Back to Journals » Cancer Management and Research » Volume 15
CXCL12 and CXCR4 as Potential Early Biomarkers for Luminal A and Luminal B Subtypes of Breast Cancer
Authors Motyka J , Gacuta E, Kicman A, Kulesza M, Malinowski P, Ławicki S
Received 9 April 2023
Accepted for publication 15 June 2023
Published 4 July 2023 Volume 2023:15 Pages 573—589
DOI https://doi.org/10.2147/CMAR.S416382
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Joanna Motyka,1 Ewa Gacuta,2 Aleksandra Kicman,3 Monika Kulesza,1 Paweł Malinowski,4 Sławomir Ławicki1
1Department of Population Medicine and Lifestyle Diseases Prevention, Medical University of Bialystok, Bialystok, Poland; 2Department of Perinatology, University Clinical Hospital of Bialystok, Bialystok, Poland; 3Department of Aesthetic Medicine, Medical University of Bialystok, Bialystok, Poland; 4Department of Oncological Surgery, Bialystok Oncology Center, Bialystok, Poland
Correspondence: Joanna Motyka, Department of Population Medicine and Lifestyle Diseases Prevention, Medical University of Bialystok, Jerzego Waszyngtona 15B str, Bialystok, 15-269, Poland, Tel +85 686 53 71, Email [email protected]
Purpose: Breast cancer is the most common type of malignancy in women. Factors that increase the risk of occurrence include chronic inflammation, with chemokines as its mediators. Therefore, the purpose of the present study was to determine the diagnostic utility of CXCL12 and CXCR4 as modern tumor markers in patients with early-stage luminal A and luminal B subtype of breast cancer and also to compare the results with the routinely used marker – CA 15-3.
Patients and Methods: The study included 100 patients with early breast cancer of luminal A and B subtypes, 50 women with benign breast lesion and 50 healthy women. The levels of CXCL12 and CXCR4 concentrations were determined by enzyme-linked immunosorbent assay (ELISA), comparative marker CA 15-3 – by electrochemiluminescence method (ECLIA).
Results: Concentrations of CXCL12 were significantly lower, while CXCR4 and CA 15-3 – significantly higher among patients with early-stage breast cancer than healthy women. CXCL12 also showed lower concentrations among fibroadenoma patients in comparison to healthy women, while CXCR4 – lower concentrations among fibroadenoma patients than cancer group. CXCL12 showed significantly higher values of sensitivity (79%), specificity (82%), positive predictive value (89.72%), negative predictive value (80%), diagnostic accuracy (80%) and diagnostic power (AUC = 0.8196) in the whole breast cancer group compared to the CA 15-3 marker (58%; 72%; 80.56%; 46.15%, 62.67%, 0.6434, resp.). Analysis of combined parameters resulted in increased sensitivity, negative predictive value and power of the test with a slight decrease in positive predictive value and a more significant decrease in specificity, reaching the best values for the three-parameter test CXCL12+CXCR4+CA15-3 (96%; 85.71%; AUC = 0.8812; 78.69%; 48%, resp.).
Conclusion: The results indicate the preliminary usefulness of CXCL12 and CXCR4 as early biomarkers in the diagnosis of breast cancer, especially in the combined panel with CA 15-3.
Keywords: breast cancer, luminal, CXCL12, CXCR4, biomarkers, early diagnosis
Introduction
Cancer is a global, social, health and economic problem and is the second most important cause of death in the world population. The incidence of various types of cancer is steadily increasing and their occurrence is influenced by a number of interrelated factors such as genetic or environmental conditions. The COVID-19 virus pandemic in 2020 has resulted in impeded access to health care, which has translated into delays in cancer diagnosis and, as a result, increased mortality among patients admitted to oncology departments.1,2 In the female population, a particularly significant health problem is breast cancer (BC) which is the most common type of malignancy in women. Despite the general availability of preventive and screening examinations such as mammography or ultrasound examinations and the constant raising of public awareness of the disease, the incidence of BC is increasing.3,4 In 2020 alone, BC was diagnosed in more than 2 million patients and, according to estimates, this number will steadily increase.1,3,4 Due to the high heterogeneity of the disease, response to treatment is variable and BC accounts for more than 685,000 deaths worldwide.4 BC detected at an early stage has a good prognosis; however, due to social, economic or geographic circumstances, women are often diagnosed at higher and thus more advanced stages of cancer.3,5 Therefore, it is expedient to search for new, accessible and minimally invasive methods to diagnose BC. Currently, high hopes are placed on the determination of tumor markers in peripheral blood. Among the commonly used markers used in the diagnosis of BC is primarily CA 15-3 and it has limited sensitivity and specificity. In addition, determination of CA 15-3 levels from peripheral blood is not sufficient to make a definite diagnosis of BC and is mainly used to monitor patients in advanced stages of the disease.3,6 New candidates being considered as potential BC markers include matrix metalloproteinases,7 cytokines8 and also chemokines.9
The pathogenesis of BC is a complex process, related to the patient’s age, genetic, hormonal and environmental factors.3,4,10 Among the most recent factors associated with an increased risk of BC is chronic inflammation – associated with both cancer formation and progression.11 This thesis is supported in part by the fact that high levels of C-reactive protein, a classic marker of inflammation, in patients with BC have been associated with a poor prognosis.12 A number of inflammatory mediators are involved in both the induction and maintenance of inflammation, which include chemokines – small-molecule proteins belonging to the cytokine family. These molecules are responsible for a number of cellular phenomena such as migration, proliferation or chemotaxis.13,14 One of the more thoroughly studied chemokine is C-X-C motif chemokine ligand 12 (CXCL12) which binds to a specific receptor - C-X-C chemokine receptor type 4 (CXCR4). The CXCL12/CXCR4 axis plays an important role in carcinogenesis where it mediates cancer cell proliferation, angiogenesis and metastatic foci formation.14 In vitro, in a BC model, activation of the CXCL12/CXCR4 axis was associated with increased migration, proliferation, invasion of cancer cells and also with induction of chemotherapy resistance and angiogenesis.15,16
Currently, the preliminary diagnostic utility of CXCL12/CXCR4 axis components as blood markers has been determined in various types of cancer such as pancreatic17 and esophageal cancer.18 In addition, there are also the first reports on the possibility of using CXL12 and CXCR4 as new tumor markers in BC.19
The purpose of our study was to determine the diagnostic utility of CXCL12 and CXCR4 as modern tumor markers in patients in first stages of luminal subtypes A or luminal subtypes B BC, and to compare the obtained results to the routinely used marker – CA 15-3. The determination of the studied parameters was also performed in a comparison group (patients with fibroadenoma) and a control group (healthy women). The present work is a continuation of the research conducted by our research team on new markers in the diagnosis of breast cancer and gynecological cancers.
Materials and Methods
Participants of the Study
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Medical University of Bialystok (R-I-002/239/2014, May 29th 2014; R-I-002/51/2015, February 26th 2015; AKP.002.104.2021 February 25th 2021). Detailed characteristics of each group can be found in Table 1. We included 100 patients with early-stage luminal type A breast cancer (50 patients) and luminal type B breast cancer (50 patients) treated at the Bialystok Oncology Center between 2017 and 2022. Control groups consisted of 50 women diagnosed with benign breast lesions (fibroadenoma) who also underwent treatment at the Bialystok Oncology Center and 50 healthy women. Clinical information – age, menopausal status, tumor diagnosis (histological type, receptor status, stage, location) and applied surgical treatment were obtained from the medical history taken in the diagnostic process by general practitioners and oncologists. For each patient, concomitant diseases, cancer in another location, as well as an active inflammatory process, was further excluded. The patients did not receive any additional preoperative therapy prior to inclusion in the study group. The healthy women participating in the study were either volunteers whose eligibility was determined on the basis of a detailed history and examination by a gynecologist at the University Clinical Hospital in Bialystok, or women participating in the BialystokPLUS cohort study on the basis of extensive examinations (including imaging) performed in the aforementioned project, which had been previously evaluated by a general practitioner or gynecologist. All participants in the study gave written informed consent prior to participation.
 |
Table 1 Descriptive Analysis of Study Participants |
Materials and Assays of the Studied Parameters
Venous blood collected for anticoagulant – sodium heparin – was the material for the study. The blood was centrifuged within 1 hour after the collection at a speed of 1810xG for 10 minutes and then separated and stored at −80°C until the day of the determinations.
The determination of CXCL12 and CXCR4 concentrations was performed by enzyme-linked immunoassay (ELISA) using kits from R&D Systems (Human CXCL12/SDF-1 alpha Quantikine ELISA Kit, Cat. No. DSA00) and FineTest (Human CXCR4 ELISA Kit, Cat. No. EH2136). The assay procedure was carried out according to the manufacturers’ instructions included with the kits. Standards and samples were assayed in duplicates. The precision of the assays was specified by the manufacturer for both kits: 3.4% (intra-assay) and 9.4% (inter-assay) for CXCL12, and <8% (intra-assay) and <10% (inter-assay) for CXCR4. The plates were read at 450 nm with the correction set to 540 nm.
Determinations of the plasma level of comparative marker CA 15-3 were performed by the electrochemiluminescence (ECLIA) method using reagents from ROCHE Diagnostics (Elecsys CA 15-3 II) on a Cobas e411 apparatus according to the instructions provided with the reagent kits.
UALCAN Analysis
The University of ALabama at Birmingham CANcer data analysis Portal (UALCAN) (http://ualcan.path.uab.edu/) is a tool that allows analysis of OMICS data available in The Cancer Genome Atlas (TCGA) or MET500 databases under open access policy.20,21 Investigation of CXCL12 and CXCR4 mRNA expression in invasive breast carcinoma compared to normal breast tissue was conducted using the built-in “TCGA Gene” explorer module.
Statistical Analysis
Statistical analysis of the results was performed using the statistical program PQStat Software (v.1.8.4.162, Poznań, Poland), while graphical processing by GraphPad Prism Software (v. 9.1.1 (225), San Diego, California, United States). Analysis of normality of distribution was assessed by the Shapiro–Wilk test. Subsequently, the analysis was based on non-parametric tests – Kruskal–Wallis test with Conover-Iman post hoc test when comparing groups, and Spearman rank correlation test when examining correlations between parameters. The analysis of diagnostic reliability and diagnostic power of the tests was based on the ROC curve with the determined optimal cut-off point of 2.733 ng/mL for CXCL12, 1337.62 pg/mL for CXCR4 and 19.7 IU/mL for CA 15-3. The comparison of ROC curves was based on the evaluation of AUC values determined by DeLong’s nonparametric method.
Results
mRNA Expression of CXCL12 and CXCR4 in Breast Cancer
Analysis of mRNA expression showed that compared to healthy tissues, CXCL12 mRNA level was significantly lower (p < 0.0001) (Figure 1A), while CXCR4 mRNA was significantly higher in BC tissues (p < 0.0001) (Figure 2A). These results have also been confirmed by other teams of scientists involved in genomic database analysis.22–29
Further analysis of individual molecular subtypes showed that CXCL12 mRNA levels were significantly lower in TNBC (p < 0.0001) and HER2 (p < 0.0001) subtypes of BC than in luminal subtypes. However, no statistically significant differences were observed between TNBC and HER2 subtype (Figure 1B). The same results were obtained by the teams of Chen et al,23 Li et al,24 Mehraj et al25 and Wu et al.29 However, the relationship between BC subtypes and CXCL12 expression was not shown by the teams of Hozhabri et al22 and Liu et al.26
We also noted a difference in CXCR4 mRNA expression in different BC subtypes (Figure 2B). TNBC showed higher expression compared to luminal (p = 0.0013) and HER2 subtypes (p = 0.0282), while there were no differences in expression levels between HER2 and luminal subtypes. These results are in agreement with those obtained by Wu et al,30 Guo et al28 and Chuan et al.27
The mRNA expression of CXCL12 and CXCR4 did not correlate among breast cancer patients, nor its level differ in individual stages (Figures 1C and 2C), which is supported by reports from other researchers,24,26 although, several scientific reports have noted an association between lower CXCL12 expression and poorly differentiated tumor cells.22,23
Plasma Concentration of CXCL12, CXCR4 and CA 15-3 in Breast Cancer Patients
Data concerning plasma level of CXCL12, CXCR4 and comparative marker CA 15-3 are presented in Table 2 and Figures 3–5. Analyzing the results in the entire study group of women with early-stage BC, we noted that CXCL12 (2.433 ng/mL) showed significantly lower plasma concentrations compared to the group of healthy women (2.920 ng/mL, p < 0.001) (Figure 3). We also noticed significant differences between the group of BC patients and healthy women in the concentrations of CXCR4 and the comparative marker CA 15-3. CXCR4 (1525.32 pg/mL) (Figure 4) and CA 15-3 (20.95 IU/mL) (Figure 5) showed significantly higher levels in the BC group than in the control group of healthy women (CXCR4 1149.23 pg/mL, p = 0.041; CA 15-3 16.65 IU/mL, p = 0.004). Solely for CXCR4, the concentrations among patients with BC were significantly higher relative to women with benign lesions - fibroadenoma (1017.64 pg/mL, p = 0.043). Additionally, we noted that the group of women with fibroadenoma showed significantly lower levels of CXCL12 (2.503 ng/mL) compared to the control group of healthy women (CXCL12, p < 0.001), which we did not observe for the CXCR4, nor the comparative marker CA 15-3 (Figures 3–5).
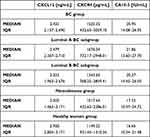 |
Table 2 Concentrations of CXCL12, CXCR4 and CA 15-3 in Individual Study Groups (Median Concentrations and Interquartile Ranges (IQR)) |
Looking at the individual luminal subgroups of BC, we noted that, compared with the group of healthy women, CXCR4 (1676.04 pg/mL, p = 0.042) and CA 15-3 (21.86 IU/mL, p = 0.012) showed significantly higher concentrations for the luminal A subgroup, while CXCL12 significantly lower concentrations in the luminal A subgroup (2.479 ng/mL, p < 0.001). Analogous results to the luminal A subgroup were obtained for CXCL12 and the comparative marker in luminal B BC subgroup – higher CA 15-3 level (20.27 IU/mL, p = 0.014) and lower CXCL12 level (2.323 ng/mL, p < 0.001) when compared to the control group of healthy women (Figures 3–5). In addition, in the luminal B BC subgroup, we observed significantly lower concentrations of CXCL12 alone (2.323 ng/mL) with respect to the group of women with benign breast lesions (2.503 ng/mL, p = 0.040) (Figure 3). CXCR4 was found to display no statistically significant differences between the luminal B subgroup of BC patients and the control groups.
Correlation Analysis on Plasma Parameters Under Study
When examining the correlations between plasma level of CXCL12, CXCR4 and CA 15-3, we observed a positive correlation only between CXCL12 and CXCR4 in the group of women with benign breast lesions (r = 0.4089, p = 0.003) and a weak negative correlation between CXCL12 and CXCR4 in the control group of healthy women (r = −0.2980, p = 0.036). In contrast, we did not observe any correlations between the parameters studied in the group of women with BC. The result of the analysis is presented as heatmaps of correlation matrix (Figure 6).
Analysis of Diagnostic Reliability and Diagnostic Power of Tests
The diagnostic reliability of a test is determined by the values of diagnostic sensitivity, diagnostic specificity, positive predictive value, negative predictive value and accuracy. Table 3 reports the values for the reliability parameters for the analyzed tests – univariate and multivariate parameters formed out of combinations of the determined compounds and a comparative marker.
 |
Table 3 Diagnostic Reliability of Univariate and Multivariate Parameters in the Respective Study Groups |
Investigating the entire group of BC patients, the highest values of diagnostic reliability were shown by CXCL12 reaching 79% for sensitivity, 82% for specificity, 89.72% for positive predictive value, 66.13% for negative predictive value and 80% accuracy, thus significantly surpassing the values shown for the comparative marker CA 15-3 (58%, 72%, 82.56%, 46.15% and 62.67% respectively). Similarly high value of diagnostic specificity as CXCL12 was achieved by CXCR4 (80%), with a simultaneously similar positive predictive value (85.32%) and much lower sensitivity (55%), negative predictive value (47.06%) and accuracy (63.33%). Despite this, all the diagnostic indicators for CXCR4, with the exception of diagnostic sensitivity, further exceeded the corresponding values obtained for the comparative marker CA 15-3. The analysis of combined parameters increased the values of sensitivity, negative predictive value and accuracy of the test at the expense of a slight decrease in positive predictive value and a more pronounced decrease in diagnostic specificity. In the case of combined panels, the highest values of sensitivity (96%) and negative predictive value (85.71%) were obtained for the three-parametric test CXCL12 + CXCR4 + CA 15-3 with a high value of accuracy of 80% and at the same time the lowest recorded specificity of the test (48%). Among the analyzed multivariate panels, the highest specificity (58%), positive predictive value (81.42%) and accuracy (80.67%) were achieved by the two-parametric test CXCL12 + CA 15-3 with simultaneous high rates of sensitivity (92%) and negative predictive value (78.38%).
Analyzing the individual luminal subgroups of BC, CXCL12 showed the highest values of all diagnostic reliability indices in both the subgroup of patients with luminal A and luminal B BC, matching the results obtained for the total study group (Table 3), in the same time outperforming the comparative marker CA 15-3 in all aspects. In the subgroup of patients with luminal A BC, CXCR4 showed higher values of positive predictive value (74.29%), negative prognostic value (65.57%) and accuracy (67%) than the comparative marker CA 15-3 (68.18%; 64.29%; 66%, respectively). However, CA 15-3 had slightly higher sensitivity value (60%) than CXCR4 (58%). Analyzing the subgroup of patients with luminal B BC, again we obtained corresponding, and only the sensitivity value of CXCR4 (52%) was lower than that of the comparative marker CA 15-3 (65%), while the rest of the CXCR4 indicators were superior to those determined for CA 15-3 (Table 3). Analysis of the combined panels of parameters in both luminal subgroups showed identical trends of change to their diagnostic reliability indicators as for the entire group of BC patients. In both the luminal A and luminal B subgroups of patients, the highest values of sensitivity (98% for luminal A and 94% for luminal B subgroup) and negative predictive value (96% for luminal A and 88.89% for luminal B subgroup) were achieved by the three-parameter test CXCL12 + CXCR4 + CA 15-3. In contrast, the highest positive predictive value (68.66% for both luminal subgroups) and accuracy (75% for both luminal subgroups) were characterized by the two-parametric test of CXCL12 + CA 15-3. It is also worth noting, that for the two-parametric test CXCL12 + CA 15-3, the values of sensitivity (92% for both luminal subgroups) and positive predictive value (87.88% for both luminal subgroups) were quite similar to those obtained for the three-parametric test.
The diagnostic power of a test is assessed by plotting sensitivity against the percentage of false positives (1 – specificity), called the receiver operating curve (ROC). The area under the ROC curve (AUC) is thus a measure of a test’s ability to accurately distinguish between normal and abnormal results, and the greater the AUC, the more powerful is the test. The AUC = 0.5 is considered to be the limit of the diagnostic usefulness of the test, to which the tested assays are compared and the p-value is determined. The results of the analysis of the ROC curves for the tested parameters (CXCL12, CXCR4, the reference marker CA 15-3) and their combinations are presented in Table 4. In addition, the ROC curves for the multivariate panels were compared to the ROC curve for the reference marker CA 15-3 in order to assess the possible improvement in the predictive ability (p-value against AUCCA 15-3).
 |
Table 4 ROC Curves Characteristics for Univariate and Multivariate Parameters in the Respective Study Groups with the Assessment of the Quality of the Test Against the Comparative Marker CA 15-3 |
ROC curve graphs for the total study group of BC patients are shown in Figure 7. In the total group of patients, significance against AUC = 0.5 was demonstrated for all tested parameters including multivariate parameters. The highest diagnostic power of the test was demonstrated by CXCL12 (AUC = 0.8196) with significantly better predictive ability than the comparative marker CA 15-3 (AUC = 0.6434, pCA 15-3 = 0.003). The diagnostic power and quality of the test demonstrated for CXCR4 was at a comparable level to the comparative marker CA 15-3 (Table 4). Analysis of combined parameters increased the value of diagnostic power reaching the maximum value for the three-component parameter CXCL12 + CXCR4 + CA 15-3 (AUC = 0.8812), significantly increasing the quality of the test of a comparative marker CA 15-3 (pCA 15-3 < 0.001). Similar AUC value to the three-parametric test was also shown by the two-parametric combination of CXCL12 + CA 15-3 reaching AUC = 0.8292.
 |
Figure 7 ROC curves of univariate and multivariate parameters in a group of BC patients. |
ROC curve plots for the subgroup of patients with luminal A BC are shown in Figure 8. All tested parameters, together with multivariate parameters, showed significant diagnostic power relative to AUC = 0.5. Similarly to the entire study group, CXCL12 (AUC = 0.7952, pCA 15-3 = 0.039) showed the highest diagnostic power superior to the comparative marker CA 15-3 (AUC = 0.6422). CXCR4 again achieved comparable, albeit lower values of diagnostic power of the assay (AUC = 0.6184) relative to CA 15-3. Combining parameters into multivariate test increased the diagnostic power of the assay, which reached the highest value again for the three-parametric combination CXCL12 + CXCR4 + CA 15-3 (AUC = 0.8736) showing a significant improvement in the quality of the assay in relation to the comparative marker CA 15-3 (pCA 15-3 < 0.001).
 |
Figure 8 ROC curves of univariate and multivariate parameters in a group of in a luminal A BC subgroup of patients. |
ROC curve graphs for the subgroup of patients with luminal B BC are shown in Figure 9. Again, the studied parameters, excluding CXCR4, along with their multivariate parameters, showed significant diagnostic power relative to AUC = 0.5. CXCL12 achieved the highest diagnostic power (AUC = 0.8440), thus significantly outperforming the comparative marker CA 15-3 (AUC = 0.6446, pCA 15-3 = 0.005). CXCR4 was once again characterized by a lower diagnostic power (AUC = 0.5924) relative to the comparative marker. In terms of combined panels, the highest value of diagnostic power was demonstrated by the three-parametric test CXCL12 + CXCR4 + CA15-3 (AUC = 0.9048), which was the only one combination that exceed the value of diagnostic power demonstrated by the both comparative marker and the best single parameter – CXCL12.
 |
Figure 9 ROC curves of univariate and multivariate parameters in a group of in a luminal B BC subgroup of patients. |
Discussion
BC is the most common malignancy among women, and many interrelated factors such as genetic or environmental conditions contribute to the onset of this disease.1,3,4 Despite the high heterogeneity of the disease, BC detected at an early stage has a good prognosis; however, most women are diagnosed at a more advanced stage – despite the existence of effective screening and preventive tests.3,5 This translates into the need to search for and introduce into health care systems, new methods of diagnosing BC. High hopes are placed on the determination of tumor markers from peripheral blood, which is a fast and minimally invasive method.3 However, the markers currently used in the diagnosis of BC are not very specific, which raises the need for the introduction into widespread use of new molecules that could be used as markers.6 These may include chemokines such as CXCL12 and its receptor, CXCR4.
The aim of the present study was to determine CXCL12 and CXCR4 concentrations in plasma and to determine the diagnostic utility of these molecules as new BC markers. In addition, the results were related to two control groups (benign breast lesions and healthy subjects) as well as compared with CA 15-3, which is a routinely used marker for BC diagnosis.
In the pathogenesis of BC, an important role is played by the CXCL12/CXCR4 axis, especially at the tumor progression. The action of this axis was primarily associated with negative cellular phenomena such as increased proliferation, migration and invasion what related to tumor weight gain in vivo.31–34 The CXCL12/CXCR4 axis is also responsible for the increased metastatic potential of cells resulting in a higher likelihood of metastatic foci in female patients.31,35,36 An especially unfavorable property of this axis arose from the induction of angiogenesis which has been linked to both an increase in tumor weight and an elevated risk of metastasis.32,37
Expression of both CXCL12 and CXCR4 has been demonstrated in various tumor types such as kidney cancer,38 esophageal cancer39 or ovarian cancer.40 In the case of BC patients, the presence of CXCL12 was found in the nucleus and cytoplasm, while CXCR4 was found in the membrane and cytoplasm of tumor cells.15 In addition, according to a study by Mirisola et al41 expression of both axis components is also found in the stroma of the cancerous lesion. Immunohistochemical CXCL12 expression positively correlated with BC stage according to the TNM classification, while CXCR4 expression was associated with the presence of lymph node metastasis and TNM stage.15 However, data analyzing CXCL12 and CXCR4 mRNA expression clearly contradicts the existing correlation between mRNA levels and BC stage.24,26 Additionally, slightly disparate information can be found on the level of CXCL12 expression and the association with overall survival and relapse-free survival in estrogen receptor-positive and -negative BC.21–26,29,30,41 In the majority of reports, nevertheless, higher CXCL12 mRNA expression is associated with a longer relapse-free survival period as well as longer overall survival time.22–24,26
We are now the first research team to determine the diagnostic utility of CXCL12 and CXCR4 in BC patients with luminal A and luminal B subtypes determined as a tumor marker from plasma. Our study is a continuation of the study conducted by Dąbrowska et al19 whose team determined the diagnostic utility of CXCL12 and CXCR4 as markers in BC without an isolated histological subtype; therefore, our results will be partially referred to the results obtained by said research team. We used plasma as the study material, which was selected on the basis of previous experiments conducted by our research team.3,7,19
In our study, the obtained concentrations of CXCL12 and CXCR4 were compared with those obtained in healthy women and patients with benign lesions - fibroadenoma, and related to the routinely used marker CA 15-3. The selection of patients with fibroadenoma as a comparison group and the routine marker CA 15-3 is dictated by previous experience and indicates the correct methodology of our study.3,7,19
In our study, the levels of CXCL12/CXCR4 axis components were dysregulated – CXCL12 concentrations were lower, while CXCR4 concentrations – higher, in patients with BC compared to healthy women and patients with benign lesions (fibroadenoma). This partly disagrees with the results obtained by Dąbrowska et al19 who finds higher concentrations of CXCL12 in BC women compared to healthy women and fibroadenoma patients. However, the results of the CXCR4 concentrations are in line with the results obtained by the team of Dąbrowska et al19 and Frayyeh et al,42 where higher concentrations of CXCR4 are also found among women with BC compared to healthy volunteers.19,42 The differences between the results may be due to several variables. First, our study included patients with BC of luminal subtype A or B, in contrast to the work of Dąbrowska et al19 who did not distinguish the molecular subtype of BC in their papers. Secondly, the patients in our study group were only in stage I or II, in contrast to the works of Dąbrowska et al19 whose studies included patients in all stages of cancer, resulting in a significantly smaller study group with early BC patients. The heterogeneity of the study groups selected by the previous research team may have resulted in such large differences between the acquired results.
Our findings revealed no difference in the concentrations of both CXCL12 and CXCR4, as well as comparative marker CA 15-3, between BC patients of luminal A and luminal B. Unfortunately, we do not have the opportunity to relate our findings to the results obtained by other teams, since we are the first research team to make this kind of finding. It should be noted, however, that in our previous studies of luminal subtypes of BC, we also did not observe dependencies of CXCL1, CXCL8 nor CA 15-3 concentration between luminal A and luminal B subgroups.3
According to our study, CA 15-3 concentrations were higher in women with BC than in healthy volunteers. This agrees with the results obtained in previous experiments and indicates that the determination of the comparative marker was performed correctly.3,7,19
Using sensitivity and specificity, positive and negative predictive value, we determined the diagnostic reliability of the studied parameters. We revealed that in the BC group, CXCL12 (sensitivity 79%; specificity 82%; positive predictive value 89.77%; negative predictive value 66.13%) and CXCR4 (55%; 80%; 84.42%; 47.06%, resp.) showed higher or comparable values for all diagnostic parameters compared to the routine marker CA 15-3 (58%; 72%; 80.56%; 46.15%, resp.). This differs from the study of Dąbrowska et al19 who found higher values for CXCL12 only for sensitivity (74.23%) and negative predictive value (64.29%) compared to CA 15-3 (59%; 58.06%, respectively) for total group of patients. Similar results to Dąbrowska et al team was provided by team Łukaszewicz-Zając et al18 who, in patients with esophageal cancer, also found higher only sensitivity and negative predictive value values for CXCL12 than for the cancer biomarker, CEA.
Viewing the different subgroups selected in the Dąbrowska et al study,19 the team observed higher sensitivity values (58.82%) and comparable negative predictive value values (76.12%) for CXCL12 relative to CA 15-3 (50%; 76.06%, respectively) for the stage I BC patients group, while specificity (71.43%) and positive predictive value (52.63%) values were lower for CXCL12 than for the comparative marker CA 15-3 (85.71%; 65.38%, resp.).19 In the stage II BC patients group, CXCL12 showed higher both sensitivity (78.05%) and negative predictive value (83.33%) and lower positive predictive value (64%) than CA 15-3 (53.66%; 73.97%; 70.97%, resp.). The results obtained for CXCL12 in the group of patients with stage II cancer in the study by Dąbrowska et al19 reached similar values or – for stage I cancer patients – lower values of diagnostic parameters compared to those obtained in our study for the whole group of patients with early-stage BC, which, despite the differences in the direction of changes in CXCL12 concentrations, seems to confirm the assumed clinical utility of CXCL12 as a marker of BC.
On the other hand, the diagnostic reliability for CXCR4 obtained by Dąbrowska et al19 for all-stages BC patients (sensitivity 59.80%; specificity 65.08%; positive predictive value 72.50%; negative predictive value 51.25%) was lower than for CA 15-3 (59.79%; 85.75%; 86.57%; 58.06%, resp.). Interestingly, when analyzing the results only for stage I and II BC patients in Dąbrowska et al study,19 CXCR4 showed a higher sensitivity (stage I: 64.71%; stage II: 60.98%), comparable negative predictive value (stage I: 77.36%; stage II: 71.93%) and lower positive predictive value (stage I: 50%; stage II: 53.19%) than CA 15-3 (stage I: 50%, 76.06%, 65.38%; stage II: 53.66%, 73.97%, 70.97%, resp.). Similar dependencies were also noted by the Łukaszewicz-Zając et al team,18 obtaining higher sensitivity (80%) and negative predictive value (63%) for CXCR4 than for the comparison marker CEA (22%; 42%, resp.). Those results are partially consistent with the results obtained for CXCR4 in our study, where we noted higher specificity (82%), positive predictive value (85.49%) and negative predictive value (47.67%) for CXCR4 than for routinely used marker CA 15-3 (72%; 80.56%; 46.15%, resp.).
For the multiparameter analysis, we observed an increase in sensitivity and negative predictive value, which was also observed by the Dąbrowska et al team.19 Combining CXCL12 and CA 15-3 into a two-parameter test resulted in an increase in sensitivity values of up to 73.53% and negative predictive value values of up to 80.85% for the subgroup of patients with stage I BC and 85.37% and 86.36% for patients with stage II BC in the Dąbrowska et al study.19 In our study, there was a stronger increase in sensitivity values up to 92%, and a comparable increase in negative predictive value values to 78.36%. It is noteworthy that combining parameters in the CXCL12 + CA 15-3 pairing slightly reduced the positive predictive value in both works, and more markedly the specificity value. Nevertheless, the specificity level in both works for CXCL12 + CA 15-3 remained around 60%. When performing combined analysis of the three parameters, we noted the highest values of sensitivity (96%) and negative predictive value (85.71%). This agrees with the results of Dąbrowska et al19 who also found an increase in sensitivity (stage I: 88.24%; stage II: 92.68%) and negative predictive value (stage I: 84.62%; stage II: 88%) when the three-parameter test was performed. Simultaneously, it is worth noting that the three-parametric analysis in our work significantly exceeded the sensitivity (96%) and positive predictive value (78.69%), as well as by a smaller margin – the specificity (48%) and negative predictive value (85.71%) than then three-parametric combination of CXCL12, CXCR4 and CA 15-3 in the work of Dąbrowska et al19 for both early BC subgroups (stage I: 88.24%, 42.25%, 34.92%, 84.62%; stage II: 92.68%, 48.1%, 34.92%, 88%, resp.).19 The study by Łukaszewicz-Zając et al18 did not analyze the combination of the three parameters; however, an evaluation of the combination of CXCL12 + CXCR4 was performed, where high values of sensitivity (84%), positive predictive value (71%) and negative predictive value (79%) were also achieved, along with a comparable low value of specificity (37%) as to both the results obtained in our study and the study by Dąbrowska et al for the CXCL12 + CXCR4 + CA 15-3 test.
With the help of the AUC, we assessed the power of the test. The highest power of the test in all study groups was revealed for CXCL12 (0.8196), which agrees with the results of Dąbrowska et al19 who also found the highest AUC, albeit lower than in our study, for CXCL12 (0.7502) in the BC total group. In our study, the AUC obtained for CXCR4 in all study groups (BC total group 0.6054; luminal A subgroup 0.6184; luminal B subgroup 0.5924) was lower to the AUC for CA 15-3 (sequentially: 0.6434; 0.6422; 0.6446) which is in agreement with the results of previous teams. Dąbrowska et al team showed that in the BC total group, as well as in stage I and stage II of BC, CXCR4 had similar AUC values (sequentially: 0.6239; 0.6629; 0.66210), which were also lower than for CA 15-3 (sequentially: 0.7354; 0.6452; 0.7163).19 Interestingly, comparable AUC values for CXCR4 and the comparative marker were also obtained by Łukaszewicz-Zając et al18 studying patients with esophageal cancer. In our study, when performing two- or three-parametric analyses, the AUC values increased, which is in line with the experience of Dąbrowska et al19 who also finds a similar relationship in all studied BC groups.
The puzzling results noted in our study, which we are unfortunate not able to confirm by the lack of other work, concerns CXCL12 and CXCR4 plasma concentrations with regard to its mutual correlation. The lack of correlation in all BC studied groups, positive correlation between CXCL12 and CXCR4 concentrations in the group of women with benign lesions and the negative correlation between the same parameters in the group of healthy women that we observed seem to be a logical consequence of the changes taking place in the breast. The reversal of the balance between CXCL12 and CXCR4 in the development of a benign lesion in relation to the physiological situation, and complete imbalance in a malignant lesion seems to be a potential new target for further expanded research. However, this thesis requires verification on larger groups of subjects and with studies conducted by other research teams.
Conclusion
According to our results, CXCL12 and CXCR4 performed simultaneously with the determination of the routine marker CA 15-3, especially combined as three-parametric test, may be considered in the future as a diagnostic marker for early BC, particularly for luminal subtypes A and B. However, it is unfortunate that our study has some limitations. First, we only included patients with luminal subtype A and B BC in the study, making us unable to determine the diagnostic utility of CXCL12 and CXCR4 in other subtypes of the disease. Secondly, in our study, we did not determine the concentrations and utility of CXCR7 (ACKR3), the atypical receptor for CXCL12, thereby not fully evaluating the overall ligand-receptor axis for CXCL12. Nevertheless, our study was a prelude to performing future determinations of other chemokines and their receptors. Thirdly, a restriction of our study was due to the relatively small size of the study groups. In order to completely confirm the results obtained by our team, it is necessary to carry out determinations in a larger number of samples and to introduce additional control groups such as women with breast cysts which are histologically different from fibroadenoma lesions, as well as to carry out the same tests among women of premenopausal status. The final limitation of our work is the lack of post-operative evaluation of the tested parameters’ concentrations, which is a direct result of the too sparse amount of post-operative material obtained (also as a result of the COVID-19 pandemic) to reliably assess the occurring changes. Despite significant limitations, we believe that our findings bring important information about the dynamics of CXCL12 and CXCR4 in early-stage BC of the most common receptor subtype.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
2. GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010–19: a systematic analysis for the global burden of disease study 2019. Lancet. 2022;400:563–591.
3. Motyka J, Gacuta E, Kicman A, et al. Plasma levels of CXC Motif Chemokine 1 (CXCL1) and chemokine 8 (CXCL8) as diagnostic biomarkers in luminal A and B breast cancer. J Clin Med. 2022;11:6694.
4. Arnold M, Morgan E, Rumgay H, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23.
5. Ginsburg O, Yip CH, Brooks A, et al. Breast cancer early detection: a phased approach to implementation. Cancer. 2020;126:2379–2393.
6. Duffy MJ, Evoy D, McDermott EW. CA 15-3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta. 2010;411:1869–1874.
7. Piskór BM, Przylipiak A, Dąbrowska E, et al. Plasma level of MMP-10 may be a prognostic marker in early stages of breast cancer. J Clin Med. 2020;9:4122.
8. Wang H, Yang X. Association between serum cytokines and progression of breast cancer in Chinese population. Medicine. 2017;96:e8840.
9. Dąbrowska E, Przylipiak A, Zajkowska M, et al. C-C motif chemokine ligand 5 and C-C chemokine receptor type 5: possible diagnostic application in breast cancer patients. Acta Biochim Pol. 2020;67:539.
10. Smolarz B, Nowak AZ, Romanowicz H, Cancer-Epidemiology B. Classification, pathogenesis and treatment (review of literature). Cancers. 2022;14:2569.
11. Danforth DN. The role of chronic inflammation in the development of breast cancer. Cancers. 2021;13:3918.
12. Han Y, Mao F, Wu Y, et al. Prognostic role of C-reactive protein in breast cancer: a systematic review and meta-analysis. Int J Biol Markers. 2011;26:209–215.
13. Shi Y, Riese DJ 2nd, Shen J. The role of the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Front Pharmacol. 2020;11:574667.
14. Masih M, Agarwal S, Kaur R, et al. Role of chemokines in breast cancer. Cytokine. 2022;155:155909.
15. Sun Y, Mao X, Fan C, et al. CXCL12-CXCR4 axis promotes the natural selection of breast cancer cell metastasis. Tumour Biol. 2014;35:7765–7773.
16. Zielińska KA, Katanaev VL. The signaling duo CXCL12 and CXCR4: chemokine fuel for breast cancer tumorigenesis. Cancers. 2020;12:3071.
17. D’Alterio C, Giardino A, Scognamiglio G, et al. CXCR4-CXCL12-CXCR7 and PD-1/PD-L1 in pancreatic cancer: CXCL12 predicts survival of radically resected patients. Cells. 2022;11:3340.
18. Łukaszewicz-zając M, Mroczko B, Kozłowski M, et al. The serum concentrations of chemokine CXCL12 and its specific receptor CXCR4 in patients with esophageal cancer. Dis Markers. 2016;2016:7963895.
19. Dąbrowska E, Przylipiak A, Zajkowska M, et al. Possible diagnostic application of CXCL12 and CXCR4 as tumor markers in breast cancer patients. Anticancer Res. 2020;40:3221–3229.
20. Chandrashekar DS, Karthikeyan SK, Korla PK, et al. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27.
21. Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658.
22. Hozhabri H, Moghaddam MM, Moghaddam MM, et al. A comprehensive bioinformatics analysis to identify potential prognostic biomarkers among CC and CXC chemokines in breast cancer. Sci Rep. 2022;12:10374.
23. Chen E, Qin X, Peng K, et al. Identification of potential therapeutic targets among CXC chemokines in breast tumor microenvironment using integrative bioinformatics analysis. Cell Physiol Biochem. 2018;45:1731–1746.
24. Li Y, Liang M, Lin Y, et al. Transcriptional expressions of CXCL9/10/12/13 as prognosis factors in breast cancer. J Oncol. 2020;2020:4270957.
25. Mehraj U, Alshehri B, Khan AA, et al. Expression pattern and prognostic significance of chemokines in breast cancer: an integrated bioinformatics analysis. Clin Breast Cancer. 2022;22:567–578.
26. Liu H, Li Z, Deng M, et al. Prognostic and clinicopathological value of CXCL12/SDF1 expression in breast cancer: a meta-analysis. Clin Chim Acta. 2018;484:72–80.
27. Chuan T, Li T, Yi C. Identification of CXCR4 and CXCL10 as potential predictive biomarkers in Triple Negative Breast Cancer (TNBC). Med Sci Monit. 2020;26:e918281.
28. Guo K, Feng G, Yan Q, et al. CXCR4 and CXCR3 are two distinct prognostic biomarkers in breast cancer: database mining for CXCR family members. Mol Med Rep. 2019;20:4791–4802.
29. Wu W, Qian L, Chen X, Ding B. Prognostic significance of CXCL12, CXCR4, and CXCR7 in patients with breast cancer. Int J Clin Exp Pathol. 2015;8:13217–13224.
30. Wu W, Qian L, Dai J, et al. 不同分子分型乳腺癌组织中趋化因子 CXCL12 及其受体 CXCR4 和 CXCR7 的表达及临床意义 [Expression of chemokine CXCL12 and its receptor (CXCR4 and CXCR7) in different molecular subtypes of human breast carcinoma and the clinical significance]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42:147–153. Chinese.
31. Pan H, Peng Z, Lin J, et al. Forkhead box C1 boosts triple-negative breast cancer metastasis through activating the transcription of chemokine receptor-4. Cancer Sci. 2018;109:3794–3804.
32. Ahirwar DK, Nasser MW, Ouseph MM, et al. Fibroblast-derived CXCL12 promotes breast cancer metastasis by facilitating tumor cell intravasation. Oncogene. 2018;37:4428–4442.
33. Helbig G, Christopherson KW, Bhat-Nakshatri P, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–21638.
34. Pasquier J, Abu-Kaoud N, Abdesselem H, et al. SDF-1alpha concentration dependent modulation of RhoA and Rac1 modifies breast cancer and stromal cells interaction. BMC Cancer. 2015;15:569.
35. Masuda T, Endo M, Yamamoto Y, et al. ANGPTL2 increases bone metastasis of breast cancer cells through enhancing CXCR4 signaling. Sci Rep. 2015;5:9170.
36. Seoane S, Martinez-Ordoñez A, Eiro N, et al. POU1F1 transcription factor promotes breast cancer metastasis via recruitment and polarization of macrophages. J Pathol. 2019;249:381–394.
37. Zhou W, Guo S, Liu M, et al. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr Med Chem. 2019;26:3026–3041.
38. Schrader AJ, Lechner O, Templin M, et al. CXCR4/CXCL12 expression and signalling in kidney cancer. Br J Cancer. 2002;86:1250–1256.
39. Sasaki K, Natsugoe S, Ishigami S, et al. Expression of CXCL12 and its receptor CXCR4 in esophageal squamous cell carcinoma. Oncol Rep. 2009;21:65–71.
40. Machelon V, Gaudin F, Camilleri-Broët S, et al. CXCL12 expression by healthy and malignant ovarian epithelial cells. BMC Cancer. 2011;11:97.
41. Mirisola V, Zuccarino A, Bachmeier BE, et al. CXCL12/SDF1 expression by breast cancers is an independent prognostic marker of disease-free and overall survival. Eur J Cancer. 2009;45:2579–2587.
42. Frayyeh A, Salman E, Melconian A. Elevated serum level of CXCR4 in breast cancer women. Res J Pharm Technol. 2019;12:3833.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.