Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
CXCL13 Is A Biomarker Of Anti-Leucine-Rich Glioma-Inactivated Protein 1 Encephalitis Patients
Authors Lin Y, Yang X, Lv J, Liu X, Wang S
Received 7 July 2019
Accepted for publication 17 September 2019
Published 11 October 2019 Volume 2019:15 Pages 2909—2915
DOI https://doi.org/10.2147/NDT.S222258
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
You-ting Lin, 1 Xue Yang, 2 Jing-wei Lv, 2 Xue-wu Liu, 2,* Sheng-jun Wang 2,*
1Department of Neurology, Shandong Provincial Hospital, Shandong University, Ji’nan, People’s Republic of China; 2Department of Neurology, Qilu Hospital, Shandong University, Ji’nan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Sheng-jun Wang;Xue-wu Liu
Department of Neurology, Qilu Hospital, Shandong University, 107# Wen Hua Xi Road, Ji’nan 250012, Peoples Republic of China
Email [email protected]; [email protected]
Background: Although antibody-mediated immune responses are considered pathogenic and responsible for neural injury in anti-leucine-rich glioma-inactivated protein 1 (anti-LGI1) encephalitis, previous studies have indicated that cytokines and chemokines might play roles in the pathogenic process by serving as B cell enhancers. In this study, we detected the profiles of cytokines and chemokines in the cerebral fluid (CSF) and serum of patients with anti-LGI1 encephalitis to identify potential biomarkers.
Methods: Sixteen patients diagnosed with anti-LGI1 encephalitis and nine patients diagnosed with noninflammatory neurologic disorders were included in the study. Cytokines and chemokines including IL-6, IL-10, IL-17, CXCL12, CXCL13, BAFF and HMGB1 in serum and CSF were measured.
Results: The serum and CSF levels of CXCL13 were significantly higher in patients with anti-LGI1 encephalitis (36.32± 34.71 pg/mL and 2.23± 2.41 pg/mL, respectively) than in controls (10.84± 5.02 pg/mL and 0.34± 0.21 pg/mL, respectively). There was no significant difference in serum or CSF levels of IL-6, IL-10, IL-17, CXCL12, BAFF and HMGB1 between the two groups.
Conclusion: CXCL13 is a potential biomarker of active inflammation in anti-LGI1 encephalitis. The distinctive response of cytokines and chemokines might be closely linked to the mechanisms underlying this condition.
Keywords: leucine-rich glioma-inactivated protein 1, CXCL13, cytokine, encephalitis, biomarker
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
Autoimmune encephalitis (AE) associated with antibodies against neuronal surface antigens has been attracting extensive attention since anti-NMDA receptor (NMDAR) encephalitis was first described a dozen years ago. This family of autoimmune encephalitis that includes anti-NMDAR, anti-leucine-rich glioma-inactivated protein 1 (anti-LGI1) encephalitis, shares some common characteristics, and this group of antibodies seems to be directly pathogenic.1–3 However, the triggers and underlying causes of the pathogenic pathways that allow antibodies to obtain access to the central nervous system (CNS) are poorly understood.4 Although the roles of several inflammatory cytokines/chemokines have been implicated in several studies about AE, their effects have not yet been fully established in detail.1
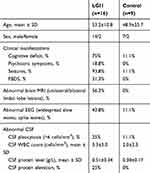 |
Table 1 Clinical Features Of Anti-LGI1 And Control Patients |
Cytokines are biologically active low molecular weight polypeptides that act as intercellular messengers at sites of inflammation. According to their cytokine profiles, naïve T cells are divided into distinct subsets that promote different types of inflammatory responses indifferent immunological disorders.5 Chemokines, a superfamily of small proteins, and their receptors play a central role in the inflammatory recruitment of leukocytes and other cell types into the CNS.6 Thus, the presence of specific cytokines/chemokines could reflect different underlying pathways and act as a biomarker of inflammatory diseases. For instance, B cell markers, such as CXCL13(B-lymphocyte chemoattractant, BLC) and BAFF (B cell activating factor), have been most extensively investigated and are found to be elevated in autoantibody-associated CNS disorders; while helper T cell2 (Th2) and Th17 cytokines, such as interleukin (IL)4, IL-5, CCL17, IL-6, IL-17, and CXCL8, are frequently elevated in acute disseminated encephalomyelitis (ADEM) and neuromyelitis optica (NMO); and Th1 and Th17 cytokines, such as tumor necrosis factorα (TNF-α), CXCL10, IL-6, IL-17, and IL-23, are more commonly elevated in multiple sclerosis.7 Compared with the widely accepted diagnostic value of antibodies in AE, few studies have explored chemokines and cytokines in this condition, and existing studies have mainly investigated anti-NMDAR encephalitis, in which both B cell- and T cell-mediated immune activation are indicated.8–11 In this study, we detected a series of cytokines and chemokines in the CSF and serum of patients with anti-LGI1 encephalitis to illustrate the underlying inflammatory mechanisms. These cytokines and chemokines included B cell markers such as CXCL12 (constitutively secrete the chemokine stromal cell-derived factor-1, SDF-1), CXCL13 and BAFF, T help cell markers such as IL-6 and IL-17, a regulatory T cell marker IL-10 and a key molecule that links tissue damage and inflammation chemokine HMGB1 (high-mobility group box protein 1).
Materials And Methods
Sixteen patients diagnosed with anti-LGI1 encephalitis at Qilu Hospital of Shandong University and Shandong Provincial Hospital from 2016 to 2018 were included in the study. The diagnosis was based on limbic encephalitis symptoms and the detection of specific anti-LGI1 antibodies as previous reported.12 Commercially available cell-based assays were used to detect anti-LGI1 antibodies. The control group consisted of nine patients with noninflammatory neurologic disorders, whose serum and CSF samples did not reveal anti-LGI1 antibodies. The diagnoses of these patients included headache (n=3), cervical radiculopathy (n=2), nonspecific dizziness (n=1), vasovagal syncope (n=1), seizure (n=1) and essential tremor (n=1). This study was approved by the Ethics Committee of Qilu Hospital of Shandong University and the Ethics Committee of Shandong Provincial Hospital. This study was conducted in accordance with the Declaration of Helsinki. Informed consents were written and obtained from all patients.
Serum and CSF samples were obtained immediately after the patients were hospitalized in an active disease state, prior to immunotherapy. Samples were stored at −80°C until the assays were performed. The levels of cytokines and chemokines, including IL-6, IL-10, IL-17, CXCL12, CXCL13, BAFF and HMGB1 were measured by enzyme-linked immunosorbent assay (ELISA) using the human ELISA kits (Abcam, Cambridge, UK). Samples were diluted and measured according to the manufacturer’s instructions. The cytokine and chemokine concentrations were calculated using standard curves.
Data are expressed as the means ±standard deviations (SD) and are statistically analyzed using SPSS version 20.0. Independent-samples t-tests were used to compare the levels of cytokines and chemokines between the anti-LGI1 antibody group and the control group. Differences with P <0.05 were considered significant.
Results
The clinical features of sixteen patients with anti-LGI1 encephalitis and nine control patients were listed in Table 1. There was no difference in age or sex between the two groups. The anti-LGI1 encephalitis patients presented with more neuropsychiatric manifestations including cognitive impairments (75%), seizures (93.8%) and faciobrachial dystonic seizures (FBDS)(31.3%) than those were found in the controls. Widespread slow waves and paroxysmal sharp/spike waves were often found on EEG (43.8%) and mesial temporal region abnormalities were often observed on MRI scans (56.3%) in anti-LGI1 encephalitis patients. CSF leukocyte counts and protein levels were not obviously elevated. Anti-LGI1 antibody tests were positive in all sixteen anti-LGI1 encephalitis patients, including ten serum samples and sixteen CSF samples.
The cytokine and chemokine concentrations were detected in ten serum samples and sixteen CSF samples of patients with anti-LGI1 encephalitis. The serum and CSF levels of CXCL13 (36.32±34.71 pg/mL; 2.23±2.41 pg/mL) were significantly higher in patients with anti-LGI1 encephalitis than those in controls (10.84±5.02 pg/mL; 0.34±0.21 pg/mL). (P<0.05; Figures 1 and 2) The serum levels of IL-6 (0.16±0.37 pg/mL), IL-10 (1.88±2.47 pg/mL), IL-17 (5.26±1.78 pg/mL), CXCL12 (2302.01±1490.43 pg/mL), BAFF (0.23±0.14 pg/mL) and HMGB1 (2901.21±985.93 pg/mL) were not significantly different between the anti-LGI1 encephalitis group and the control group (0.19±0.21 pg/mL; 0.59±0.91 pg/mL; 7.92±4.67 pg/mL; 2313.33±722.29 pg/mL; 0.23±0.15 pg/mL; 3122.78±545.33 pg/mL, respectively). (Figure 1) The CSF levels of IL-6 (4.91±6.38 pg/mL), IL-10 (1.44±1.21pg/mL), IL-17 (8.39±2.66pg/mL), CXCL12 (912.51±642.99 pg/mL), BAFF (0.10±0.07 pg/mL) and HMGB1 (4277.56±1168.29 pg/mL) were not different between the anti-LGI1 encephalitis group and the control group (1.51±0.64 pg/mL; 0.75±0.53 pg/mL; 11.85±4.09 pg/mL; 1322.22±773.14 pg/mL; 0.13±0.23 pg/mL; 3735.33±243.88 pg/mL, respectively). (Figure 2)
Discussion
In this study, patients with anti‑LGI1 antibody encephalitis have prominent clinical manifestations including seizures, memory deficits and FBDS. About one half patients showed epileptic or slow waves on EEG exams and temporal region abnormalities by MRI scans. We revealed that levels of CXCL13 were higher in both serum and CSF of patients with acute anti-LGI1 encephalitis than in controls. There was no difference in serum/CSF levels of other cytokines or chemokines, including IL-6, IL-10, IL-17, CXCL12, BAFF and HMGB1, between the two groups. We propose that CXCL13might be a potential biomarker for active neuroinflammation in anti-LGI1 encephalitis.
CXCL13 is undetectable in the normal CNS, and localizes to infiltrating immune cells in active CNS inflammation.13 CXCR5, the receptor for CXCL13, is expressed on virtually all B cells as well as, naïve and memory T cells.14,15 CXCL13 has been shown to be broadly elevated in both inflammatory and infectious CNS conditions, including multiple sclerosis,13,16 neuroborreliosis,17,18 anti-NMDAR encephalitis9,19 and other neurological diseases.20 The level of CXCL13 is most consistently correlated with CSF B cells, plasmablasts and intrathecal immunoglobulin production,9,21 suggesting that CXCL13 maybe a biomarker for neuroinflammation, especially B cell recruitment to the CSF. It seems that elevated CXCL13 is associated with inflammatory activity, but not with a specific pathogen. In this study, we found that CXCL13 levels were higher in anti-LGI1 encephalitis group than in controls, indicating the presence of active inflammation despite the unremarkable pleocytosis observed in the majority of our patients. The close linkage between CXCL13 and B cell function also corresponded with the widely accepted point that humoral immunity played a critical role in anti-LGI1 encephalitis.1–3 A different conclusion was drawn in another study in which CXCL13 was unchanged in either serum or CSF samples obtained from an anti-LGI1 encephalitis group.22 We assume that this might be partially due to small sample bias, and the course of the disease could also be a factor that influences the level of cytokine/chemokines.11,19 Based on these considerations, we speculate that serum/CSF levels of CXCL13 could be a potential biomarker to evaluate the inflammatory response in anti-LGI1 encephalitis.
One characteristic that deserves consideration is that CXCL13 levels were increased in both serum and CSF in anti-LGI1 group, with higher level in serum than CSF. Interestingly, in patients with anti-NMDAR encephalitis, the increases of CXCL13 and other cytokines/chemokines were observed only in CSF, and no dynamic changes were observed in the peripheral blood.9,19,22 These distinctions might provide clues suggesting that the intrathecal synthesis of NMDAR antibodies is the main source of pathogenic attacks in anti-NMDAR encephalitis, while the CXCL13 and LGI1 antibodies detected in CSF probably resulted from leakage through the blood brain barrier (BBB) in anti-LGI1 encephalitis.
Limited neuropathological studies have revealed variably intense T cell infiltration in the brain tissues of patients with anti-LGI1 encephalitis, although T cell were not considered a major contributer.23 This observation raised questions regarding the still largely unknown mechanisms underlying the recruitment of B and T cells into the inflamed CNS. Cytokines/chemokines might be key components of the crosstalk between humoral and cell-mediated immunity. In a previous study, AE patients with antibodies to cell surface antigens (undistinguished LGI1 and NMDAR) had higher serum Th17 cytokine (IL-17, IL-23) levels than those were found in patients with antibodies against intracellular antigens,24 indicating a role for Th cells in supporting B cell reactions. However, this family of AE is not absolutely the same, even with regard to characteristics of immune activation and pathological features. In this study of anti-LGI1 encephalitis, we failed to find difference in the level of Th cell markers (such as IL-6,10, 17) between the anti-LGI1 group and the control group, though these factors are important for the activation of B cells. Interestingly, in anti-NMDAR encephalitis, apart from an increase in the level of CXCL13, a wider range of cytokines/chemokines associated with Th cells (also known as B cell enhancers), including IL-6, IL-17, IL-10 and HMGB1, was upregulated.8,10,19,22 This finding could be of great clinical significance in anti-NMDA encephalitis in light of the fact that these cytokines/chemokines were correlated with both inflammation severity and prognosis. We assumed that the profiles of cytokines/chemokines in CSF and serum might reflect the different immunological mechanisms that underlie anti-NMDAR and anti-LGI1 encephalitis and could correspond to some of their clinical characteristics. For example, patients with anti-LGI1encephalitis usually seem to respond faster than those with anti-NMDAR encephalitis at the beginning of immunotherapy.25–27 Obtaining additional details could promote the evaluation of disease severity more precisely and the exploration of therapeutic targets in the future.
In conclusion, serum/CSF CXCL13 level is a potential biomarker of active inflammation in anti-LGI1 encephalitis. The distinctive responses of cytokines and chemokines might be closely linked to underlying mechanisms. Further exploration might provide more information about pathogenic mechanisms, diagnostic biomarkers, prognosis prediction, and potential targets for individual therapy.
Acknowledgment
This work is supported by grants from Natural Science Foundation of China (No.81873786), Natural Science Foundation of Shandong Province, China (No.ZR2017MH082).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Weissert R. Adaptive immunity is the key to the understanding of autoimmune and paraneoplastic inflammatory central nervous system disorders. Front Immunol. 2017;8:336. doi:10.3389/fimmu.2017.00336
2. Höftberger R. Neuroimmunology: an expanding frontier in autoimmunity. Front Immunol. 2015;6:206. doi:10.3389/fimmu.2015.00206
3. Martinez-Hernandez E, Horvath J, Shiloh-Malawsky Y, Sangha N, Martinez-Lage M, Dalmau J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77:589–593. doi:10.1212/WNL.0b013e318228c136
4. Platt MP, Agalliu D, Cutforth T. Hello from the other side: how autoantibodies circumvent the blood-brain barrier in autoimmune encephalitis. Front Immunol. 2017;8:442. doi:10.3389/fimmu.2017.00442
5. Akdis M, Burgler S, Crameri R, et al. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127:701–721. doi:10.1016/j.jaci.2010.11.050
6. Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi:10.1016/j.immuni.2012.05.008
7. Kothur K, Wienholt L, Brilot F, Dale RC. CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine. 2016;77:227–237. doi:10.1016/j.cyto.2015.10.001
8. Ai P, Zhang X, Xie Z, et al. The HMGB1 is increased in CSF of patients with an Anti-NMDAR encephalitis. Acta Neurologica Scandinavica. 2018;137:277–282. doi:10.1111/ane.12850
9. Leypoldt F, Hoftberger R, Titulaer MJ, et al. Investigations on CXCL13 in anti-N-methyl-D-aspartate receptor encephalitis: a potential biomarker of treatment response. JAMA Neurology. 2015;72:180–186. doi:10.1001/jamaneurol.2014.2956
10. Kothur K, Wienholt L, Mohammad SS, et al. Utility of CSF cytokine/chemokines as markers of active intrathecal inflammation: comparison of demyelinating, Anti-NMDAR and enteroviral encephalitis. PloS One. 2016;11:e0161656. doi:10.1371/journal.pone.0161656
11. Omae T, Saito Y, Tsuchie H, Ohno K, Maegaki Y, Sakuma H. Cytokine/chemokine elevation during the transition phase from HSV encephalitis to autoimmune anti-NMDA receptor encephalitis. Brain Dev. 2018;40:361–365. doi:10.1016/j.braindev.2017.12.007
12. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi:10.1016/S1474-4422(15)00401-9
13. Krumbholz M, Theil D, Cepok S, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–211. doi:10.1093/brain/awh680
14. Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi:10.1084/jem.193.12.1373
15. Anja L, Kinya N, Kristine M, et al. Kinetics and expression patterns of chemokine receptors in human CD4+T lymphocytes primed by myeloid or plasmacytoid dendritic cells. Eur J Immunol. 2003;33(2):474–482. doi:10.1002/immu.200310023
16. Olesen MN, Soelberg K, Debrabant B, et al. Cerebrospinal fluid biomarkers for predicting development of multiple sclerosis in acute optic neuritis: a population-based prospective cohort study. J Neuroinflammation. 2019;16:59. doi:10.1186/s12974-019-1440-5
17. Markowicz M, Schotta AM, Kundi M, et al. CXCL13 concentrations in cerebrospinal fluid of patients with lyme neuroborreliosis and other neurological disorders determined by luminex and ELISA. Ticks Tick Borne Dis. 2018;9:1137–1142. doi:10.1016/j.ttbdis.2018.04.008
18. Wagner JN, Weis S, Kubasta C, Panholzer J, von Oertzen TJ. CXCL13 as a diagnostic marker of neuroborreliosis and other neuroinflammatory disorders in an unselected group of patients. Journal of Neurology. 2018;265:74–81. doi:10.1007/s00415-017-8669-7
19. Liba Z, Kayserova J, Elisak M, et al. Anti-N-methyl-D-aspartate receptor encephalitis: the clinical course in light of the chemokine and cytokine levels in cerebrospinal fluid. J Neuroinflammation. 2016;13:55. doi:10.1186/s12974-016-0507-9
20. Fujimori J, Nakashima I, Kuroda H, Fujihara K, Aoki M. Cerebrospinal fluid CXCL13 is a prognostic marker for aseptic meningitis. Journal of Neuroimmunology. 2014;273:77–84. doi:10.1016/j.jneuroim.2014.05.008
21. Kowarik MC, Cepok S, Sellner J, et al. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflammation. 2012;9:93. doi:10.1186/1742-2094-9-93
22. Byun JI, Lee ST, Moon J, et al. Distinct intrathecal interleukin-17/interleukin-6 activation in anti-N-methyl-d-aspartate receptor encephalitis. Journal of Neuroimmunology. 2016;297:141–147. doi:10.1016/j.jneuroim.2016.05.023
23. Bien CG, Vincent A, Barnett MH, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–1638. doi:10.1093/brain/aws082
24. Ulusoy C, Tuzun E, Kurtuncu M, Turkoglu R, Akman-Demir G, Eraksoy M. Comparison of the cytokine profiles of patients with neuronal-antibody-associated central nervous system disorders. Int J Neurosci. 2012;122:284–289. doi:10.3109/00207454.2011.648762
25. Hermetter C, Fazekas F, Hochmeister S. Systematic review: syndromes, early diagnosis, and treatment in autoimmune encephalitis. Front Neurol. 2018;9:706. doi:10.3389/fneur.2018.00706
26. Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies. Ann N Y Acad Sci. 2015;1338:94–114. doi:10.1111/nyas.12553
27. Newman MP, Blum S, Wong RC, et al. Autoimmune encephalitis. Intern Med J. 2016;46:148–157. doi:10.1111/imj.12974
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


