Back to Journals » ImmunoTargets and Therapy » Volume 11
Current Strategies in Treating Cytokine Release Syndrome Triggered by Coronavirus SARS-CoV-2
Received 27 January 2022
Accepted for publication 7 May 2022
Published 18 May 2022 Volume 2022:11 Pages 23—35
DOI https://doi.org/10.2147/ITT.S360151
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Michael Shurin
Long G Wang,1 Luxi Wang2
1Department of Research and Development, Natrogen Therapeutics International, Inc., Valhalla, NY, USA; 2Department of Clinical Research, Clinipace Clinical Research, Morrisville, NC, USA
Correspondence: Long G Wang, Department of Research and Development, Natrogen Therapeutics International, Inc., Vosburgh 213, NYMC Campus, Valhalla, NY, 10595, USA, Tel +1 646 229-7583, Email [email protected]
Abstract: Since the beginning of the SARS-CoV-2 pandemic, the treatments and management of the deadly COVID-19 disease have made great progress. The strategies for developing novel treatments against COVID-19 include antiviral small molecule drugs, cell and gene therapies, immunomodulators, neutralizing antibodies, and combination therapies. Among them, immunomodulators are the most studied treatments. The small molecule antiviral drugs and immunoregulators are expected to be effective against viral variants of SARS-CoV-2 as these drugs target either conservative parts of the virus or common pathways of inflammation. Although the immunoregulators have shown benefits in reducing mortality of cytokine release syndrome (CRS) triggered by SARS-CoV-2 infections, extensive investigations on this class of treatment to launch novel therapies that substantially improve efficacy and reduce side effects are still warranted. Moreover, great challenges have emerged as the SARS-CoV-2 virus quickly, frequently, and continuously evolved. This review provides an update and summarizes the recent advances in the treatment of COVID-19 and in particular emphasized the strategies in managing CRS triggered by SARS-CoV-2. A brief perspective in the battle against the deadly disease was also provided.
Keywords: COVID-19, cytokine storm, cytokine release syndrome, antiviral drug, neutralizing antibodies, immunoregulators
Since the beginning of the SARS-CoV-2 pandemic, the treatments and management of the deadly COVID-19 disease have made great progress. According to the dashboard from FDA’s (Food and Drug Administration) Coronavirus Treatment Acceleration Program (CTAP) on March 11, 2022, by February 28, 2022, there are over 690 drug development programs in planning stages, more than 470 clinical trials have been reviewed by the FDA, 15 COVID-19 treatments have been authorized for emergency use (EUA), and 1 treatment has been approved by the FDA. The treatment strategies being studied include antiviral, cell and gene therapies, immunomodulators, neutralizing antibodies, and combination therapies. Among them, immunomodulators are the most studied treatment. Many aspects on the treatments and management of COVID-19 have been extensively reviewed.1–6 This review is intended to provide an update and summarize the recent advances in the treatment of COVID-19 and in particular to emphasize on the strategies in managing cytokine release syndrome (CRS) triggered by the coronavirus SARS-CoV-2. For vaccine developments, please see reviews by Kyriakidis et al and Li et al.7,8
Antiviral Drugs
SARS-CoV-2 virus belongs to a coronavirus family of enveloped viruses with a positive sense, single-stranded RNA genome and infects animal species and humans.9 Studies have shown that several key phases are involved in the SARS-CoV-2 life cycle, including virus binding, entry, proteolysis, RNA replication, and then exit.9–12 After binding of the virus via its S proteins to cellular ACE2 (angiotensin-converting enzyme 2) receptor on the cell membrane, the virus enters the cell, viral genome is released via endosomal acid protease cleavage and translated into viral replicase polyproteins PP1a and PP1ab. The viral proteins are then cleaved into functional proteins by viral proteases. Viral genome replication is initiated following proteolysis, which is mediated by viral replication complexes, including RNA-dependent RNA polymerase (RdRp), helicase, exonuclease N, and other accessory proteins. Subsequently, the new packaged virus is assembled in endoplasmic reticulum (ER) and Golgi containing viral genomes and translated viral structural proteins exits from the cell through exocytosis and repeats the life cycle. Understanding this full viral life cycle enable us to find potential therapeutic targets for pharmaceutical interventions.10,11 For example, blocking the viral RNA replication can be achieved by using RdRp inhibitors and halting viral replication by a protease inhibitor.10–12
RdRp Inhibitors – Remdesivir and Molnupiravir
Coronaviruses use an RdRp for their replication and transcription of the RNA genome, thus RdRp becomes an important target for the development of antiviral drugs against coronaviruses.13–17 Both remdesivir and molnupiravir are nucleoside analogs and act as inhibitors of RdRp thereby blocking RNA-dependent RNA replication of coronaviruses including SARS-CoV-2.
Remdesivir, an antiviral small molecule drug from Gilead Sciences has been fully approved by the FDA for the treatment of hospitalized patients with COVID-19. The drug is administered intravenously. The active form of remdesivir is a nucleoside analog and thus acts as a pseudo substrate of RdRp and interferes with the RNA-dependent RNA replication of SARS-CoV-2.18 As a pseudo substrate, Kokic et al reported that remdesivir is able to incorporate via the RdRp complex into the newly synthesized RNA product and allows for addition of three more nucleotides before RNA synthesis stalls. Interestingly however, the study showed that the pseudo nucleoside incorporated RNA product failed to elongate the RNA chain due to a barrier of RNA translocation. This impairment causes retention of the RNA 3ʹ-nucleotide in the substrate-binding site of the RdRp and thereby interfering with entry of the next nucleoside triphosphate, and consequently blocks the RNA replication by terminating its elongation.
Molnupiravir (MK-4482, EIDD-2801) from Merck is an oral antiviral drug recently completed a Phase 3 trial for the treatment of patients with COVID-19.19 As an oral drug, molnupiravir is more convenient to use particularly for patients with mild-to-moderate diseases who are not hospitalized. Merck announced on October 1, 2021, that molnupiravir reduced the risk of hospitalization or death by approximately 50% compared to placebo for patients with mild or moderate COVID-19 in a positive interim analysis of the phase 3 study. More recently however, the data from the completed phase 3 trial showed the reduction of the risk of hospitalization or death by only approximately 30%. Nevertheless, UK’s Medicines and Healthcare Products Regulatory Agency has authorized molnupiravir for the treatment of mild-to-moderate COVID-19 in adults with a positive SARS-CoV-2 diagnostic test and who have at least one risk factor for developing severe illness. FDA also issued EUA for molnupiravir on December 23, 2021, for the treatment of mild-to-moderate COVID-19 in adults with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19.
Molnupiravir metabolizes into its active form β-d-N4-hydroxycytidine (NHC) triphosphate after oral administration. Similar to remdesivir, NHC triphosphate (MTP) also acts as a pseudo substrate of the RdRp that replaces cytidine triphosphate (CTP) or uridine triphosphate (UTP) in the RNA replication, which leads to mutated RNA products. This mechanism of mutagenesis is distinct from that of remdesivir that impairs RdRp mediated RNA elongation. It possibly applies to various viral polymerases and thus explains the broad-spectrum antiviral activity of molnupiravir.15
It is noted that although molnupiravir showed effectiveness against COVID-19, potential risks need to be addressed as host RNA polymerases may also apply MTP for substrate, and indeed the mitochondrial DNA-dependent RNA polymerase can use EIDD-1931 and incorporate MTP into RNA in vitro.15,20
Proteinase Inhibitors – Paxlovid
Paxlovid from Pfizer is an oral antiviral drug which has completed a Phase 2/3 trial for the treatment of patients with COVID-19.21 In the final analysis of the data from the clinical study, the antiviral drug candidate was found to be 89% effective at preventing high-risk patients from hospitalization or death from COVID-19.22 Pfizer announced on December 16, 2021, that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued advice on the use of Paxlovid to treat adults with COVID-19 who do not require supplemental oxygen and who are at increased risk of progressing to severe disease.
Paxlovid contains two drugs nirmatrelvir and ritonavir, in which nirmatrelvir acts as 3CL protease inhibitor and ritonavir is an inhibitor of protease and cytochrome P450-3A4 used for the treatment of HIV/AIDS.21,23
Proteolysis including the cleavage, or breakdown, of polyproteins mediated by viral proteases is a critical step in the SARS-CoV-2 life cycle. This process is driven by two virally encoded proteases, the 3C-like (3CL) protease, and the papain-like protease. The 3CL protease cleaves the viral pp1ab polyprotein at 11 sites and the papain-like protease cleaves at 3 sites. Consequently, it transforms the viral RNA translated polyproteins into functional non-structural proteins (NSPs), which are critical for viral replication since enzymes such as RdRp or NSP13 from the virus are not able to fully function without the proteolysis.10–12 Interestingly, studies have shown that although coronaviruses are frequently mutated, proteases are highly conserved among coronaviruses including SARS-CoV-2, and moreover there are no human host proteases showing similar specificity of cleavage,24 which makes the viral proteases potential and ideal targets for therapeutic interventions by blocking the viral replication.10,11,25,26 On the basis of this scientific background, we expect that Paxlovid should also be effective and well tolerated against the new omicron variant of SARS-CoV-2.
Neutralizing Antibodies
The SARS-CoV-2 genome encodes structural, non-structural, as well as accessory proteins. Four major structural proteins ie, spike glycoprotein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N) are encoded by the virus genome at the 3’-end. The S protein consists of 2 subunits, S1 and S2, in which S1 binds to angiotensin-converting enzyme 2 (ACE2) on the host cell via its receptor-binding domain (RBD), and subsequently it causes a conformational change in S2 that results in virus-host cell membrane fusion followed by viral entry.27,28 Thus, S protein has become an ideal target for monoclonal antibody (mAb) intervention therapy. Therapeutic monoclonal antibodies can bind to and “neutralize” the virus and thus preventing target cell binding and/or fusion thereby blocking the virus entry into the host cell in infected patients.29,30 For more details on the mechanism of action of monoclonal antibodies against SARS-CoV-2, please read the articles by Lee and Taylor et al.31,32 Since the mAb therapy against SARS-CoV-2 is highly specific, this approach currently faces huge challenges as the virus frequently mutates its S protein that may significantly cause the therapeutic antibodies to lose their viral neutralizing capacity.
Currently, three anti-SARS-CoV-2 mAb products have received EUA from FDA for the treatment of mild-to-moderate COVID-19 in non-hospitalized patients with laboratory-confirmed SARS-CoV-2 infection who are at high risk for progressing to severe disease and/or hospitalization (https://www.covid19treatmentguidelines.nih.gov/). These products are: Casirivimab plus Imdevimab from Regeneron; Bamlanivimab plus Etesevimab from Eli Lilly; and Sotrovimab from GlaxoSmithKline.
Casirivimab Plus Imdevimab
Casirivimab and Imdevimab are recombinant human mAbs against SARS-CoV-2. These antibodies recognize different RBD regions of the virus S protein that are not overlapped thereby enhancing the binding affinity.31 FDA issued an EUA in Nov 21, 2020, for casirivimab and imdevimab to be administered together by i.v. for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (≥12 years with least 40 kilograms) with positive results of direct SARS-CoV-2 viral testing and who are at high risk for progressing to severe COVID-19.
Clinical study showed that treatment for 28 days of casirivimab plus imdevimab in patients with COVID-19 at high risk for disease progression reduced COVID-19-related hospitalization or emergency room visits.33
Bamlanivimab Plus Etesevimab
Bamlanivimab and Etesevimab are neutralizing mAbs that bind to different RBD regions in the S protein of SARS-CoV-2 that have overlapped each other.
Clinical studies have demonstrated that the combination of Bamlanivimab and Etesevimab resulted in a clinical benefit in people with mild to moderate COVID-19 who are at high risk for progression to severe disease and/or hospitalization.34,35 Consequently, FDA authorized emergency use of bamlanivimab plus etesevimab to prevent disease progression for COVID-19 on Sept 16, 2021.
Sotrovimab
Sotrovimab was developed by GlaxoSmithKline after its identification in 2003 from a SARS-CoV survivor.36 The mAb was authorized for emergency use by FDA in May 26, 2021 for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients with confirmed SARS-CoV-2 infection who are at high risk for progression to severe COVID-19, including hospitalization or death. Issuance of the EUA was based on the results from a double-blind trial (COMET-ICE) in 583 adult outpatients with mild-to-moderate COVID-19 who were ≥55 years old or had at least one comorbidity (diabetes, obesity, chronic kidney disease, heart failure, COPD, or moderate to severe asthma). This clinical trial showed that sotrovimab reduced the risk of disease progression while it was well tolerated.37
Sotrovimab binds to a conserved region between SARS-CoV and SARS-CoV-2 in the RBD of the S protein.36 Its exact mechanism of action is unknown, but it appears to prevent membrane fusion after the virus binds to the human ACE2 receptor.38
Immunomodulators
The hyperactive inflammatory response to SARS-CoV-2 infection plays a central role in the severity and mortality of COVID-19, thus immunomodulators become the most investigated approaches in treating moderate-to-severe Covid-19 according to summary of FDA Coronavirus Treatment Acceleration Program (CTAP) on March 11, 2022.
Cytokine Storm in COVID-19
The phenomenon “cytokine storm, or cytokine storm syndrome (CSS) or cytokine release syndrome (CRS)” which is generally described as an excessive or uncontrolled release of proinflammatory cytokines, has captured much attention because of its potential link to the deadly coronavirus infection.39,40 Cytokine storms can be induced by both chronic and acute infections, and have been linked to a wide variety of severe infections, such as SARS,41 MERS,42 influenza A virus and bacteria like Francisella tularensis.43 These pathogens disrupt the delicate balance necessary for a normal inflammatory response and instead produce a destructive response by creating a positive feedback in immune cells and an upregulation of proinflammatory mediators, in particular cytokines and chemokines TNF-α, IL-1β, IL-6, IL-8 and IL-17,39,40,43,44 with parallel increases in serum D-dimer, fibrinogen, ferritin, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). The consequence is multiple organ (cardiac, liver and kidney, etc) dysfunction/failure, sepsis syndrome, acute respiratory distress syndrome (ARDS), coagulopathy, cytopenias and death.45–48 The current operating definition of CSS by Cron and Behrens defines cytokine storm as an “activation cascade of auto-amplifying cytokine production due to unregulated host immune response to different triggers”.49 CSS involves both innate and adaptive immunity and associates with a wide range of pro-inflammatory cytokines/chemokines, such as IL-1, IL-6, IL-8, IL-12 and TNF-α.50 Also observed are increases of proinflammatory subsets of T-helper 17 cells (Th17) and cytotoxic T cells, whose actions are partly responsible for severe immune injury in the lungs of CSS patients.51 In a large prospective observational study from New York City, 22% of 1150 adults with COVID-19 admitted to the hospital had acute hypoxemic respiratory failure at admission. Of these 257 patients, 127 required immediate invasive mechanical ventilation (IMV) and of the remaining 130 patients, 76 required IMV after a median of 3 days.52 Overall, 39% of patients in this study died after a median of 9 days, including 41% of those who had received IMV and 31% of those who did not. It is speculated that a high number of these patients suffered from CSS. Indeed, patients demonstrate elevated levels of inflammatory cytokines, including IL-6, which was associated with poor disease course. Mean IL-6 concentration was 26 pg/mL and higher concentrations were associated with in-hospital mortality, an observation reported in other studies as well. A meta-analysis of 7 COVID-19 studies found that the mean serum IL-6 was 57 pg/mL among severely ill COVID-19 patients and 17 pg/mL among non-severe patients. Furthermore, higher IL-6 levels on admission were associated with higher mortality.53 These observations have promoted the use of anti-IL-6 therapy in treating moderate to severe COVID-19. Clinical utility of IL-6 levels as a predictor of progression to more severe disease is echoed in other publications54 and has led to the EUA issuance of Elecsys (Roche), an immunoassay for IL-6 to identify COVID-19 patients at high risk for IMV. According to Roche, in a study of 49 hospitalized COVID-19 patients, 16 of 19 (84.2%) patients who required IMV showed IL-6 levels >35 pg/mL whereas only 11 of 30 (36.7%) patients with IMV showed the same IL-6 levels.55 Positive predictive value of the test (% of patients with baseline IL-6 >35 pg/mL who would require IMV) was 59%.
Current Treatments of CRS
Consequences of COVID-19 triggered CRS include serious organ damage (cardiovascular, liver, neurologic manifestations, and renal injuries) that result in organ failure and death.56–61 Indeed, severe pulmonary inflammation causes activation of endothelial, damage to pulmonary vasculature and may trigger pulmonary thrombosis early in the disease course.62 There is a high incidence of venous thromboembolism in hospitalized COVID-19 patients, particularly those with severe illness. This coagulopathy is likely the result of the profound inflammatory response and endothelial activation/damage.46,63 Clinical and lab parameters including levels of D-dimer, LDH, C-reactive protein, and neutrophil: lymphocyte ratio, etc., are associated with risk of progression to critical illness and elevated mortality in COVID-19. Current methods for treating CRS focus mainly on immunosuppression. Several studies have investigated strategies targeting the overactive immune response,43,64 including stimulation of the cholinergic anti-inflammatory pathway, inhibition of prostaglandins, cyclooxygenase and platelet-activating factor, manipulation of chemokines, stimulation of Treg cells, etc. Novel therapies include monoclonal antibodies against INF-1, IL-6, GM-CSF, and TNF-α, as well as small molecules such as kinase inhibitors, and corticosteroids.65,66 Consequently, IL-6 antibodies, JAK inhibitors, Bruton’s tyrosine kinase (BTK) inhibitors, and corticosteroids have been authorized or recommended by FDA for emergency use in treating moderate-to-severe COVID-19. While these therapies do provide some clinical benefits including lower risk of death or respiratory failure,67–70 there is still a desperate need for interventions that can prevent progression to and treatment of COVID-19-associated CRS, and in particular to assist in healing/resolving organ damages.
IL-6 Antibody – Tocilizumab (Actemra)
Actemra was first approved by FDA in year 2010 for the treatment of rheumatoid arthritis (RA); systemic juvenile idiopathic arthritis (SJIA); polyarticular juvenile idiopathic arthritis (PJIA); giant cell arteritis; CRS; and systemic sclerosis-associated interstitial lung disease https://www.drugs.com/history/actemra.html. It is a monoclonal antibody that reduces inflammation by blocking the IL-6 receptor. FDA issued EUA of Actemra on June 21, 2021, for the treatment of COVID-19 in hospitalized adults and pediatric patients (2 years of age and older) who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). This EUA was based on review of the data from the RECOVERY clinical trial (NCT #04381936),71 the COVACTA clinical trial (NCT #04320615),72 the EMPACTA clinical trial (NCT #04372186),69 and the REMDACTA clinical trial (NCT #04409262).73
A variety of small observational and retrospective studies have reported encouraging results from the use of targeted IL-6 therapies in COVID-19 participants, including anti-IL-6 monoclonal antibodies tocilizumab (Actemra, Roche), siltuximab (Sylvant, EUSA Pharma) and the anti-IL-6 receptor monoclonal sarilumab (Kevzara, Sanofi/Regeneron).74 Earlier sponsor’s large trials of tocilizumab and sarilumab have reported disappointing results from confirmatory, prospective clinical trials of these agents in COVID-19 participants of varying degrees of severity,69,72 suggesting that an exclusive focus on IL-6 inhibition may not be sufficient. However, additional multiple large trials demonstrated benefits of Actemra in hospitalized COVID-19 patients that eventually has led to FDA issuance of EUA.
IL-6 functions through two main signaling pathways: cis and trans. In cis signaling, IL-6 forms a complex with its member receptor (mIL-6R) and gp130 which then activates downstream JAKs, signal transducer, and activator of transcription 3 (STAT3).75 The activation of this signal cascade leads to pleiotropic effects on the acquired immune system (B and T cells) as well as the innate immune system (neutrophils, macrophages, and natural killer cells) which contribute to CRS. In trans signaling, high concentration of circulating IL-6 bind to the soluble form of IL-6 receptor (sIL-6R) and form a complex with a gp130 dimer on most somatic cell types.76 The resultant IL-6–sIL-6R–JAK-STAT3 signaling is then activated in cells that do not express mIL-6R, such as endothelial cells. Considering IL-6 mAbs are big molecules that are difficult to pass through cell membrane, thus it would be less effective to block the sIL-6R signaling. This is probably why the therapeutic efficacy of IL-6 mAbs against COVID-19 is less effective as expected.
JAK-STAT Signaling and JAK Inhibitors
The Janus kinase (JAK)-STAT system involved in regulation of the immune system consists of 3 components: a receptor, JAK, and a STAT. Following binding of infectious agents, cytokines, or growth factors to its receptors, JAK activates its downstream proteins of the STAT family by phosphorylation, thereby promoting cellular proliferation, survival, and other biological processes. Various cytokines and mediators, including TNF-α, IFNγ, IL-1, IL-2, IL-6, IL-12, IL-15, IL-17, and IL-23, and chemokines IL-8, MCP-1, etc. will be produced and released during these processes. Elevated levels of some pro-inflammatory cytokines have been observed in various autoimmune/inflammatory diseases, as well as infectious diseases including CRS triggered by SARS-Cov-2.77,78 Among them, IL-6 seems to play a crucial role, whose increased serum levels have been correlated with ARDS.77,78
JAK inhibitors block JAK/STAT signaling thereby preventing phosphorylation of STAT proteins79 that play critical roles in cellular functions, such as growth, survival, metabolism, immune modulation, and inflammation.80 Thus, JAK inhibitors mediated suppression of inflammation could potentially lower the risk of CRS triggered by SARS-CoV-2 in patients with COVID-19. Moreover, it has been shown that baricitinib at clinical doses for the treatment of patients with rheumatoid arthritis (RA) affected viral endocytosis through its inhibition on members of numb-associated kinase family, AAK1 and GAK, therefore, it has been assumed that this JAK inhibitor may have a potential direct antiviral activity.81 There are 7 members of STATs family, ie, STAT 1–6, STAT5A&B, that are downstream of the JAK kinases. Each STAT has its own function. For example, STAT1, 2 and 4 are critical responders to viral or bacterial infection,82–84 thus they are critical in fighting microbe invaders; STAT5 is a critical transcriptional factor for T regulatory cells (Treg) cells,85 a normal function of STAT5s is important in maintaining balance between inflammatory T helper cells and anti-inflammatory Treg; STAT6 plays an important role in the metabolism of glucose and lipid,86–88 thus blocking normal activity of STAT6 will cause impairments of glucose and lipid metabolism. STAT3 has two activation sites, ie, STAT3-Y705 and STAT3-Ser727. While STAT3-Y705 plays an important role in autoimmune/inflammatory diseases STAT3-Ser727 site is important in maintaining normal mitochondrion function.89
Therefore, application of a JAK inhibitor will suppress JAK activation, followed by blocking activations of all STAT family proteins. The consequence of the signaling interruption is that it relieves inflammations or CRS while it may cause several unwanted side or even toxic effects, such as secondary infections, including severe infection and reactivating TB, increases of serum levels of cholesterol and triglycerides, and potential heart attack or stroke.90
Tofacitinib
Tofacitinib is the first oral JAK inhibitor approved by FDA for the treatment of RA in 2012, and psoriatic arthritis (PsA) in 2017, and then expanded to ulcerative colitis (UC) in 2018 (https://www.fda.gov/drugs/drug-safety-and-availability/initial-safety-trial-results-find-increased-risk-serious-heart-related-problems-and-cancer-arthritis). It is a prototypical JAK inhibitor, selectively targeting JAK1 and JAK3, with modest activity against JAK2 and TYK2.91 The FDA issued an EUA for the use of tofacitinib or baricitinib in combination with remdesivir to treat COVID-19 in November 2020. In the double-blind, placebo controlled STOP-COVID trial, 289 hospitalized patients with COVID-19 in Brazil were randomized to receive tofacitinib 10 mg or placebo orally twice daily for up to 14 days (or until hospital discharge). Among patients hospitalized with COVID-19 pneumonia, tofacitinib reduced risk of death or respiratory failure through day 28 as compared with placebo.68,92
Baricitinib
Baricitinib is an oral JAK inhibitor that selectively inhibits JAK1 and JAK2. FDA approved it for the treatment of adult patients with moderately to severely active rheumatoid arthritis (RA) in 2018. Baricitinib has been shown to modulate downstream inflammatory responses via JAK1/JAK2 inhibition and in particular, has exhibited dose-dependent inhibition of IL-6-induced STAT3 phosphorylation.93 It may also affect viral endocytosis via inhibitions of AAK1 and GAK, thereby potentially preventing SARS-CoV-2 from entering and infecting susceptible cells.81,94 Overall, reduction in the frequency of disease progression in patients treated with baricitinib plus standard of care (SOC) was not significant, however the treatment was found to reduce a risk of mortality in hospitalized patients with COVID-19.95 Thus, FDA has issued an EUA of baricitinib for treatment of COVID-19 in hospitalized adults and pediatric patients 2 years of age or older requiring supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Ruxolitinib
Ruxolitinib is another oral JAK inhibitor that selectively inhibits JAK1 and JAK2. It is initially approved for the treatment of myelofibrosis, polycythemia vera, and acute graft-versus-host disease.96 Similar to baricitinib, it can suppress downstream inflammatory responses, such as IL-6-induced STAT3 phosphorylation97 via inhibition of JAK1/JAK2. Ruxolitinib also has speculated antiviral effects via a possible inhibition on viral endocytosis regulators of AAK1 and GAK thereby blocking SARS-CoV-2 from entering and infecting host cells.
In a study of ruxolitinib versus dexamethasone trial in hospitalized adults with COVID-19, ruxolitinib treatment showed fatality rate similar to dexamethasone treatment. There was also no difference in median time to discharge without requirement of oxygen support between ruxolitinib treated group and control. Subgroup analysis, however showed a reduced mortality in ruxolitinib-treated patients with a high fever. This trial also showed that ruxolitinib therapy exhibited a better safety profile as a result of a reduction of severe cardiovascular adverse events (6.8% vs 15%, p=0.025) except higher incidence of grade 1 thrombocytopenia. Thus, it is concluded that ruxolitinib may be an alternative initial anti-cytokine therapy with comparable benefits in patients with potential risks of steroid administration.98 A Phase 3 trial of ruxolitinib in patients with COVID-19-associated ARDS is currently in progress (ClinicalTrials.gov Identifier NCT04377620).
NAT-528
NAT-528 developed by Natrogen Therapeutics International, is currently approved by FDA for phase 2 clinical trial to treat patients with moderate-to-severe COVID-19 who developed or are developing CRS. NAT-528 acts as a selective inhibitor of the phosphorylation of STAT3 at the Try705 (Y705) site (STAT3-Y705), but not STAT3-Ser727 nor other members of STAT family proteins to restore immune hemostasis. A phase 2 clinical trial in treating patients with moderate to severe UC demonstrated that NAT-528 not only relieves clinical symptoms by 82% and 91%, respectively at doses of 10mg and 20mg, BID for 4 weeks, but also significantly heals or resolves mucosa lesions that parallels the inhibition of IL-2R, IL-6 and phosphorylation of STAT3-Y705. NAT-528 significantly inhibited pathogen-stimulated expression of pro-inflammatory cytokines including IL-1β, IL-6, IL-12, IL-17, and TNF-α while increasing expression of anti-inflammatory cytokine IL-10 in a concentration-dependent manner in human monocyte THP 1 cells and increasing Treg cell population in vivo. The activity of NAT-528 on inhibition of IL-6 was found to be 500–700× stronger than that of tofacitinib (EC50 of NAT-528 was 0.39 nM vs 280 nM for tofacitinib). Thus, it is expected that NAT-528 will consequently attenuate lethal CRS triggered by SARS-CoV-2 infection, thereby improving survival rate, reducing need of ventilation, and preventing/resolving complications and sequelae of COVID-19. It may also have less side effects than JAK inhibitors because of its high specificity only against STAT3-Y705.
Bruton’s Tyrosine Kinase Inhibitors
Bruton’s tyrosine kinase (BTK) is a nonreceptor tyrosine kinase that interconnects B cell receptor (BCR) signaling, Toll-like receptor (TLR) signaling, and chemokine receptor signaling.99 While BCR signaling drives the proliferation of malignant B cells, such as chronic lymphocytic leukemia (CLL) cells, TLR and chemokine receptor signaling are highly associated with inflammation and thus, BTK inhibitors become potential therapeutic agents for COVID-19. During the last decade, BTK inhibitors have been increasingly used in replacement of conventional chemotherapies, especially in patients with CLL and mantle cell lymphoma (MCL).100 Overreactive immune response to SARS-CoV-2 leading to CRS in COVID-19 patients has promoted clinical studies to use BTK inhibitors in treating moderate to severe COVID-19 patients.101,102 These studies have showed promising results in reducing the severity of the immune response to the infection, and mortality.103–105
Ibrutinib
Ibrutinib is a first-generation small molecule BTK inhibitor by irreversibly binding to the protein. FDA initially approved it in 2013 and then expanded its applications during 2013 to 2020 to treat CLL, Waldenström’s macroglobulinemia (WM), and as a second-line treatment for mantle cell lymphoma, marginal zone lymphoma, and chronic graft vs host disease. Based on results from a small case study, ibrutinib has been speculated to be able to reduce severity of inflammation triggered by SARS-CoV-2 infection, and thus protect patients with COVID-19 from lung injury.102
Acalabrutinib and Zanubrutinib
Acalabrutinib and zanubrutinib are second-generation BTK inhibitors with more selective and more potent than the first generation inhibitor ibrutinib.106,107 FDA approved acalabrutinib in year 2017 to treat B-cell malignancies (ie, chronic lymphocytic leukemia/small lymphocytic lymphoma, mantle cell lymphoma),108 and zanubrutinib in August 2021 for adult patients with WM. The second-generation BTK inhibitors show a better toxicity profile than first-generation BTK inhibitors due to their more selective and less off-target activities against other kinases.106,109 A small clinical trial using acalabrutinib showed that this BTK inhibitor decreased the duration of mechanical ventilation and mortality rate for hospitalized patients with severe COVID-19, paralleled with the normalization of IL-6 and a reduction of lymphopenia in most cases.101,103 However, larger confirmatory clinical trials are needed to demonstrate the benefits of this BTK inhibitor in treating moderate to severe COVID-19 patients. There are currently no clinical data available on the use of zanubrutinib to treat COVID-19.
Metformin
Metformin is the most proscribed drug world-wide for type 2 diabetes (T2D) with an ideal safety profile over 50 years. Beyond activity against T2D, metformin has also been shown to be effective for treating various chronic diseases, such as cardiovascular disease, kidney disease, neurodegenerative disorders, and cancer, etc. Interestingly, while metformin has been found to regulate energy metabolism via inhibiting glucose absorption, transport, utilization, and gluconeogenesis,110,111 it acts as an anti-informatory agent through regulation of expression of pro-inflammatory and anti-inflammatory cytokines.111,112 Hyun et al reported that using LPS to stimulate RAW 264.7 cells and peritoneal macrophages, authors studied the effects of metformin on the expression of cytokines and the possible mechanisms behind it. They found that metformin inhibited the production of various pro-inflammatory cytokines including IL-1β, IL-6 and TNF-α as well as NO and PGE2 via down-regulation of NF-κB translocation. Metformin also stimulated expressions of anti-inflammatory cytokines IL-4 and IL-10 concurrently. Moreover, in a high-fat diet induced T2D C57BL/6N mouse model, metformin was found to suppress the secretion of TNF-α and expression of TNF-α at both the protein and mRNA levels in obese mice and macrophages.112 Earlier retrospective analysis has shown that metformin was associated with significantly lower mortality in older patients who were hospitalized with pneumonia and prior history of diabetes.113 Moreover, it has been generally recognized that pre-existing or newly diagnosed diabetes are often associated with an adverse prognosis in patients with COVID-19. Therefore, a suggestion has been made to FDA to use metformin as adjuvant therapy to decrease the mortality rate of COVID-19 in elderly, obese and diabetic patients through the reduction of weight and pneumonia.113 This suggestion is indeed supported by several retrospective analyses that showed metformin reduced admission into intensive care, and mortality, especially in diabetic women.114–116
Corticosteroids and Dexamethasone
Corticosteroids are the most effective anti-inflammatory therapy for many inflammatory diseases and systemic corticosteroids have been used in the treatment of numerous medical conditions for more than a half century.117 Dexamethasone is a long-acting, systemic corticosteroid and has been studied to treat hospitalized COVID-19 patients.118 The study showed that in patients hospitalized with COVID-19, dexamethasone reduced mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at randomization but not among those receiving no respiratory support. In addition, systemic corticosteroids used in combination with IL-6 antibodies, such as tocilizumab71,119 or JAK inhibitors like baricitinib,95 have demonstrated clinical benefit in subsets of hospitalized patients with COVID-19, especially those with early critical illness and/or with signs of cytokine storm.
Perspective and Conclusion
Since the pandemic, the race on COVID-19 vaccines and drug development has become a hotspot, and thus far a lot of progress has been made in the battle against the deadly SARS-CoV-2 infections. However, great challenges have emerged as the SARS-CoV-2 virus quickly, frequently, and continuously evolved120–123 (Figure 1). In case of variant omicron (BA.1, its subvariant, BA.2, and BA.3), it carries about 30 mutations in the gene for the S-protein.121 Since the majority of COVID-19 vaccines and human made therapeutic antibodies targeted S protein of the virus,121,124 these mutations not only make the virus more infectious or transmissible but also enable the virus to overcome a host’s immune response,120 vaccination-enhanced immunity, and antibody therapies.121
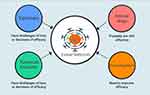 |
Figure 1 A scheme presentation of the perspective activity of vaccines, therapeutic antibodies, small molecule antiviral drugs, and immunoregulators against evolved SARS-CoV-2. |
The small molecule antiviral drugs including those repurposed drugs like remdesivir4,5,125 and immunoregulators are expected to still be effective against viral variants of SARS-CoV-2 as these drugs target either conservative parts of the virus or common pathways of inflammation. Although the immunoregulators have been shown benefits in reducing mortality of CRS triggered by SARS-CoV-2 infections, extensive investigations on this class of treatment to launch novel therapies that substantially improve efficacy and reduce side effects are still warranted (Figure 1).
The present practices to manage CSS or CRS triggered by SARS-CoV-2 mainly focus on kinase inhibitors that suppress signal pathways involved in inflammation, such as JAK inhibitors, Bruton’s tyrosine kinase (BTK) inhibitors, or wide-spectrum immunosuppressants like corticosteroids. While these immunosuppressive therapies may relieve symptoms of CRS, besides other side effects or toxicities, they may also cause secondary infections or delay patients’ recovery from the viral infections. Since CRS involves both innate immunity and adaptive immunity and is associated with dysregulated immune system, restoring immune homeostasis through therapeutic intervention, such as metformin and NAT-528 may be proven to have advantages over immunosuppressive therapies. Investigations on this aspect need to be paid more attention.
Therefore, great efforts need to be made continuously before we are able to declare a victory in the battle against the SARS-CoV-2.
Disclosure
Long G Wang was a formal employee of the Natrogen Therapeutics International. The authors report no other conflicts of interest in this work.
References
1. Rodriguez-Guerra M, Jadhav P, Vittorio TJ. Current treatment in COVID-19 disease: a rapid review. Drugs Context. 2021;10:1–8. doi:10.7573/dic.2020-10-3
2. Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). In: StatPearls. Treasure Island (FL): StatPearls; 2022.
3. Chibber P, Haq SA, Ahmed I, Andrabi NI, Singh G. Advances in the possible treatment of COVID-19: a review. Eur J Pharmacol. 2020;883:173372. doi:10.1016/j.ejphar.2020.173372
4. Nasir M, Perveen RA, Saha SK, Talha KA, Selina F, Islam MA. Systematic review on repurposing use of favipiravir against SARS-CoV-2. Mymensingh Med J. 2020;29(3):747–754.
5. Perveen RA, Nasir M, Talha KA, Selina F, Islam MA. Systematic review on current antiviral therapy in COVID-19 pandemic. Med J Malaysia. 2020;75(6):710–716.
6. Pascarella G, Strumia A, Piliego C, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi:10.1111/joim.13091
7. Kyriakidis NC, Lopez-Cortes A, Gonzalez EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):28. doi:10.1038/s41541-021-00292-w
8. Li Y, Tenchov R, Smoot J, Liu C, Watkins S, Zhou Q. A comprehensive review of the global efforts on COVID-19 vaccine development. ACS Cent Sci. 2021;7(4):512–533. doi:10.1021/acscentsci.1c00120
9. Eastman RT, Roth JS, Brimacombe KR, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6(5):672–683. doi:10.1021/acscentsci.0c00489
10. Senger MR, Evangelista TCS, Dantas RF, et al. COVID-19: molecular targets, drug repurposing and new avenues for drug discovery. Mem Inst Oswaldo Cruz. 2020;115:e200254. doi:10.1590/0074-02760200254
11. Simabuco FM, Tamura RE, Pavan ICB, Morale MG, Ventura AM. Molecular mechanisms and pharmacological interventions in the replication cycle of human coronaviruses. Genet Mol Biol. 2020;44(1 Suppl 1):e20200212. doi:10.1590/1678-4685-gmb-2020-0212
12. Pluskota-Karwatka D, Hoffmann M, Barciszewski J. Reducing SARS-CoV-2 pathological protein activity with small molecules. J Pharm Anal. 2021;11(4):383–397. doi:10.1016/j.jpha.2021.03.012
13. Hilgenfeld R, Peiris M. From SARS to MERS: 10 years of research on highly pathogenic human coronaviruses. Antiviral Res. 2013;100(1):286–295. doi:10.1016/j.antiviral.2013.08.015
14. Dolgin E. The race for antiviral drugs to beat COVID - and the next pandemic. Nature. 2021;592(7854):340–343. doi:10.1038/d41586-021-00958-4
15. Kabinger F, Stiller C, Schmitzova J, et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28(9):740–746. doi:10.1038/s41594-021-00651-0
16. Posthuma CC, Te Velthuis AJW, Snijder EJ. Nidovirus RNA polymerases: complex enzymes handling exceptional RNA genomes. Virus Res. 2017;234:58–73. doi:10.1016/j.virusres.2017.01.023
17. Vicenti I, Zazzi M, Saladini F. SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert Opin Ther Pat. 2021;31(4):325–337. doi:10.1080/13543776.2021.1880568
18. Kokic G, Hillen HS, Tegunov D, et al. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat Commun. 2021;12(1):279. doi:10.1038/s41467-020-20542-0
19. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi:10.1056/NEJMoa2116044
20. Sticher ZM, Lu G, Mitchell DG, et al. Analysis of the potential for N (4)-hydroxycytidine to inhibit mitochondrial replication and function. Antimicrob Agents Chemother. 2020;64(2). doi:10.1128/AAC.01719-19
21. Eng H, Dantonio AL, Kadar EP, et al. Disposition of PF-07321332 (Nirmatrelvir), an orally bioavailable inhibitor of SARS-CoV-2 3CL protease, across animals and humans. Drug Metab Dispos. 2022;
22. Mahase E. Covid-19: pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. MBJ. 2021;375:n2713.
23. Renjifo B, van Wyk J, Salem AH, Bow D, Ng J, Norton M. Pharmacokinetic enhancement in HIV antiretroviral therapy: a comparison of ritonavir and cobicistat. AIDS Rev. 2015;17(1):37–46.
24. Zhang L, Lin D, Sun X, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science. 2020;368(6489):409–412. doi:10.1126/science.abb3405
25. Wong JP, Damania B. SARS-CoV-2 dependence on host pathways. Science. 2021;371(6532):884–885. doi:10.1126/science.abg6837
26. Goyal B, Goyal D. Targeting the dimerization of the main protease of coronaviruses: a potential broad-spectrum therapeutic strategy. ACS Comb Sci. 2020;22(6):297–305. doi:10.1021/acscombsci.0c00058
27. Mariano G, Farthing RJ, Lale-Farjat SLM, Bergeron JRC. Structural characterization of SARS-CoV-2: where we are, and where we need to be. Front Mol Biosci. 2020;7:605236. doi:10.3389/fmolb.2020.605236
28. Paules CI, Marston HD, Fauci AS. Coronavirus infections-more than just the common cold. JAMA. 2020;323(8):707–708. doi:10.1001/jama.2020.0757
29. Renn A, Fu Y, Hu X, Hall MD, Simeonov A. Fruitful neutralizing antibody pipeline brings hope to defeat SARS-Cov-2. Trends Pharmacol Sci. 2020;41(11):815–829. doi:10.1016/j.tips.2020.07.004
30. Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol. 2020;38(1):10–18. doi:10.12932/AP-200220-0773
31. Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21(6):382–393. doi:10.1038/s41577-021-00542-x
32. Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5(10):1185–1191. doi:10.1038/s41564-020-00789-5
33. Razonable RR, Pawlowski C, O’Horo JC, et al. Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine. 2021;40:101102. doi:10.1016/j.eclinm.2021.101102
34. Dougan M, Azizad M, Mocherla B, et al. A randomized, placebo-controlled clinical trial of bamlanivimab and etesevimab together in high-risk ambulatory patients with COVID-19 and validation of the prognostic value of persistently high viral load. Clin Infect Dis. 2021. doi:10.1093/cid/ciab912
35. Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate covid-19. N Engl J Med. 2021;385(15):1382–1392. doi:10.1056/NEJMoa2102685
36. Quiros-Roldan E, Amadasi S, Zanella I, et al. Monoclonal antibodies against SARS-CoV-2: current scenario and future perspectives. Pharmaceuticals. 2021;14(12). doi:10.3390/ph14121272
37. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi:10.1056/NEJMoa2107934
38. Orders M. An EUA for sotrovimab for treatment of COVID-19. Med Lett Drugs Ther. 2021;63(1627):97–xx98.
39. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
40. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. Hlh Across Speciality Collaboration UK: COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi:10.1016/S0140-6736(20)30628-0
41. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi:10.1128/MMBR.05015-11
42. Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi:10.1016/j.cyto.2018.01.025
43. D’Elia RV, Harrison K, Oyston PC, Lukaszewski RA, Clark GC. Targeting the “cytokine storm” for therapeutic benefit. Clin Vaccine Immunol. 2013;20(3):319–327. doi:10.1128/CVI.00636-12
44. Lancet T. COVID-19: fighting panic with information. Lancet. 2020;395(10224):537. doi:10.1016/S0140-6736(20)30379-2
45. Carmi O, Berla M, Shoenfeld Y, Levy Y. Diagnosis and management of catastrophic antiphospholipid syndrome. Expert Rev Hematol. 2017;10(4):365–374. doi:10.1080/17474086.2017.1300522
46. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi:10.1182/blood.2020006000
47. Wujtewicz M, Dylczyk-Sommer A, Aszkielowicz A, Zdanowski S, Piwowarczyk S, Owczuk R. COVID-19 - what should anaethesiologists and intensivists know about it? Anaesthesiol Intensive Ther. 2020;52(1):34–41. doi:10.5114/ait.2020.93756
48. Ghahramani S, Tabrizi R, Lankarani KB, et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):30. doi:10.1186/s40001-020-00432-3
49. Cron EM. Cytokine storm syndrome. 2019.
50. Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2(7):e428–e436. doi:10.1016/S2665-9913(20)30120-X
51. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi:10.1016/S2213-2600(20)30076-X
52. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi:10.1016/S0140-6736(20)31189-2
53. Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020;92:2283–2285. doi:10.1002/jmv.25948
54. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi:10.1515/cclm-2020-0369
55. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1):128–136 e124. doi:10.1016/j.jaci.2020.05.008
56. Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5(7):751–753. doi:10.1001/jamacardio.2020.1105
57. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–818. doi:10.1001/jamacardio.2020.1017
58. Li J, Fan JG. Characteristics and mechanism of liver injury in 2019 coronavirus disease. J Clin Transl Hepatol. 2020;8(1):13–17. doi:10.14218/JCTH.2020.00019
59. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–391. doi:10.1016/j.pcad.2020.03.001
60. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi:10.1001/jamaneurol.2020.1127
61. Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40(6):1321–1326. doi:10.1111/liv.14449
62. Thachil J, Cushman M, Srivastava A. A proposal for staging COVID-19 coagulopathy. Res Pract Thromb Haemost. 2020;4(5):731–736. doi:10.1002/rth2.12372
63. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18(7):1559–1561. doi:10.1111/jth.14849
64. Behrens EM, Koretzky GA. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 2017;69(6):1135–1143. doi:10.1002/art.40071
65. Wang X, He Z, Zhao X. Immunoregulatory therapy strategies that target cytokine storms in patients with COVID-19 (Review). Exp Ther Med. 2021;21(4):319. doi:10.3892/etm.2021.9750
66. Mehta P, Porter JC, Manson JJ, et al. Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities. Lancet Respir Med. 2020;8(8):822–830. doi:10.1016/S2213-2600(20)30267-8
67. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N Engl J Med. 2021;384(9):795–807. doi:10.1056/NEJMoa2031994
68. Guimaraes PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with covid-19 pneumonia. N Engl J Med. 2021;385(5):406–415. doi:10.1056/NEJMoa2101643
69. Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi:10.1056/NEJMoa2030340
70. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704.
71. Group RC. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Lancent. 2021;397:1637–1645.
72. Rosas IO, Brau N, Waters M, et al. Tocilizumab in hospitalized patients with severe covid-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi:10.1056/NEJMoa2028700
73. Tatham KC, Shankar-Hari M, Arabi YM. The REMDACTA trial: do interleukin receptor antagonists provide additional benefit in COVID-19? Intensive Care Med. 2021;47(11):1315–1318. doi:10.1007/s00134-021-06540-w
74. Khan FA, Stewart I, Fabbri L, et al. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax. 2021;76(9):907–919. doi:10.1136/thoraxjnl-2020-215266
75. Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019;50(4):1007–1023. doi:10.1016/j.immuni.2019.03.026
76. Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8(9):1237–1247. doi:10.7150/ijbs.4989
77. Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi:10.1016/j.immuni.2020.04.003
78. Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368–370. doi:10.1016/j.jmii.2020.03.005
79. Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. Biochem J. 2014;462(1):1–13. doi:10.1042/BJ20140712
80. Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi:10.1016/j.clim.2020.108393
81. Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi:10.1016/S1473-3099(20)30132-8
82. Wang Y, Song Q, Huang W, et al. A virus-induced conformational switch of STAT1-STAT2 dimers boosts antiviral defenses. Cell Res. 2021;31(2):206–218. doi:10.1038/s41422-020-0386-6
83. Deng JC, Zeng X, Newstead M, et al. STAT4 is a critical mediator of early innate immune responses against pulmonary Klebsiella infection. J Immunol. 2004;173(6):4075–4083. doi:10.4049/jimmunol.173.6.4075
84. Mehrpouya-Bahrami P, Moriarty AK, De Melo P, et al. STAT4 is expressed in neutrophils and promotes antimicrobial immunity. JCI Insight. 2021;6(14):e141326. doi:10.1172/jci.insight.141326
85. Passerini L, Allan SE, Battaglia M, et al. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25- effector T cells. Int Immunol. 2008;20(3):421–431. doi:10.1093/intimm/dxn002
86. Iff J, Wang W, Sajic T, et al. Differential proteomic analysis of STAT6 knockout mice reveals new regulatory function in liver lipid homeostasis. J Proteome Res. 2009;8(10):4511–4524. doi:10.1021/pr9003272
87. Kang SG, Lee SE, Choi MJ, et al. Th2 cytokines increase the expression of fibroblast growth factor 21 in the liver. Cells. 2021;10(6):1298. doi:10.3390/cells10061298
88. Tsao CH, Shiau MY, Chuang PH, Chang YH, Hwang J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J Lipid Res. 2014;55(3):385–397. doi:10.1194/jlr.M041392
89. Nassal D, Mohta S, Patel N, et al. Regulation of STAT3 phosphorylation through βIV-spectrin interaction and its role in concentric versus eccentric hypertrophy. Circulation. 2021;144:A13911.
90. Kotyla PJ, Islam MA, Engelmann M. Clinical aspects of Janus Kinase (JAK) inhibitors in the cardiovascular system in patients with rheumatoid arthritis. Int J Mol Sci. 2020;21(19):7390. doi:10.3390/ijms21197390
91. Tanaka Y, Luo Y, O’Shea JJ, Nakayamada S. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022;18(3):133–145. doi:10.1038/s41584-021-00726-8
92. van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, et al. A guide to immunotherapy for COVID-19. Nat Med. 2022;28(1):39–50. doi:10.1038/s41591-021-01643-9
93. McInnes IB, Byers NL, Higgs RE, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 2019;21(1):183. doi:10.1186/s13075-019-1964-1
94. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi:10.1016/S0140-6736(20)30304-4
95. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407–1418. doi:10.1016/S2213-2600(21)00331-3
96. Ali H, Salhotra A, Modi B, Nakamura R. Ruxolitinib for the treatment of graft-versus-host disease. Expert Rev Clin Immunol. 2020;16(4):347–359. doi:10.1080/1744666X.2020.1740592
97. Vaddi K, Sarlis NJ, Gupta V. Ruxolitinib, an oral JAK1 and JAK2 inhibitor, in myelofibrosis. Expert Opin Pharmacother. 2012;13(16):2397–2407. doi:10.1517/14656566.2012.732998
98. Stanevich OV, Fomina DS, Bakulin IG, et al. Ruxolitinib versus dexamethasone in hospitalized adults with COVID-19: multicenter matched cohort study. BMC Infect Dis. 2021;21(1):1277. doi:10.1186/s12879-021-06982-z
99. Wen T, Wang J, Shi Y, Qian H, Liu P. Inhibitors targeting Bruton’s tyrosine kinase in cancers: drug development advances. Leukemia. 2021;35(2):312–332. doi:10.1038/s41375-020-01072-6
100. Burger JA. Bruton tyrosine kinase inhibitors: present and future. Cancer J. 2019;25(6):386–393. doi:10.1097/PPO.0000000000000412
101. Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020;5(48). doi:10.1126/sciimmunol.abd0110
102. Treon SP, Castillo JJ, Skarbnik AP, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135(21):1912–1915. doi:10.1182/blood.2020006288
103. Rada M, Qusairy Z, Massip-Salcedo M, Macip S. Relevance of the Bruton tyrosine kinase as a target for COVID-19 therapy. Mol Cancer Res. 2021;19(4):549–554. doi:10.1158/1541-7786.MCR-20-0814
104. Kifle ZD. Bruton tyrosine kinase inhibitors as potential therapeutic agents for COVID-19: a review. Metabol Open. 2021;11:100116. doi:10.1016/j.metop.2021.100116
105. Rezaei M, Babamahmoodi A, Marjani M. Bruton’s tyrosine kinase: a promising target for the treatment of COVID-19. Tanaffos. 2020;19(2):85–88.
106. Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol. 2016;9:21. doi:10.1186/s13045-016-0250-9
107. Tam CS, Opat S, Simpson D, et al. Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. 2021;5(12):2577–2585. doi:10.1182/bloodadvances.2020004074
108. Markham A, Dhillon S. Acalabrutinib: first global approval. Drugs. 2018;78(1):139–145. doi:10.1007/s40265-017-0852-8
109. Owen C, Berinstein NL, Christofides A, Sehn LH. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol. 2019;26(2):e233–e240. doi:10.3747/co.26.4345
110. Horakova O, Kroupova P, Bardova K, et al. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci Rep. 2019;9(1):6156. doi:10.1038/s41598-019-42531-0
111. Bai B, Chen H. Metformin: a novel weapon against inflammation. Front Pharmacol. 2021;12:622262. doi:10.3389/fphar.2021.622262
112. Hyun B, Shin S, Lee A, et al. Metformin down-regulates TNF-alpha secretion via suppression of scavenger receptors in macrophages. Immun Netw. 2013;13(4):123–132. doi:10.4110/in.2013.13.4.123
113. El-Arabey AA, Abdalla M. Metformin and COVID-19: a novel deal of an old drug. J Med Virol. 2020;92(11):2293–2294. doi:10.1002/jmv.25958
114. Bailey CJ, Gwilt M. Diabetes, metformin and the clinical course of Covid-19: outcomes, mechanisms and suggestions on the therapeutic use of metformin. Front Pharmacol. 2022;13:784459. doi:10.3389/fphar.2022.784459
115. Bramante CT, Ingraham NE, Murray TA, et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev. 2021;2(1):e34–e41. doi:10.1016/S2666-7568(20)30033-7
116. Zangiabadian M, Nejadghaderi SA, Zahmatkesh MM, Hajikhani B, Mirsaeidi M, Nasiri MJ. The efficacy and potential mechanisms of metformin in the treatment of COVID-19 in the diabetics: a systematic review. Front Endocrinol. 2021;12:645194. doi:10.3389/fendo.2021.645194
117. Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol. 2006;148(3):245–254. doi:10.1038/sj.bjp.0706736
118. Group TRC. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):12.
119. Gordon AC, Mouncey PR, Al-Beidh F, et al.; Investigators R-C. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502.
120. Callaway E. Beyond Omicron: what’s next for COVID’s viral evolution. Nature. 2021;600(7888):204–207. doi:10.1038/d41586-021-03619-8
121. Chen J, Wei GW. Omicron BA.2 (B.1.1.529.2): high potential to becoming the next dominating variant. ArXiv. 2022. doi:10.1021/acs.jpclett.2c00469
122. El-Arabey AA, Abdalla M. In the face of the future, what do we learn from COVID-19? Hum Vaccin Immunother. 2021;17(11):4119–4120. doi:10.1080/21645515.2021.1963174
123. Abdalla M, El-Arabey AA, Jiang X. Are the new SARS-CoV-2 variants resistant against the vaccine? Hum Vaccin Immunother. 2021;17(10):3489–3490. doi:10.1080/21645515.2021.1925503
124. Abdalla M, El-Arabey AA, Jiang X. What are the challenges faced by COVID-19 vaccines? Expert Rev Vaccines. 2022;21(1):5–7. doi:10.1080/14760584.2022.2008245
125. Nasir M, Talha KA, Islam T, Saha SK, Selina F, Parveen RA. Use of remdesivir in the management of COVID-19: a systematic review on current evidences. Mymensingh Med J. 2020;29(2):481–487.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
