Figure 1A). Transfection efficiency was confirmed by RT-qPCR and Western blot (Figure 3A). We then performed CCK8 and colony formation assays. CCK-8 assays showed that Daam1 depletion downregulated the growth rate, while Daam1 plasmid transfection increased the cellular growth rate (Figure 3B). Colony formation assays demonstrated that Daam1 knockdown decreased colony formation ability, while Daam1 transfection upregulated colony formation ability (Figure 3C). In addition, the effect of Daam1 was also validated in NCI-N87 and BGC-823 cell lines. As shown in Supplementary Figure 1B–D, CCK-8 and colony formation assay showed that Daam1 knockdown decreased growth rate and colony number in NCI-N87 cell line. Daam1 overexpression increased growth rate and colony formation ability in BGC-823 cell line.
Daam1 Regulates Cisplatin Sensitivity and Apoptosis
Although Daam1 has been shown to regulate invasion and migration in various cells, its involvement in apoptosis and drug sensitivity has not been fully explored. Cisplatin is one of the most common drugs used in GC chemotherapeutic regimens. We therefore explored the involvement of Daam1 in the regulation of cisplatin sensitivity of GC cells.
Using CCK-8 cell viability assays, we examined changes in the 50% inhibitory concentration (IC50) after Daam1 overexpression and knockdown. AGS cells with Daam1 depletion showed increased cisplatin sensitivity and decreased IC50 value compared with the control group (Figure 4A). SGC-7901 cells with ectopic Daam1 expression showed decreased sensitivity and increased IC50 compared with the control. CCK-8 assay also showed that Daam1 knockdown increased the cisplatin inhibition rate, while the Daam1 overexpression decreased it after 24 and 48 h of treatment (Figure 4B). Annexin V/PI staining showed that Daam1 depletion increased the rate of cisplatin-induced apoptosis in AGS cell line, while Daam1 overexpression decreased the cisplatin-induced apoptosis in SGC-7901 cell line (Figure 4C). We also tested if Daam1 could change inhibition rate of GC cells treated by 5-Fu. As shown in Supplementary Figure 2, Daam1 knockdown increased the 5-Fu inhibition rate, while Daam1 overexpression decreased it after 24 and 48 h of 5-Fu treatment.
Cisplatin sensitivity is closely related to the status of mitochondria. Thus, we checked for changes in mitochondrial membrane potential using JC-1 staining with flow cytometry, which showed red fluorescence under normal conditions and green fluorescence when mitochondrial membrane potential was decreased.
As shown in Figure 4D, ectopic Daam1 expression downregulated the rate of green fluorescence, suggesting that Daam1 upregulated mitochondrial membrane potential in SGC-7901 cells treated with cisplatin. On the other hand, Daam1 depletion downregulated mitochondrial membrane potential in AGS cells treated with cisplatin.
Daam1 Positively Regulates ERK and AKT Pathways in GC Cells
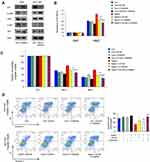
To determine the possible mechanism of Daam1 in GC cells, we explored potential downstream proteins and pathways. Western blotting showed that Daam1 decreased p21 protein levels, while Daam1 depletion increased p21 expression (Figure 5A). We further confirmed that ectopic Daam1 expression significantly enhanced p-ERK and p-AKT in SGC-7901 cells without significant change in total ERK and AKT. In contrast, Daam1 depletion downregulated p-ERK and p-AKT protein in AGS cells (Figure 5A).
To further validate that ERK and AKT signaling pathways were involved in GC cell proliferation and cisplatin sensitivity regulated by Daam1, we treated Daam1 transfected SGC7901 cells with AKT inhibitor LY294002 (20 μM) and ERK inhibitor U0126 (20 μM). CCK-8 assay demonstrated that increased proliferation rate induced by Daam1 was abolished by treatment with U0126 and LY294002 (Figure 5B). In addition, AKT and ERK inhibitors also reversed the effect of Daam1 on cisplatin sensitivity (Figure 5C). Annexin V/PI apoptosis assay showed that ERK and AKT inhibition upregulated cisplatin-induced apoptosis rate that was decreased by Daam1 (Figure 5D). Moreover, the combination effect of ERK and AKT inhibition was greater than either inhibitor alone. The effect of Daam1 on apoptosis reduction was largely abolished in cells treated with combination of ERK and AKT inhibitor. These results indicated that Daam1 regulated GC growth and drug sensitivity through ERK and AKT signaling.
Discussion
Recent studies have reported that Daam1 functioned as cancer-related protein and participates in cell migration and invasion in various types of cancers such as breast cancer, ovarian cancer and glioblastoma.10–12 As the role of Daam1 on carcinogenesis has mainly focused on its regulation of invasion and migration in previous studies,10–13 its impact on other biological activity, such as chemo-sensitivity, has not been fully investigated. For the first time to our knowledge, our study has shown the involvement of Daam1 in the regulation of proliferation and chemo-sensitivity in GC cells.
It has been recently reported that Daam1 upregulation correlated with poor prognosis in breast cancer.9 However, its expression in gastric cancers remains unexplored. Here, our clinical data showed Daam1 overexpression in 40.3% of human GC specimens, which was positively associated with TNM stage, T stage and lymph node metastasis. In addition, we demonstrated that high Daam1 expression status was associated with poor patient prognosis. Our results were further supported by analyses of TCGA and Oncomine, indicating that Daam1 could be a promising biomarker for the diagnosis of aggressive gastric cancer.
To explore the biological roles of Daam1, we upregulated Daam1 and knocked down Daam1 in GC cell lines. CCK-8 and colony formation assays indicated that Daam1 positively regulated GC cell proliferation. Previous studies cited above reported that Daam1 participated in oncogenic processes including migration and invasion. However, its involvement in chemo-sensitivity and apoptosis remains unclear. Cisplatin is currently one of the most widely used chemotherapeutic drugs for GC treatment. Common regimens such as ECF (epirubicin, cisplatin and fluorouracil) and ECX (epirubicin, cisplatin and capecitabine) contain cisplatin. We found that Daam1 overexpression downregulated cisplatin sensitivity and cisplatin-induced apoptosis, while Daam1 depletion increased cisplatin sensitivity and apoptosis. Our data showed, for the first time, the involvement of Daam1 in chemo-sensitivity of cancer cells. The pathways of chemotherapeutic drugs, such as cisplatin, which induce apoptosis are mainly dependent on changes in mitochondrial function.14–17 Using JC-1 staining, we showed that Daam1 was able to increase mitochondrial membrane potential in GC cells treated with cisplatin, suggesting Daam1 could stabilize mitochondrial function.
We further explored the underlying mechanisms by which Daam1 regulated proliferation and chemosensitivity. After screening a series of possibly related proteins, we found that Daam1 overexpression upregulated both AKT and ERK signaling pathways. ERK and AKT signaling have been reported to be crucial for proliferation and chemoresistance in various cancers, including GC. ERK signaling can lead to activation of the proto-oncogenes c-Fos, c-Jun, and c-Myc, which coordinates growth signals.18 AKT signaling regulates the cell cycle through GSK3 phosphorylation and cell survival through Bcl family proteins.4,19 In addition, ERK and AKT signaling cooperate to translationally regulate Survivin protein, an important inhibitor of drug-induced apoptosis.20 Our data further showed that enhanced proliferation and decreased chemo-sensitivity induced by Daam1 were greatly impaired by AKT and ERK inhibitors. The combination of AKT and ERK inhibitors in Daam1-overexpressing SGC-7901 cells showed a greater effect than either inhibitor alone. These results indicated that Daam1 modulated cancer cell proliferation and cisplatin sensitivity at least partly through regulation of ERK/AKT signaling.
In conclusion, the present study revealed the clinical significance and biological functions of Daam1 in human gastric cancer. Our results indicated that Daam1 overexpression promoted proliferation and reduced cisplatin sensitivity in GC. We also linked its oncogenic effects with ERK/AKT signaling, suggesting the therapeutic possibility of targeting Daam1 in GC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi:10.1016/S0140-6736(16)30354-3
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi:10.3322/caac.21387
3. Yoon C, Cho S-J, Aksoy BA, et al. Chemotherapy resistance in diffuse-type gastric adenocarcinoma is mediated by RhoA activation in cancer stem-like cells. Clin Cancer Res. 2016;22(4):971–983. doi:10.1158/1078-0432.CCR-15-1356
4. Du J, Fu L, Hao J, Lin X, Dong Q. Rab11a is overexpressed in gastric cancer and regulates FAK/AKT signaling. J Oncol. 2020;2020:3494396. doi:10.1155/2020/3494396
5. Aspenstrom P, Richnau N, Johansson AS. The diaphanous-related formin DAAM1 collaborates with the Rho GTPases RhoA and Cdc42, CIP4 and Src in regulating cell morphogenesis and actin dynamics. Exp Cell Res. 2006;312:2180–2194. doi:10.1016/j.yexcr.2006.03.013
6. Hoffmann AK, Naj X, Linder S. Daam1 is a regulator of filopodia formation and phagocytic uptake of Borrelia burgdorferi by primary human macrophages. FASEB J. 2014;28:3075–3089. doi:10.1096/fj.13-247049
7. Luo W, Lieu ZZ, Manser E, Bershadsky AD, Sheetz MP. Formin DAAM1 organizes actin filaments in the cytoplasmic nodal actin network. PLoS One. 2016;11:e0163915. doi:10.1371/journal.pone.0163915
8. Nishimura T, Ito S, Saito H, et al. DAAM1 stabilizes epithelial junctions by restraining WAVE complex-dependent lateral membrane motility. J Cell Biol. 2016;215:559–573. doi:10.1083/jcb.201603107
9. Mei J, Xu B, Hao L, et al. Overexpressed DAAM1 correlates with metastasis and predicts poor prognosis in breast cancer. Pathol Res Pract. 2020;216:152736. doi:10.1016/j.prp.2019.152736
10. Xiong H, Yan T, Zhang W, et al. miR-613 inhibits cell migration and invasion by downregulating Daam1 in triple-negative breast cancer. Cell Signal. 2018;44:33–42. doi:10.1016/j.cellsig.2018.01.013
11. Liu G, Yan T, Li X, et al. Daam1 activates RhoA to regulate Wnt5ainduced glioblastoma cell invasion. Oncol Rep. 2018;39:465–472.
12. Mei J, Huang Y, Hao L, et al. DAAM1-mediated migration and invasion of ovarian cancer cells are suppressed by miR-208a-5p. Pathol Res Pract. 2019;215:152452. doi:10.1016/j.prp.2019.152452
13. Rodriguez-Hernandez I, Maiques O, Kohlhammer L, et al. WNT11-FZD7-DAAM1 signalling supports tumour initiating abilities and melanoma amoeboid invasion. Nat Commun. 2020;11:5315. doi:10.1038/s41467-020-18951-2
14. Guerra F, Arbini AA, Moro L. Mitochondria and cancer chemoresistance. Biochim Biophys Acta. 2017;1858:686–699. doi:10.1016/j.bbabio.2017.01.012
15. Kim JS, Lee JM, Chwae YJ, et al. Cisplatin-induced apoptosis in Hep3B cells: mitochondria-dependent and -independent pathways. Biochem Pharmacol. 2004;67:1459–1468. doi:10.1016/j.bcp.2003.12.013
16. Zhao W, You CC, Zhuang JP, et al. Viability inhibition effect of gambogic acid combined with cisplatin on osteosarcoma cells via mitochondria-independent apoptotic pathway. Mol Cell Biochem. 2013;382(1–2):243–252. doi:10.1007/s11010-013-1740-5
17. Li H, Fu L, Liu B, Lin X, Dong Q, Wang E. Ajuba overexpression regulates mitochondrial potential and glucose uptake through YAP/Bcl-xL/GLUT1 in human gastric cancer. Gene. 2019;693:16–24. doi:10.1016/j.gene.2019.01.018
18. Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19:1997–2007.
19. Dong Q, Fu L, Zhao Y, Tan S, Wang E. Derlin-1 overexpression confers poor prognosis in muscle invasive bladder cancer and contributes to chemoresistance and invasion through PI3K/AKT and ERK/MMP signaling. Oncotarget. 2017;8(10):17059–17069. doi:10.18632/oncotarget.15001
20. Ye Q, Cai W, Zheng Y, Evers BM, She QB. ERK and AKT signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer. Oncogene. 2014;33:1828–1839. doi:10.1038/onc.2013.122
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.