Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Current Strategies and Potential Prospects for Nanoparticle-Mediated Treatment of Diabetic Nephropathy
Authors Liu C, Wu K, Gao H, Li J, Xu X
Received 29 June 2022
Accepted for publication 20 August 2022
Published 31 August 2022 Volume 2022:15 Pages 2653—2673
DOI https://doi.org/10.2147/DMSO.S380550
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Chunkang Liu,1,* Kunzhe Wu,2,* Huan Gao,3 Jianyang Li,3 Xiaohua Xu3
1Department of Gastrointestinal Surgery, China-Japan Union Hospital of Jilin University, Changchun, People’s Republic of China; 2Department of Scientific Research Center, China-Japan Union Hospital of Jilin University, Changchun, People’s Republic of China; 3Department of Nephrology, China-Japan Union Hospital of Jilin University, Changchun, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaohua Xu, Email [email protected]
Abstract: Diabetic nephropathy (DN), a severe microvascular complication of diabetes mellitus (DM), is the most common form of chronic kidney disease (CKD) and a leading cause of renal failure in end-stage renal disease. No currently available treatment can achieve complete cure. Traditional treatments have many limitations, such as painful subcutaneous insulin injections, nephrotoxicity and hepatotoxicity with oral medication, and poor patient compliance with continual medication intake. Given the known drawbacks, recent research has suggested that nanoparticle-based drug delivery platforms as therapeutics may provide a promising strategy for treating debilitating diseases such as DN in the future. This administration method provides multiple advantages, such as delivering the loaded drug to the precise target of action and enabling early prevention of CKD progression. This article discusses the development of the main currently used nanoplatforms, such as liposomes, polymeric NPs, and inorganic NPs, as well as the prospects and drawbacks of nanoplatform application in the treatment of CKD.
Keywords: diabetic nephropathy, DN, nanoplatform, route of administration
Introductions
DN is a chronic complication of diabetes and the leading cause of end-stage kidney disease, a specific microvascular disease that is the main cause of death in type 1 DM (T1DM).1,2 In type 2 DM (T2DM), DN greatly increases mortality and disability in patients with diabetes, and its degree of malignancy is second only to that of cardiovascular disease.3 Recent data has indicated that approximately 9% of the world’s adult population is affected by DN, and the prevalence of DN is nearly 70% in patients with diabetes; 592 million people have been predicted to have diabetes worldwide by the year 2035.4–6 As many as 47.66% of patients with diabetes have been estimated to have DN, thus posing a major challenge in treating DM. DN is also the primary cause of kidney failure in patients with diabetes.7
DN is more common in patients who have had T2DM for more than a decade, whose vascular damage is caused by hyperglycemia and affected by several pathways, such as oxidative stress, inflammatory response, endoplasmic reticulum (ER) stress.8 Hyperglycemia may be a direct trigger of inflammation, endoplasmic reticulum stress, and mitochondrial dysfunction, thus leading to excessive reactive oxygen species (ROS) production. Diabetic complications occur as a result of the accumulation of cellular damage caused by oxidative stress, and inflammatory infiltrates further lead to the release of ROS and proinflammatory cytokines.9,10 Glycemic control often relies on several strategies, such as lifestyle changes, low-sugar diets, chemical hypoglycemic agents, and herbal preparations; multiple medications are often required to achieve adequate glycemic control in most patients.11 Diabetes-induced fibrogenesis is driven by inflammation. Statins, vitamin D, and sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as glibenclamide, are now routinely considered anti-inflammatory medications.12 MCP-1, also known as chemokine ligand 2 (CCL2), produced by podocytes, plays a central role in DN inflammation. Elevated TRB3 expression significantly inhibits MCP-1 production and may be a therapeutic target for DN anti-inflammation.13 A relationship between oxygenation stress and ER stress is often thought to exist, because increased ROS production destabilizes the luminal environment of the ER, thereby causing ER stress.14 In addition, antioxidants have been found to decrease ER stress through the elimination of ROS and oxidative stress.15
The occurrence and development of DN are also thought to be related to genetic susceptibility, insulin resistance, low-grade inflammatory state, vascular endothelial cell dysfunction, and coagulation abnormalities.16 A major pathological abnormality in DN is mesangial expansion, the accumulation of extracellular matrix (ECM) within the mesangium, followed by tubulointerstitial fibrosis and glomerulosclerosis.17 The above pathological features may be adverse reactions of long-term extensive use of hypoglycemic drugs. At present, conventional treatments include strict weight control, blood sugar control, lipid and blood pressure reduction, protein intake restriction, smoking cessation, treatment goals of A1c < 7% and <130/80 mmHg, and elimination of albuminuria.18 However, commonly used drugs such as renin-angiotensin system blockers, metformin, long-and short-acting insulin often have high blood clearance, low effective circulating drug concentrations, and non-targeted drug delivery, thus leading to the development of end-stage renal disease in patients with DN.19 The end-stage renal disease stage of DN requires dialysis treatment, which accounts for 35% of new cases of dialysis treatment worldwide, and 13–25% of patients experience a fall after starting chronic hemodialysis, thus severely affecting quality of life in patients with DN.20
With developments in nanotechnology and biochemistry, a nanoplatform drug delivery system can be built in which anti-diabetic medicine can be broken down, implanted, encapsulated, or attached to nanoparticles.21,22 In addition, nanoparticles modified by active targeting molecules can also contribute to efficient drug delivery. Transported drugs pass through the immune clearance system, thus eliminating unwanted off-target effects and improving treatment effects.22,23 Therefore, the development of nanoparticle-mediated treatments is urgently required to overcome the adverse effects of conventional drug delivery methods. The current status of research and development in DN treatment is introduced and described in Table 1. This review presents the most recent developments in various nanoparticle technologies and their applications in DN treatment, as well as a new vision for the future development of nanoplatforms.
 |  |  |  |
Table 1 Examples of Preclinical Studies About Different Nanoplatform in the Treatment of DN |
Research Progress in Nanoplatforms in the Treatment of DN
Liposomes
The first synthesis of lipid NPs (LNs) occurred in the early 1990s, in a new era of searching for a new generation of nanocarriers. Given their multiple advantages of biodegradability, biocompatibility and low immunogenicity, LNs are regarded as a highly promising route of drug delivery. A liposome consists of either one (unilamellar liposomes) or several (multilamellar liposomes) lipid bilayers.24 Liposomes encapsulate hydrophilic drugs within the aqueous core of the bilayer membrane, and hydrophobic drugs are incorporated within the acyl chains.25 Relevant studies have indicated that liposomes allow for efficient incorporation of lipophilic drugs, thus not only enhancing membrane permeability, and consequently improving controlled release of active ingredients and increasing drug bioavailability, but also enabling the controlled delivery of a drug to specific tissues through surface functionalization with targeting molecules.26,27
Optimal nanoparticle sizes are also important, because the kidneys rapidly clear NPs less than 10 nm in size. However, NPs larger than 150 nm are recognized by the immune system and cleared.28 In addition, NPs of certain sizes can be selected by the kidneys. Owing to the presence of the glomerular filtration membrane in the kidney, particles of specific sizes can be isolated in the renal thylakoid membrane. Particles of 30–80 nm are effectively retained in the kidney, thus avoiding excretion from the urinary system and low bioavailability due to capture by the liver and spleen.4 Given that hyperglycemia promotes high levels of ROS, thus resulting in glucose-mediated damage, the mechanisms of damage include an increase in polyol pathway flux, and AGE formation and hexosamine pathway flux with activation of receptor of advanced glycation end-products (RAGE) and protein kinase C isoforms.29 Among the many antioxidants that may delay overproduction of ROS, phenolic compounds play important roles in ROS detoxification while maintaining the oxidation reduction (redox) balance to facilitate cellular homeostasis. Notably, in some cell lines and tissues, myricitrin appears to be able to decrease oxidative stress and cytotoxicity.30 To overcome the problem of low bioavailability of polyphenols with high polarity, research has been conducted on combining polyphenols. Ahangarpour et al31 have designed and used the cold homogenization method to prepare SLNs of myricitrin. In a mouse model of DN induced by streptozotocin-nicotinamide (STZ-NA), compared with a diabetes group, moderate and low doses of myricitrin have been found to be more effective in decreasing expression of the TGF-β gene. TGF-β is a key intermediary that exerts its effects by binding its receptors on the cell membrane, TGF-β R I and RII, thus promoting the phosphorylation of downstream factors, such as SMAD proteins, and stimulating glomerular cells to synthesize ECM, and TGF-β is associated with abnormal renal cell proliferation, glomerular hypertrophy, ECM protein accumulation, renal interstitial fibrosis, and damage to the glomerular filtration barrier.32 In SLN-treated diabetic mice, by decreasing oxidative stress through increasing antioxidant enzyme levels, LNs clearly ameliorate various pathological conditions in DN.31 This superior therapeutic effect by improving drug bioavailability is often found in the use of LNs in targeted organ therapy.33 Compared with conventional NPs, which have low bioavailability because of their low retention in the kidneys, LNs can be flexibly sized to enhance retention on the basis of differences in target organ filtration, and can be chemically modified to enhance their affinity toward target sites, to minimize drug loss from the bloodstream.34 Evidence indicates that rhubarb acid has antidiabetic effects, as well as anticancer, renoprotective, cardioprotective, and hepatoprotective activities. This plant may have the potential to protect body tissues, particularly the liver and kidneys, from environmental toxins and drug side effects.35 Wang et al4 have used a yolk-shell structure with polycaprolactone-polyethyleneimine (PCL-PEI) as the core and lipid layer modified with kidney-targeting peptide (KTP) and successfully fabricated kidney-targeted rhein (RH)-loaded LNs (KLPPR). KTP (sequence: CSAVPLC) was modified to promote rapid cellular internalization by renal tubular cells, endothelial cells, mesangial cells, and podocytes through uptake and endocytosis via a non-lysosomal pathway (Figure 1). The size of 75.4 ± 2.9 nm has been demonstrated to determine the kidney tissue distribution and specificity of NPs in DN model mice.4,36 In the case of TGF-β1, KLPPR shows more favorable pharmacological efficacy of RH than LPPR in a mouse model of DN; moreover, KLPPR significantly regulates the abnormal expression of proteins, thereby improving renal function in the kidney. The drug delivery system not only alleviates the hepatotoxic adverse effects of RH in clinical settings but also increases bioavailability through excellent kidney-targeted distribution. Notably, apigenin NPs are approximately 4.96 times more bioavailable than raw apigenin, but they have no toxic effects on rats’ organs.37 To decrease the high oral intake of apigenin and overcome the limitations of its poor stability in the blood, low absorption and high loss from the urinary system, Li et al38 have artificially increased the bioavailability of apigenin by mixing it into solid LNs through a microemulsification method. They have demonstrated that apigenin-SLNP activates the expression of nuclear factor-red lineage-2 related factor 2 and haematoxylin-1, thus resulting in an anti-apoptotic effect. Simultaneously, the anti-inflammatory effect increases the expression of Nrf2 and HO-1. The combination of apigenin and LNs is less toxic than other engineered formulations, thus indicating great potential for clinical applications. More promisingly, such combinations with weak toxic effects have shown similar pharmacological activity to that of metformin (MET), thus encouraging the application of NPs in renal injury studies. According to the results outlined above, the biodistribution sensitivity and specificity in lipid-modifying therapy can be significantly improved while decreasing nephrotoxic adverse effects. In vitro and in vivo studies have been performed primarily in mice and rats in preclinical trials. Nonetheless, before human testing, the drug will require a comprehensive evaluation of safety pharmacology and drug toxicology.
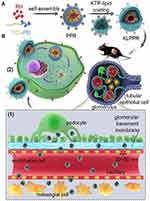 |
Figure 1 Generalized map of kidney-targeted drug transport based on KLPPR lipid nanoparticles. Abbreviations: KTP, kidney-targeting peptide; KLPPR, kidney-targeted RH-loaded liponano particles with a yolk-shell structure composed by PCL-PEI-based cores and KTP-modified lipid layers. Notes: (A). KLPPR composed of a negatively charged KTP-modified lipid layer and a positively charged rhein -loaded polycaprolactone-polyethyleneimine nanocore by electrostatic interaction. (B) Application of the two-step nanoscale cascade concept: (1) control the size of KLPPR in the range of 30–80 nm to ensure transport across the glomerular filtration membrane to the kidney; (2) KTP-modified membrane fusion promotes uptake and internalization by renal cells and increases the renal retention of KLPPR. Reproduced from Wang G, Li Q, Chen D et al, Kidney-targeted rhein-loaded liponanoparticles for diabetic nephropathy therapy via size control and enhancement of renal cellular uptake. Theranostics. 2019;9(21):6191–62084. |
Liposomes were the first nanomedicine technology to reach the clinical application stage in the past decade.39 Liposomes’ biocompatibility and biodegradability make them suitable for clinical applications.40 However, long-term stability and membrane permeability are major challenges with liposome systems. Some studies have shown that polyethylene glycol (PEG) chains may be covalently linked to increase liposome stability and in vivo circulation time, a possibility that warrants further study.
Polymeric NPs
NPs are currently favored because of their controlled drug release properties. Among the various types of nano-loaded systems, polymeric NPS have received widespread interest in the field of medical biology for their high efficiency, ease of preparation, long-term circulation, biodegradability, low toxicity, and ability to absorb and carry other molecules, in contrast to other nano-sized drug carriers.26,41 Polymeric NPs can be roughly subdivided into chitosan, polylactic acid and other branches such as dendrimers and nanogels.
Chitosan
Chitosan (CS), a non-toxic mucoadhesive polymer with biocompatibility and sites for easy modification, has excellent biodegradability, and has been shown to be a reliable, valid intestinal permeation enhancer favoring the absorption of protein drugs.9,42 This natural polysaccharide made from crustaceans and insects has been widely applied in various drug delivery systems because of its biocompatibility, safety, and mucoadhesive properties.6 The achievement of glycemic control remains the most important therapeutic goal for preventing DN and other complications of diabetes, because chronic hyperglycemia is closely associated with microvascular inflammation.43 Traditional blood glucose control methods, such as insulin administered subcutaneously, have been used as an invasive method to control glycemia in DN; its many drawbacks include needle phobia, pain, skin bulges, infections, hypoglycemia, peripheral hyperinsulinemia, and poor pharmacodynamics. Moreover, engineered formulations of oral insulin tend to have relatively poor absorption in the gastrointestinal tract.9,44 CS NPs thus provide a new avenue for oral insulin administration.
A derivative of CS known as trimethyl CS has superior solubility to that of CS.45 Ghavimishamekh et al9 have collected trimethyl CS carrying insulin and demonstrated that, in rats, oral therapy with oral insulin-loaded trimethyl CS NPs decreases fasting blood glucose (FBG) to a similar extent as that after injection of insulin; oral administration of insulin-loaded trimethyl CS NPs clearly decreases serum TGF-β1 levels, whereas subcutaneous insulin injection does not. This finding has been attributed to CS, as a natural cationic polysaccharide, facilitating mucosal adhesion and increasing absorption through the ionic interaction between its cationic amine groups and the anionic groups at the epithelial cell surface. Consequently, tight junctions of the intestinal epithelium temporarily open, thus supporting inter-epithelial insulin transport. The response of alginate to pH is reflected in that it dissolves in the alkaline pH of the intestine, but is much more stable under the acidic pH values of other organs, thus resulting in retention of insulin molecules in the gastrointestinal tract.6,9 When designing new formulations in future studies, considering the pH sensitivity of NPS is essential to identifying approaches for drug delivery systems, and avoiding significant washout of the drug after oral administration. As a non-toxic, biodegradable cationic polymer, CS is relatively immunogenic and has especially good macro-adhesion that can prolong the presence of drugs in the GI tract. CS binds negatively charged insulin and forms polyanion NPs with a size of 210 nm; these features can overcome the short duration of subcutaneous insulin injection.46,47 Additionally, CS NPs’ increased retention time, ultrafine size, and ability to release drugs for longer periods of time make them ideal oral delivery vehicles.48 Asal et al46 have used an emulsion solvent diffusion method to functionalize CS NPs (ChNPs) with poly lactic-co-glycolic acid (PLGA). They successfully fabricated CS gold NPs functionalized with poly lactic-PLGA (ChAuNP/PLGA). The zeta potential values for the prepared ChAuNPs decorated with PLGA changed from +37 ± 4.3 to −26 ± 2.1 mv after loading with insulin, thus indicating the better stability of the NPs in the final product. The positive charge of Ch enables NPs to traverse cell membranes or facilitate endocytosis. Research has demonstrated that the pharmacological availability and bioavailability of orally administered insulin-loaded ChAuNP/PLGA are clearly higher than those of orally administered insulin solution. This nano-delivery method similarly promotes the sustained release of the drug. Traditional Chinese medicine is considered to have strong development potential for DN therapy, because of its lower adverse effects and higher safety than conventional insulin and MET therapy. Polydatin (resveratrol-3-O-β-mono-D-glucoside) is well known as a polypeptide NP with antiglycation, antioxidant, anti-inflammatory, and nephroprotective effects, which can be easily prepared from common sources such as peanuts, grapes, and red wine.49–51 Abeer M. Abd El-Hameed have synthesized polydatin-loaded CS NPs (POL-NPs) through a modified ionotropic gelation method. The POL-NPs have been found to downregulate proinflammatory responses and mediators during the treatment of diabetes-associated complications.52 Notably, the hypoglycemic efficacy of POL-NPs is more pronounced than that of free POL. The mechanism of the renal protective effect of POL-NPs against DN involves regulating blood glucose and HbA1c by promoting insulin secretion, thus resulting in antidiabetic function; improving oxidative stress status and suppressing AGE formation, thus resulting in antioxidant and anti-inflammatory function; and improving the absorption and prolonged-release properties of NPs. POL-NP formulas substituting conventional hypoglycemic agents have received attention because of the protective roles of NPs loaded with traditional Chinese medicines in renal disease. Berberine (BBR), a natural isoquinoline alkaloid obtained from Chinese rhizomacoptidis, cortex phellodendri, berberis, and other types of plants, has a hypoglycemic effect through promoting insulin receptor expression, thus enhancing the sensitivity of the body to insulin; its hypoglycemic effect is similar to that of MET, the most commonly prescribed oral hypoglycemic agent.53,54 In addition, BBR also significantly ameliorates glucose metabolism and is therefore considered a promising anti-diabetic drug55. P-glycoprotein (Pgp-mediated drug efflux combined with tight junctions in the intestines clearly prevents BBR across the intestinal epithelium. Given that CS has been described to facilitate paracellular drug transport through opening intercellular tight junctions, Xiong et al6 have synthesized and characterized Brij-grafted-CS (BC). They innovatively grafted Brij-S20 (polyethylene glycol octadecyl ether) onto the amino group of CS to reverse the Pgp-mediated drug efflux and found that BC12 (BC with 12% grafting) exhibits excellent drug loading capacity and encapsulation efficacy. Moreover, they have demonstrated that BBR-BC12-NPs greatly increase the oral absorption of BBR through promoting intestinal permeability by facilitating paracellular transport and inhibiting Pgp-mediated drug efflux. CS is expected to become one of the most promising drug delivery pathways in the future, because of its various functions, which deserve further research.
CS has a number of advantages in formulation development, including its biocompatibility, biodegradability, and low immunogenicity.56 However, the low bioactivity and poor water solubility of CS may greatly limit its clinical application.57 Some studies have indicated that chemical modification can enhance the properties of CS without losing its unique characteristics, thus enabling a wider range of applications.58 Therefore, structural and functional modifications of CS to obtain different CS derivatives with good water solubility and bioactivity are a focus of research.
Polylactic Acid
Among biodegradable materials, polylactide or poly(lactic acid) are most commonly used, because they are derived from renewable resources such as sugar, corn or vegetables.59 Polylactoses, such as poly (D, L-lactide) (PLA), PLGA, poly-ɛ-caprolactone, gelatin, CS, and gelatin poly (alkyl cyanoacrylates), as biodegradable polymeric NPs, have been widely used as a platform for controlled drug release delivery systems for a variety of diseases such as diabetes and other malignant diseases, as well as kidney tissue engineering, because of their low toxicity, biocompatibility, and biodegradability. Some nondegradable polymers tend to have the disadvantage of requiring removal after loss of insulin effectiveness, eg, ethylene vinyl acetate.60 Because of its longer duration of action than that of typical subcutaneous injection of free insulin, polylactose has gradually attracted attention as a sustained-release drug delivery system.
Xiong et al61 have attached PLA segments to both ends of a Pluronic P85 copolymer to generate an amphiphilic PLA-P85-PLA block copolymer. Vesicular NPs have been demonstrated to deliver hydrophobic drugs and hydrophilic drugs. Moreover, degradation products produced by PLA block copolymers can enter the tricarboxylic acid cycle or be eliminated through the kidneys.62 Insulin-loaded PLA-P85-PLA vesicles have been found to be used to maintain blood glucose homeostasis for longer than normal subcutaneous injection of free insulin. The mechanism of prolonging hypoglycemic effects may involve the following. 1. PLA-P85-PLA vesicles protect insulin against enzymatic degradation in the GI tract. 2. PLA-P85-PLA vesicles (178 nm) with suitable particle size are conducive to insulin absorption in the intestines. 3. P85, owing to its amphiphilic properties, shows high cell membrane permeability in the intestines. 4. The small size and amphiphilic characteristics of vesicles delay macromolecular transport across the intestinal epithelium of insulin-loaded PLA-P85-PLA vesicles.61,63 In addition to enhancing the bioavailability of insulin, the development of polylactose in certain herbal medicines has also been investigated, because chemical-based dosing regimens are not only complex to synthesize and costly, but also ineffective and have substantial toxic adverse effects on the human body. Studies have indicated that that some Chinese herbs significantly decrease both structural damage and functional changes in DN, but clinical applications are severely hampered by their low solubility in water and poor bioavailability. A recent study has reported that Tinospora cordifolia contains antioxidants, radical scavengers, hepatoprotective agents, anticancer agents, antiallergics, immune modulators, and anti-inflammatory compounds.64 Tinospora cordifolia (Willd.) has been used as an antihypertensive and anti-inflammatory therapeutic agent by Ambalavanand et al65 for the synthesis of TC-PLA NPs through a double-emulsion solvent evaporation method. In rats treated with TC-PLA NPs, a significant decrease in the expression of inflammatory cytokines has been achieved, and inflammation and fibrosis in DN have been reported to be caused by ROS and pro-inflammatory cytokines. TC-PLA NPs effectively control DN by enhancing the active antioxidant function of plant components and decreasing their inflammation.65,66 Overall, TC‐PLA NPs protected renal function mainly through decreasing levels of blood glucose, stabilizing renal parameters, decreasing levels of inflammatory cytokines, and regulating gene expression levels. Studies have also shed light on modern approaches to promote the development of traditional Chinese medicine. Although biodegradable polymers such as PLA are generally considered to be safe, their immunotoxicity should still not be neglected, because smaller NPs appear to result in more damage to normal cells.67
Depending on the choice of polymer and method of nanocarrier fabrication and drug incorporation, polymeric nanocarriers constitute an important class of materials for biomedical applications.68 However, the safety and effectiveness of polymeric nanocarriers in the human body have not yet been demonstrated in a systematic review, and high-grade evidence is still lacking. The next research steps should involve studying this aspect in greater detail.
Inorganic NPs
Inorganic NPs involve the application of inorganic or hybrid nanomaterials and nanosized objects in the field of molecular medicine, including imaging examinations, medical diagnosis, therapy, clinical research, and basic science. Inorganic NPs have been developed in various imaging applications leading to medical diagnosis, therapy, or combination therapy.61,69 Inorganic NPs generally consist of metal, silica, nano-oxides, nano-composite oxides, nanometals, or alloys.69
Nano-Oxides
Inorganic nanomedicine has achieved innovative medical breakthroughs for biomarker discovery and molecular diagnostics. For example, iron oxide magnetic NPs (MNPs), composed of iron oxide NPs, have been found to improve the practicality and accuracy of magnetic resonance imaging, and have been approved for use as signal enhancers in tumor imaging and therapy.69
Islet autoantibodies can be measured before the appearance of some clinical symptoms, thus indicating that DN is a predictable disease. Inspired by this finding, LOX-1 has been validated as a biomarker in early stages of DN and has been found to mediate a vast array of inflammatory changes in renal tissue.70 Breil et al71 have synthesized anti-LOX-1-ultrasmall superparamagnetic iron oxide PEG-coated NPs (USPIO-PEG NPs) to detect noninvasively inflammatory renal lesions in early stages of DN. In DN mice, the signal intensity has been found to significantly decrease in T2*-weighted cortical MR, but has not shown any changes in normal mice injected with anti-LOX-1 USPIOs or in DN mice injected with untargeted USPIO NPs.71,72 Renal LOX-1 upregulation is associated with lipid peroxidation stress, inflammation, and fibrosis. Activated macrophages expressing LOX-1 selectively accumulate LOX-1-targeted PEG-coated USPIO NPs simultaneously. Signal reduction induced by LOX-1-targeted USPIO administration has been demonstrated to reflect the inflammatory response—an important feature of early stage of DN. Li et al73 have also noted that USPIO-PEG exhibits good cellular internalization and long-term MRI tracking properties. However, the lack of information regarding the safety and toxicity of LOX-1 targeted USPIOs in humans requires further investigation.71,74
After the heart, the kidneys contain the second most mitochondria and have the second highest level of energy metabolism. The main function of mitochondria, the most susceptible organelles to oxidative damage, is to generate large amounts of ATP via OXPHOS, thus ensuring the operation of normal physiological kidney function.75,76 Mitochondrial dysfunction caused by hyperglycemia plays a role in promoting the development of DN. Altered energy metabolism or altered mitochondrial dysfunction in DN can lead to renal dysfunction and eventually CKD.75 A dynamic balance between oxidative eustress and distress (ie, cellular redox homeostasis) is the basis for proper mitochondrial function. Biocompatible transition metal oxide NPs are highly sought after for their wide range of remarkable properties and applications, particularly their electron-donating and accepting activity, which is considered the best type of NP to maintain the balance of the redox potential of cellular mitochondria.77 C-Mn3O4 NP has become one of the least expensive and most effective materials for oxidative stress, owing to its free radical scavenging activity and good biocompatibility. C-Mn3O4 NP is autocatalytic and has protective effects through preventing mitochondrial permeability transition pore (mPTP) opening and ATP depletion. C-Mn3O4 NP can reduce oxidative stress by controlling the redox homeostasis, while the functionalization of citric acid greatly increases its biocompatibility. Simultaneously the Mn2+ ions produce harmless free radicals and can be well controlled by biological systems. Thus, C-Mn3O4 NPs show favorable biological properties and low toxicity, and are novel nanoplatforms for anti-DN drugs78.Adhikari et al77 have evaluated the potential of citrate functionalized Mn3O4 NPs (C-Mn3O4NPs) to alleviate mitochondrial injury and have indicated that C-Mn3O4 NPs protect mitochondria—the master regulator of cellular redox equilibrium, which promote high cell viability through scavenging of intracellular ROS, inhibit apoptotic triggers, and prevent loss of antioxidant enzymes (Figure 2). In vivo animal experiments have indicated that, according to drug elimination curve analysis of C-Mn3O4 NPs, the NPs in the blood of a CKD mouse model begin to be eliminated after 12 hours, or even after 24 hours in blood near the kidneys. Nanodrug concentrations in the therapeutic range are 0.95 ± 0.09 μg−1. The above results suggest that C-Mn3O4 NPs eliminate ROS—signaling molecules that enhance cell growth—while maintaining persistent long-term release in the kidneys. More interestingly in mice, C-Mn3O4 NPs do not have any toxic side effects.79 Given these advantages, C-Mn3O4 NPs may be superior to traditional oral hypoglycemic drugs.
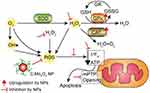 |
Figure 2 Schematic diagram of the pathway by which C-Mn3O4 NPs maintain redox homeostasis by counteracting H2O2 distress. Abbreviation: C- Mn3O4 NPs, citrate functionalized Mn3O4 nanoparticles. Notes: Adapted from Adhikari A, Mondal S, Chatterjee T et al, Redox nanomedicine ameliorates chronic kidney disease (CKD) by mitochondrial reconditioning in mice. Commun Biol. 2021;4(1): Article 101377. |
Zinc oxide NPs (ZnO NPs), among the most abundant and functionally diverse metal oxide NPs, are widely used in biomedical purposes for anticancer, drug delivery, antibacterial, and diabetes treatment, and antiinflammation.80 NPs made of ZnO are among the most important nanomaterials in this context, owing to their high electron mobility, good chemical stability, low toxicity, and biological compatibility.81 ZnO NPs regulate glucose levels through the following mechanisms: 1. decreasing glucose absorption by inhibiting the intestinal alpha-glucosidase enzyme, ameliorating hepatic glycogenesis by acting on insulin signaling pathways, and enhancing glycolysis, thereby improving glucose disposal; 2. a potential antiglycation effect through inhibition of AGE formation and prevention of changes in protein structure that may result from a consequence of covering of amino groups in protein structures or sequestration of the reacting group in glycating species by ZnO NPs; and 3. increasing Zn concentration, because strong antioxidants attenuate oxidative stress in diabetic rats.82–84 In diabetic kidneys, Alomari et al85 have demonstrated that ZnO NP treatment not only suppresses the expression of TGF‐β1, collagen IV, and fibronectin, but also increases the expression of MMP‐9, a key enzyme that downregulates the level of ECM and is associated with the occurrence and development of DN.66,85 Of note, the accumulation of Zn in kidney tissues and the development of toxicity must be avoided, which requires the regulation of the dose of the drug to the organism within a strict safety threshold.86
Nano-Composite Oxides
Drug-loaded NPs tend to unload drugs either slowly or prematurely while circulating in the bloodstream at the target tissues; thus, slow unloading effects are expected.87 Nano-composite oxides are a new type of functional material widely used in many industries, including chemical, medical, and electronics applications.88 The pathogenesis of DN often does not occur through a single pathway, and nanocomposites have gradually received attention because of their superior characteristics to simultaneously combat multiple pathogenesis.
The reducing activity of antioxidant enzymes combined with enhanced free radical generation and lipid peroxidation are major causative factors in CKD.89 Antioxidant therapy plays an important role in patients with diabetes, whereas traditional antioxidants, such as vitamins E and C, do not appear helpful.90 Zhong et al91 have designed hollow mesoporous silica nanocomposite (HMSN) particles doped with trace cerium oxide. In contrast to the poor safety of organic and inorganic NPs, such as potential carcinogenicity, statistically significant chronic toxicity has not been observed for small non-porous silica nanocomposites (SNPs) and MSNPs.91,92 The authors aimed to construct an oxidative stress-targeted multifunctional nanocomposite, fabricated by doping with trace cerium oxide to achieve long-term cyclic scavenging of free radicals in the body. CeO2 provides high oxygen storage abilities and enhances redox properties.93 The hollow structure of silica was etched to hold MET. In vitro drug release studies of MET from MET-HMSN-CeO2 NPs developed by Zhong et al have indicated that, at 24 hours, only 40% of the total drug is released from MET-HMSN-CeO2 NPs, thus implying sustained release of MET. The authors have observed the apoptosis of NRK-52E cells with TUNEL assays; the changes in HG-induced apoptosis of NRK-52E cells were ameliorated by treatment with MET-HMSN-CeO2 NPs. Thus, the results also demonstrated that HMSN-CeO2 NPs can achieve long-term cyclic scavenging of free radicals in vivo. Additionally, the antioxidative effect of cerium loaded HMSN occurs in vivo, as observed in pathological detection. Such nanocomplexes are used to treat DN by controlling hypoglycemia, inhibiting oxidative stress, and improving renal apoptosis, thereby also providing ideas and application prospects for treating other diseases with multiple pathogenic mechanisms.
Nanometals and Nanoalloys
During the new millennium, NPs of noble metals such as silver, gold, and platinum have been developed in medical and pharmaceutical applications; these NPs have received extensive attention in biomedicine, such as CKD treatment, because of their easy synthesis and stability in long-term storage.94,95 Nanoparticle preparations of metals have advantages of stability, lower dosages, easy storability, and sustained availability, which surpass the low mechanical properties of polymeric NPs. However, heavy metals are difficult to remove by biodegradation, and heavy metal NPs often accumulate in the kidneys.96 Therefore, how to avoid the adverse effects caused by heavy metals to the greatest extent possible has become a focus of current nanoparticle research.
For example, gold NPs (AuNPs) are metal NPs ranging in size from 2 to 100 nanometers.97 AuNPs are commonly used in medical molecular diagnosis and treatment and drug delivery, and the chemical modification of AuNPs can decrease toxicity.98,99 To overcome the limitation of ionic gold easily inactivated by complexation, Alomari et al100 have used a modified sodium citrate approach to synthesize 50 nm AuNPs, which have long blood circulation times.101 Under high glucose, TGF-β1 is released from glomerular mesangial cells, thus initiating epithelial-mesenchymal transition (EMT) and the accumulation of ECM proteins, an important characteristic of early DN, and driving renal fibrosis. TGF-β1 is linked to glomerular sclerosis, loss of podocytes, and DN.102–104 In a DN mouse model induced by STZ, AuNPs have been found to decrease TGF-β1 mRNA and protein levels, thereby decreasing ECM proteins, collagen IV, and fibronectin. These results indicate that AuNPs maintain the integrity and permeability of the glomerular filtration barrier through limiting mesangial matrix expansion and ameliorating podocyte damage in the diabetic condition.99 In another study, Manna et al95 have demonstrated that peel extract–stabilized gold nanoparticles (PPE-AuNPs) inhibit rage-induced hyperactivation of NOX-4/p47phox, thereby decreasing ROS production by inhibiting protein glycation and dephosphorylating the MAPK/NF-κB/Stat3-mediated proinflammatory response (Figure 3). Some studies have shown that pomegranate peel extract (PPE) inhibits lipid peroxidation in vivo and has antioxidant effects on removing ROS.101 In addition, exosomal urinary microRNAs protected by RNase activity have gradually become an accurate new biomarker for the early diagnosis of CKD, in which metal nanoparticle-based diagnosis is playing an increasingly important role.105 Tan et al106 have reported a sensor consisting of AuNPs and a composite under an aptamer panel to detect cancer biomarkers for cancer diagnosis. Compared with expensive qRT-PCR miRNA detection kits, the AuNP detection method is simple, economical, and low-tech, and does not require complex equipment or trained personnel to detect the expression of microRNAs.5,107 Nossier et al5 have used a simple AuNP colorimetric assay to diagnose DN; the test solution is red because the miRNA-probe adsorbs on AuNPs, thus preventing salt-induced aggregation of AuNPs. When non-specific miRNAs are present, only short probe molecules are adsorbed on AuNPs, and the test solution is blue. The assay’s sensitivity for detecting miRNAs is comparable to that of qRT-PCR, but the NPs are less expensive and easier to use. Furthermore, silver NPs are most commonly used, owing to their high antimicrobial activity.108 Olalekan et al109 have synthesized bitter melon NPs that significantly upregulate SOCS3 expression in STZ-induced diabetic rats, thereby downregulating the JAK/STAT signaling pathway and inhibiting its activation. This cascade of signaling is associated with the proliferation of mesangial cells. The NPs significantly upregulate the expression of PTEN in non-treated diabetic rats. The PTEN gene, a negative regulator of the PI3K/Akt pathway, visibly alleviates the inflammatory response, although the DNA damage and mutations easily caused by the properties of nanomaterials limit clinical use.110 Silver ions can easily cause DNA damage and mutation, thus limiting its clinical application. However, creatinine is the most important indicator in DN and is often used as a sub-index for DN to assess renal insufficiency in clinical practice. Silver ions can be used in in vitro biological tests of creatinine levels.111 Olalekan et al112 have used polyvinyl alcohol (PVA) and polyvinyl pyrrolidone (PVP) to modify silver NPs, whose color is related to the concentration of creatinine in the solution, and blood creatinine content can be quickly measured by an image processing system (colorimetry). Thus, PVP-coated silver NPs (AgNPs@PVP) and PVA-coated silver NPs (AgNPs@PVA) are expected to be applied in clinical settings to detect creatinine concentration in patients in the near future.
 |
Figure 3 The diagram illustrates the possible potential pathways of action of PPE-AuNP in reversing STZ-induced DN. Abbreviations: AuNP, gold nanoparticle; PPE, pomegranate peel extract; STZ, streptozotocin; DN, Diabetic nephropathy. Notes: Reproduced from Manna K, Mishra S, Saha M et al. Amelioration of diabetic nephropathy using pomegranate peel extract-stabilized gold nanoparticles: assessment of NF-kappaB and Nrf2 signaling system. Int J Nanomedicine. 2019;14:1753–177795. |
Quantum Dots
Quantum dots (QDs) are luminescent NPs that have been used extensively in QD displays and in a variety of bioimaging procedures.113 Although the pathogenesis of DN is complex, QD NPs are developing into a new research field for diseases such as DN.114–116
The polyethylene glycol coated QDs described by Pollinger et al114 prolong drug release time in the circulatory system and can also be enriched in the renal mesangium for targeted therapy. In addition, to overcome the limitations of traditional immunohistochemical detection of multiple biomarkers, Liu et al117 have established a dual-color immunofluorescence labeling technology for the application of QD primary antibody probes. Recognizing and binding Aldose reductase (AR) and Toll-like receptor 4 (TLR4), the linked probe QDs can yield stable and strong fluorescence and provide clearer imaging. However, the application of dual-color immunofluorescence labeling with QD cell therapy requires enhanced efficiency of conjugating QDs with the primary Ab. Therefore, further study is warranted.
Although nanotechnology has become increasingly dependent on QDs, as a result of increasing concerns regarding toxicity and chemical instability, QDs have been restricted to long-term usage.118 Two strategies are currently used to decrease the cytotoxicity of QDs, including the use of an aqueous synthesis method, as well as the formation of core/shell QDs at the core to protect against oxidation.119 We hope that in the future, more effective ways to further reduce the toxicity of QDs will be identified.
Biological NPs
Biomimetic nanomedicine is a bioengineered preparation that combines the physicochemical properties of various functional materials and the advantages of biomaterials, especially mammalian cells and pathogens (such as viruses) in biomaterials are the most commonly used components for synthesizing NPs with good properties. Its biocompatibility and high accumulation capacity are becoming a promising therapeutic approach.120 Compared with traditional biomedical nanomaterials, viral nanomaterials, as biological nanomaterials, can integrate the nucleic acid of the virus into the nucleic acid of the host cell, with higher specificity and stronger targeting specificity.121–123 Figueroa et al124 used influenza A virus Nps, which drives cinaciguat (CCG) (degraded by endolysosomes) into renal mesangial sites. CCG from the cytoplasm can specifically bind and activate the soluble form of guanylate cyclase (sGC), which then converts guanosine triphosphate (GTP) to 30.50-cyclic guanosine monophosphate (cGMP), thereby downregulating excessive glomerular fibrosis (Figure 4).124,125 The results showed that the same therapeutic effect was achieved despite only 10% of the administered dose carried by NPs compared to free ccg concentrations, and the drug delivery system appeared to significantly reduce the biological toxicity of the carried drug. In a word, particles with mimetic virus triploidy as a more accurate and stable recognition strategy showed better efficacy than traditional NPs.123 How to solve the biotoxicity of bio-nano drug-loaded particles and their degradation products is still one of the main challenges in the clinical application of nano-biomedicine, which deserves further research.
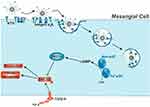 |
Figure 4 Schematic diagram of treatment principle. Nanoparticle (NP)-assisted cinaciguat (CCG) delivery to intracellular Apo-/Fe3+-sGC of target mesangial cells. Abbreviation: sGC, the soluble form of guanylate cyclase. Notes: After a sequential and thereby highly cell-selective mesangial NP uptake, CCG is released into the cytosol due to endolysosomal degradation of the NP. Here, CCG binds and thus activates both oxidized and heme-free sGC, leading to an increased production of 30.50-cyclic guanosine monophosphate (cGMP) and a protein kinase 1 α (PKG1-α) mediated inhibition of transforming growth factor β (TGF-β)-induced pathological remodeling. Adapted from Fleischmann D, Harloff M, Figueroa SM, Schlossmann J, Goepferich A. Targeted Delivery of Soluble Guanylate Cyclase (sGC) Activator Cinaciguat to Renal Mesangial Cells via Virus-Mimetic Nanoparticles Potentiates Anti-Fibrotic Effects by cGMP-Mediated Suppression of the TGF-beta Pathway. Int J Mol Sci. 2021;22(5):2557124. |
Challenges and Future Perspectives
DN has evolved into a major condition in type 1 and type 2 diabetes worldwide. It is a complicated disorder, and given that the number of people with diabetes is increasing, the incidence of DN is expected to soar. Currently, the goal of halting DN progression and achieving regression of albuminuria focuses on glucose, blood pressure, and lipid control, which remain standard therapy for DN. Oral hypoglycemic drugs, such as MET, often aggravate kidney damage through adverse reactions.126 The molecular targets of DN are crucial for site-specific targeted delivery, and future studies are expected to increasingly focus on drug delivery systems based on NPs. NPs can eliminate the toxicity characteristics of oral drugs, such as short duration of action, low oral bioavailability, and toxic adverse effects, thus providing new effective, and feasible therapeutic strategies for DN treatment.
Early DN often does not show any clinical symptoms. Because it is a progressive nephropathy, early detection and treatment of DN is particularly recommended. However, routine clinical examinations for DN, such as renal biopsy, are invasive and this histopathologic examination are considered for only patients with clinical presentation or disease progression.127 Therefore, new NP-based methods may offer a new approach to the diagnosis of DN, such as the use of lox-1-targeted iron oxide NPs for non-invasive imaging of DN. Renal damage is often key for the early diagnosis of DN. However, cells are susceptible to programmed cell death when large amounts of iron accumulate, and small NPs are associated with greater cytotoxicity.128 When the toxicity of iron ions is overcome, we believe that NPs such as lox-1-targeted iron oxide NPs will have bright prospects.
Multiple factors and mechanisms leading to DN interact and develop together, such as vasoactive pathways, including alterations in the renin-angiotensin-aldosterone system, hypertrophy of mesangial and tubular epithelial cells, endothelin receptor-mediated DN progression, and metabolic pathways, including the generation of ROS, activation of PKC, advanced glycation end products, and polyol pathways, and the release of transcription factors such as NF-IIB. Therefore, treating a single disease pathway with a single drug may not be sufficient to produce a therapeutic effect. Polymer-carrier NPs may play an important role in overcoming the renal filtration threshold, because their low molecular weight allows them to be filtered through the kidneys and retained in the kidneys through post-glomerular processes. In addition, MET-HMSN-CeO2, as a multifunctional NPs, and MET interacts with CeO2, thus forming aggregates, enhancing antioxidant activity of MET, and protecting the kidneys by enhancing resistance to cellular death. This approach unequivocally addresses the problem of the single hypoglycemic effect of MET and additionally shows potential effects leading to apoptotic resistance. Therefore, other properties of multifunctional NPs deserve further exploration. From a synergistic therapy perspective, these approaches may present new therapeutic opportunities to treat complex heterogeneous diseases.129 However, nanocarriers for therapeutic purposes must be safe in vivo, which requires that the concentration of silica be kept within safety limits.
As discussed previously, research on innovative nano-drug delivery platforms has begun, but further exploration in DN is needed to assess the pharmacokinetics and pharmacodynamics of characteristic proteinuria in pathological conditions, as well as changes in the GFR in pathological conditions, and to design an optimal intrarenal drug delivery platform to treat DN.130,131 Among the numerous NPs, biological NPs, such as those mimicking the sequence recognition strategy of influenza A virus, tend to be more kidney specific, thereby increasing their bioavailability. Unfortunately, little research has been performed on the application of viral NPs as drug delivery vehicles. Furthermore, evaluating the toxicity and biosafety of viral NPs requires extended trials, and in vitro and vivo studies. DN therapy requires high renal drug concentrations, thus necessitating the development of concentration-dependent targeted drug delivery systems, such as peptides, CS, and other nanoplatforms.
Compared with other DN treatments, the aim of nanoplatform-based drug delivery is to deliver drugs precisely to kidney sites and inhibit multiple pathogenic pathways. The application of multifunctional targeted NPs in DN diagnosis is a development trend whose successful clinical translation will require long-range action and safety assessment through in vitro and in vivo studies. In DN NP drug development, drug-loaded NPs are modified on the basis of the directional properties of DN pathological mechanisms, thereby increasing the interaction of nanodrugs with renal cells, and improving the intracellular growth of GBM epithelial cells, podocytes, mesangial cells, and proximal tubule cells. The development of nano-drug delivery platforms provides higher drug efficacy and safety, as well as new precise therapeutic measures to treat patients with DN.
Abbreviations
DN, Diabetic nephropathy; CKD, chronic kidney disease; DM, diabetes mellitus; T1DM, type 1 DM; T2DM, type 2 DM; ECM, extracellular matrix; LNs, lipid nanoparticles; NPs, nanoparticles; STZ-NA, streptozotocin-nicotinamide; PCL-PEI, polycaprolactone-polyethyleneimine; KTP, kidney-targeting peptide; RH, rhein; KLPPR, kidney-targeted RH-loaded liponanoparticles with a yolk-shell structure composed by PCL-PEI-based cores and KTP-modified lipid layers; SLN, Solid lipid nanoparticle; MET, metformin; PEG, Polyethylene glycol; CS, chitosan; FBG, fasting blood glucose; ChnPs, CS NPs; PLGA, poly lactic-co-glycolic acid; ChAuNP/PLGACS gold NPs functionalized with PLGA; POL-NPs, polydatinloaded CS NPs; BBR, Berberine; BC, Brij-grafted-CS; BC12, BC with 12% grafting; Pgp, P-glycoprotein; Brij-S20, Polyethylene glycol octadecyl ether; PLA, poly D, L-lactide; PLA-P85-PLA, PLA segments to both ends of a Pluronic P85 copolymer to generate an amphiphilic vesicles; TC, Tinospora cordifolia; TC-PLA NPs, TC-PLA nanoparticles; MNPs, magnetic nanoparticles; USPIO-PEG NPs, ultrasmall superparamagnetic iron oxide PEG-coated NPs; C- Mn3O4NPs, citrate func-tionalized Mn3O4 nanoparticles; ROS, reactive oxygen species; ZnO NPs, Zinc oxide NPs; RAGE, receptor of advanced glycation end-products; HMSN, hollow mesoporous silica nanocomposite; AuNPs, Gold NPs; EMT, epithelial-mesenchymal transition; QDs, quantum dots; AR, Aldose reductase; TLR4, Toll-like receptor 4; CCG, cinaciguat; SGc, soluble form of guanylate cyclase; GTP, guanosine triphosphate; cGMP, cyclic guanosine monophosphate; PVP, Polyvinyl pyrrolidone; PVA, Polyvinyl alcohol; AgNPs@PVP, PVP-coated silver nanoparticles; AgNPs@PVA, PVA-coated silver nanoparticles; PPE-AuNP, peel extract–stabilized gold nanoparticle.
Acknowledgments
Previously reported data were used to support this study and are available at DOI. These prior studies (and datasets) are cited at relevant places within the text as references.
Funding
This study was financially supported by the Science and Technology Development Plan Projects of Jilin Province (Grant No. 20210101294JC and Grant No.20220204032YY).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Zhao H, Cui Y, Dong F, et al. lncRNA MSC-AS1 aggravates diabetic nephropathy by regulating the miR-325/CCNG1 axis. J Healthc Eng. 2022;2022:2279072. doi:10.1155/2022/2279072
2. Manazir S, Durrani HM, Jawed F, et al. Concurrent presentation of diabetic nephropathy and type 1 diabetes mellitus in a pediatric patient. Cureus. 2021;13(12):e20831. doi:10.7759/cureus.20831
3. Allen A, Iqbal Z, Green-Saxena A, et al. Prediction of diabetic kidney disease with machine learning algorithms, upon the initial diagnosis of type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2022;10(1):e002560. doi:10.1136/bmjdrc-2021-002560
4. Wang G, Li Q, Chen D, et al. Kidney-targeted rhein-loaded liponanoparticles for diabetic nephropathy therapy via size control and enhancement of renal cellular uptake. Theranostics. 2019;9(21):6191–6208. doi:10.7150/thno.37538
5. Nossier AI, Shehata NI, Morsy SM, et al. Determination of certain urinary microRNAs as promising biomarkers in diabetic nephropathy patients using gold nanoparticles. Anal Biochem. 2020;609:113967. doi:10.1016/j.ab.2020.113967
6. Xiong W, Xiong SH, Chen QL, et al. Brij-functionalized chitosan nanocarrier system enhances the intestinal permeability of P-glycoprotein substrate-like drugs. Carbohydr Polym. 2021;266:118112. doi:10.1016/j.carbpol.2021.118112
7. Lin S, Yang J, Wu G, et al. Preventive effect of taurine on experimental type II diabetic nephropathy. J Biomed Sci. 2010;17(Suppl 1):S46. doi:10.1186/1423-0127-17-S1-S46
8. Meng X, Ma J, Kang AN, et al. A novel approach based on metabolomics coupled with intestinal flora analysis and network pharmacology to explain the mechanisms of action of bekhogainsam decoction in the improvement of symptoms of streptozotocin-induced diabetic nephropathy in mice. Front Pharmacol. 2020;11:633. doi:10.3389/fphar.2020.00633
9. Ghavimishamekh A, Ziamajidi N, Dehghan A, et al. Study of insulin-loaded chitosan nanoparticle effects on TGF-beta1 and fibronectin expression in kidney tissue of type 1 diabetic rats. Indian J Clin Biochem. 2019;34(4):418–426. doi:10.1007/s12291-018-0771-9
10. Tsai JL, Chen C-H, Wu M-J, et al. New approaches to diabetic nephropathy from bed to bench. Biomedicines. 2022;10(4):876. doi:10.3390/biomedicines10040876
11. Banu S, Jabir NR, Manjunath NC, et al. Reduction of post-prandial hyperglycemia by mulberry tea in type-2 diabetes patients. Saudi J Biol Sci. 2015;22(1):32–36. doi:10.1016/j.sjbs.2014.04.005
12. Hernandez LF, Eguchi N, Whaley D, et al. Anti-oxidative therapy in diabetic nephropathy. Front Biosci. 2022;14(2):14. doi:10.31083/j.fbs1402014
13. Morse E, Schroth J, You Y-H, et al. TRB3 is stimulated in diabetic kidneys, regulated by the ER stress marker CHOP, and is a suppressor of podocyte MCP-1. Am J Physiol Renal Physiol. 2010;299(5):F965–72. doi:10.1152/ajprenal.00236.2010
14. Roe ND, Ren J. Oxidative activation of Ca(2+)/calmodulin-activated kinase II mediates ER stress-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol. 2013;304(6):H828–39. doi:10.1152/ajpheart.00752.2012
15. Kang K, Tarchick MJ, Yu X, et al. Carnosic acid slows photoreceptor degeneration in the Pde6b(rd10) mouse model of retinitis pigmentosa. Sci Rep. 2016;6:22632. doi:10.1038/srep22632
16. Samsu N, Bellini MI. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1497449. doi:10.1155/2021/1497449
17. Xu Z, Zhang M, Wang Y, et al. Gentiopicroside ameliorates diabetic renal tubulointerstitial fibrosis via inhibiting the AT1R/CK2/NF-κB pathway. Front Pharmacol. 2022;13:848915. doi:10.3389/fphar.2022.848915
18. Wang J, Zhang L, Qin W, et al. Near-infrared probe for early diagnosis of diabetic complications-nephropathy and in vivo visualization fluorescence imaging research. Anal Chim Acta. 2022;1221:340147. doi:10.1016/j.aca.2022.340147
19. Zakiyanov O, Kalousová M, Zima T, et al. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in kidney disease. Adv Clin Chem. 2021;105:141–212. doi:10.1016/bs.acc.2021.02.003
20. Tuttle KR, Wong L, St. Peter W, et al. Moving from evidence to implementation of breakthrough therapies for diabetic kidney disease. Clin J Am Soc Nephrol. 2022;17(7):1092–1103. doi:10.2215/CJN.02980322
21. Yu B, Wu K, Xu X, et al. Recent advances in nanoplatforms for the treatment of neuropathic pain. Spinal Cord. 2022;60(7):594–603. doi:10.1038/s41393-021-00746-x
22. Lin B, Ma YY, Wang JW. Nano-technological approaches for targeting kidney diseases with focus on diabetic nephropathy: recent progress, and future perspectives. Front Bioeng Biotechnol. 2022;10:870049. doi:10.3389/fbioe.2022.870049
23. Wu K, Yu B, Li D, et al. Recent advances in nanoplatforms for the treatment of osteosarcoma. Front Oncol. 2022;12:805978. doi:10.3389/fonc.2022.805978
24. Juszkiewicz K, Sikorski AF, Czogalla A. Building blocks to design liposomal delivery systems. Int J Mol Sci. 2020;21(24):9559. doi:10.3390/ijms21249559
25. AlSawaftah NM, Awad NS, Paul V, et al. Transferrin-modified liposomes triggered with ultrasound to treat HeLa cells. Sci Rep. 2021;11(1):11589. doi:10.1038/s41598-021-90349-6
26. Fonseca-Gomes J, Loureiro JA, Tanqueiro SR, et al. In vivo bio-distribution and toxicity evaluation of polymeric and lipid-based nanoparticles: a potential approach for chronic diseases treatment. Int J Nanomedicine. 2020;15:8609–8621. doi:10.2147/IJN.S267007
27. Musielak E, Feliczak-Guzik A, Nowak I. Optimization of the conditions of solid lipid nanoparticles (SLN) synthesis. Molecules. 2022;27(7):2202.
28. Akhtar A, Wang S, Ghali L, et al. Effective delivery of arsenic trioxide to HPV-positive cervical cancer cells using optimised liposomes: a size and charge study. Int J Mol Sci. 2018;19(4):1081. doi:10.3390/ijms19041081
29. De Geest B, Mishra M. Role of oxidative stress in diabetic cardiomyopathy. Antioxidants. 2022;11(4):784. doi:10.3390/antiox11040784
30. Chen W, Feng L, Shen Y, et al. Myricitrin inhibits acrylamide-mediated cytotoxicity in human Caco-2 cells by preventing oxidative stress. Biomed Res Int. 2013;2013:724183. doi:10.1155/2013/724183
31. Ahangarpour A, Oroojan AA, Khorsandi L, et al. Antioxidant, anti-apoptotic, and protective effects of myricitrin and its solid lipid nanoparticle on streptozotocin-nicotinamide-induced diabetic nephropathy in type 2 diabetic male mice. Iran J Basic Med Sci. 2019;22(12):1424–1431. doi:10.22038/IJBMS.2019.13989
32. Xu ZJ, Shu S, Li ZJ, et al. Liuwei Dihuang pill treats diabetic nephropathy in rats by inhibiting of TGF-beta/SMADS, MAPK, and NF-kB and upregulating expression of cytoglobin in renal tissues. Medicine. 2017;96(3):e5879. doi:10.1097/MD.0000000000005879
33. Du B, Yan Y, Li Y, et al. Preparation and passive target of 5-fluorouracil solid lipid nanoparticles. Pharm Dev Technol. 2009;00(00):090921101957048. doi:10.1080/10837450903246390
34. Wang Z, Shi J, Pan H, et al. Membrane-cloaked polydopamine modified mesoporous silica nanoparticles for cancer therapy. Nanotechnology. 2022;33(34):345101. doi:10.1088/1361-6528/ac6fee
35. Ghorbani A, Amiri MS, Hosseini A. Pharmacological properties of Rheum turkestanicum Janisch. Heliyon. 2019;5(6):e01986. doi:10.1016/j.heliyon.2019.e01986
36. Chen D, Han S, Zhu Y, et al. Kidney-targeted drug delivery via rhein-loaded polyethyleneglycol-co-polycaprolactone-co-polyethylenimine nanoparticles for diabetic nephropathy therapy. Int J Nanomedicine. 2018;13:3507–3527. doi:10.2147/IJN.S166445
37. Wu W, Zu Y, Wang L, et al. Preparation, characterization and antitumor activity evaluation of apigenin nanoparticles by the liquid antisolvent precipitation technique. Drug Deliv. 2017;24(1):1713–1720. doi:10.1080/10717544.2017.1399302
38. Li P, Bukhari SNA, Khan T, et al. apigenin-loaded solid lipid nanoparticle attenuates diabetic nephropathy induced by streptozotocin nicotinamide through Nrf2/HO-1/NF-kB signalling pathway. Int J Nanomedicine. 2020;15:9115–9124. doi:10.2147/IJN.S256494
39. van Alem CMA, Metselaar JM, van Kooten C, et al. Recent advances in liposomal-based anti-inflammatory therapy. Pharmaceutics. 2021;13(7):1004. doi:10.3390/pharmaceutics13071004
40. Deng W, Chen W, Clement S, et al. Controlled gene and drug release from a liposomal delivery platform triggered by X-ray radiation. Nat Commun. 2018;9(1):2713. doi:10.1038/s41467-018-05118-3
41. Wu S, Helal-Neto E, Matos APDS, et al. Radioactive polymeric nanoparticles for biomedical application. Drug Deliv. 2020;27(1):1544–1561. doi:10.1080/10717544.2020.1837296
42. Li X, Wang Y, Feng C, et al. Chemical modification of chitosan for developing cancer nanotheranostics. Biomacromolecules. 2022;23:2197–2218.
43. Yamabe N, Yokozawa T, Oya T, et al. Therapeutic Potential of (-)-Epigallocatechin 3- O -Gallate on Renal Damage in Diabetic Nephropathy Model Rats. J Pharmacol Exp Ther. 2006;319(1):228–236. doi:10.1124/jpet.106.107029
44. Gordon Still J. Development of oral insulin: progress and current status. Diabetes Metab Res Rev. 2002;18(Suppl 1):S29–37. doi:10.1002/dmrr.207
45. Li Z, Li X, Cao Z, et al. Camptothecin nanocolloids based on N,N,N-trimethyl chitosan: efficient suppression of growth of multiple myeloma in a murine model. Oncol Rep. 2012;27(4):1035–1040. doi:10.3892/or.2012.1635
46. Asal HA, Shoueir KR, El-Hagrasy MA, et al. Controlled synthesis of in-situ gold nanoparticles onto chitosan functionalized PLGA nanoparticles for oral insulin delivery. Int J Biol Macromol. 2022;209:2188–2196. doi:10.1016/j.ijbiomac.2022.04.200
47. Mukhopadhyay P, Sarkar K, Chakraborty M, et al. Oral insulin delivery by self-assembled chitosan nanoparticles: in vitro and in vivo studies in diabetic animal model. Mater Sci Eng C Mater Biol Appl. 2013;33(1):376–382. doi:10.1016/j.msec.2012.09.001
48. Khan N, Bharali DJ, Adhami VM, et al. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis. 2014;35(2):415–423. doi:10.1093/carcin/bgt321
49. Abd El-Hameed AM. Polydatin-loaded chitosan nanoparticles ameliorates early diabetic nephropathy by attenuating oxidative stress and inflammatory responses in streptozotocin-induced diabetic rat. J Diabetes Metab Disord. 2020;19(2):1599–1607. doi:10.1007/s40200-020-00699-7
50. Kim HR, Jung WK, Park S-B, et al. Polydatin alleviates diabetes-induced hyposalivation through anti-glycation activity in db/db mouse. Pharmaceutics. 2021;14(1):51. doi:10.3390/pharmaceutics14010051
51. Latos-Brozio M, Masek A. The application of (+)-catechin and polydatin as functional additives for biodegradable polyesters. Int J Mol Sci. 2020;21(2):414.
52. Nagpal K, Singh SK, Mishra DN. Optimization of brain targeted chitosan nanoparticles of Rivastigmine for improved efficacy and safety. Int J Biol Macromol. 2013;59:72–83. doi:10.1016/j.ijbiomac.2013.04.024
53. Li Y, Chen X, Chen Y, et al. Berberine improves TNF-alpha-induced hepatic insulin resistance by targeting MEKK1/MEK pathway. Inflammation. 2022;22:1.
54. Zhang B, Zhang X, Zhang C, et al. Berberine improves the protective effects of metformin on diabetic nephropathy in db/db mice through Trib1-dependent inhibiting inflammation. Pharm Res. 2021;38(11):1807–1820. doi:10.1007/s11095-021-03104-x
55. Yu M, Alimujiang M, Hu L, et al. Berberine alleviates lipid metabolism disorders via inhibition of mitochondrial complex I in gut and liver. Int J Biol Sci. 2021;17(7):1693–1707. doi:10.7150/ijbs.54604
56. Agrahari V, Meng J, Purohit SS, et al. Real-time analysis of tenofovir release kinetics using quantitative phosphorus ((31)P) nuclear magnetic resonance spectroscopy. J Pharm Sci. 2017;106(10):3005–3015. doi:10.1016/j.xphs.2017.03.043
57. Mi Y, Tan W, Zhang J, et al. Modification of hydroxypropyltrimethyl ammonium chitosan with organic acid: synthesis, characterization, and antioxidant activity. Polymers. 2020;12(11):2460. doi:10.3390/polym12112460
58. Wang W, Meng Q, Li Q, et al. Chitosan derivatives and their application in biomedicine. Int J Mol Sci. 2020;21(2):487.
59. Gieldowska M, Puchalski M, Szparaga G, et al. Investigation of the influence of PLA molecular and supramolecular structure on the kinetics of thermal-supported hydrolytic degradation of wet spinning fibres. Materials. 2020;13(9):2111. doi:10.3390/ma13092111
60. Ambalavanan R, John AD, Selvaraj AD. Nano-encapsulated Tinospora cordifolia (Willd.) using poly (D, L-lactide) nanoparticles reduce effective control in streptozotocin-induced type 2 diabetic rats. IET Nanobiotechnol. 2020;14(9):803–808. doi:10.1049/iet-nbt.2020.0085
61. Xiong XY, Li QH, Li YP, et al. Pluronic P85/poly(lactic acid) vesicles as novel carrier for oral insulin delivery. Colloids Surf B Biointerfaces. 2013;111:282–288. doi:10.1016/j.colsurfb.2013.06.019
62. Mohamed EA, Zhao Y, Meshali MM, et al. Vorinostat with sustained exposure and high solubility in poly(ethylene glycol)-b-poly(DL-lactic acid) micelle nanocarriers: characterization and effects on pharmacokinetics in rat serum and urine. J Pharm Sci. 2012;101(10):3787–3798. doi:10.1002/jps.23265
63. De Marchi JGB, Cé R, Onzi G, et al. IgG functionalized polymeric nanoparticles for oral insulin administration. Int J Pharm. 2022;622:121829. doi:10.1016/j.ijpharm.2022.121829
64. Alajmi MF, Mothana RA, Al-Rehaily AJ, et al. Antimycobacterial activity and safety profile assessment of alpinia galanga and tinospora cordifolia. Evid Based Complement Alternat Med. 2018;2018:2934583. doi:10.1155/2018/2934583
65. Ambalavanan R, John AD, Selvaraj AD. Nephroprotective role of nanoencapsulated Tinospora cordifolia (Willd.) using polylactic acid nanoparticles in streptozotocin-induced diabetic nephropathy rats. IET Nanobiotechnol. 2021;15(4):411–417. doi:10.1049/nbt2.12030
66. Pal PB, Sinha K, Sil PC, Srinivasula SM. Mangiferin attenuates diabetic nephropathy by inhibiting oxidative stress mediated signaling cascade, TNFalpha related and mitochondrial dependent apoptotic pathways in streptozotocin-induced diabetic rats. PLoS One. 2014;9(9):e107220. doi:10.1371/journal.pone.0107220
67. Da Silva J, Jesus S, Bernardi N, et al. Poly(D,L-lactic acid) nanoparticle size reduction increases its immunotoxicity. Front Bioeng Biotechnol. 2019;7:137. doi:10.3389/fbioe.2019.00137
68. Sunoqrot S, Alfaraj M, Hammad AM, et al. Development of a thymoquinone polymeric anticancer nanomedicine through optimization of polymer molecular weight and nanoparticle architecture. Pharmaceutics. 2020;12(9):811. doi:10.3390/pharmaceutics12090811
69. Sekhon BS, Kamboj SR. Inorganic nanomedicine–part 1. Nanomedicine. 2010;6(4):516–522. doi:10.1016/j.nano.2010.04.004
70. Simmons KM, Michels AW. Type 1 diabetes: a predictable disease. World J Diabetes. 2015;6(3):380–390. doi:10.4239/wjd.v6.i3.380
71. Luo B, Wen S, Chen Y-C, et al. LOX-1-targeted iron oxide nanoparticles detect early diabetic nephropathy in db/db mice. Mol Imaging Biol. 2015;17(5):652–660. doi:10.1007/s11307-015-0829-5
72. Dubreil C, Sainte Catherine O, Lalatonne Y, et al. Tolerogenic iron oxide nanoparticles in type 1 diabetes: biodistribution and pharmacokinetics studies in nonobese diabetic mice. Small. 2018;14(40):e1802053. doi:10.1002/smll.201802053
73. An L, Tao Q, Wu Y, et al. Synthesis of SPIO nanoparticles and the subsequent applications in stem cell labeling for parkinson’s disease. Nanoscale Res Lett. 2021;16(1):107. doi:10.1186/s11671-021-03540-z
74. Dominguez JH, Mehta JL, Li D, et al. Anti-LOX-1 therapy in rats with diabetes and dyslipidemia: ablation of renal vascular and epithelial manifestations. Am J Physiol Renal Physiol. 2008;294(1):F110–9. doi:10.1152/ajprenal.00013.2007
75. Liu X, Du H, Sun Y, et al. Role of abnormal energy metabolism in the progression of chronic kidney disease and drug intervention. Ren Fail. 2022;44(1):790–805. doi:10.1080/0886022X.2022.2072743
76. Nissanka N, Moraes CT. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018;592(5):728–742. doi:10.1002/1873-3468.12956
77. Adhikari A, Mondal S, Chatterjee T, et al. Redox nanomedicine ameliorates chronic kidney disease (CKD) by mitochondrial reconditioning in mice. Commun Biol. 2021;4(1):1013. doi:10.1038/s42003-021-02546-8
78. Shaik MR, Syed R, Adil SF, et al. Mn3O4 nanoparticles: synthesis, characterization and their antimicrobial and anticancer activity against A549 and MCF-7 cell lines. Saudi J Biol Sci. 2021;28(2):1196–1202. doi:10.1016/j.sjbs.2020.11.087
79. Adhikari A, Polley N, Darbar S, et al. Citrate functionalized Mn(3)O(4) in nanotherapy of hepatic fibrosis by oral administration. Future Sci OA. 2016;2(4):Fso146. doi:10.4155/fsoa-2016-0029
80. Jiang J, Pi J, Cai J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl. 2018;2018:1062562. doi:10.1155/2018/1062562
81. Cao L, Kiely J, Piano M, et al. Facile and inexpensive fabrication of zinc oxide based bio-surfaces for C-reactive protein detection. Sci Rep. 2018;8(1):12687. doi:10.1038/s41598-018-30793-z
82. Abd El-Khalik SR, Ragab S, Nasif E, et al. The prospective ameliorative role of zinc oxide nanoparticles in STZ-induced diabetic nephropathy in rats: mechanistic targeting of autophagy and regulating Nrf2/TXNIP/NLRP3 inflammasome signaling. Biol Trace Elem Res. 2022;200(4):1677–1687. doi:10.1007/s12011-021-02773-4
83. Ashraf JM, Ansari MA, Fatma S, et al. Inhibiting effect of zinc oxide nanoparticles on advanced glycation products and oxidative modifications: a potential tool to counteract oxidative stress in neurodegenerative diseases. Mol Neurobiol. 2018;55(9):7438–7452.
84. Umrani RD, Paknikar KM. Zinc oxide nanoparticles show antidiabetic activity in streptozotocin-induced Type 1 and 2 diabetic rats. Nanomedicine. 2014;9(1):89–104. doi:10.2217/nnm.12.205
85. Alomari G, Al‐Trad B, Hamdan S, et al. Alleviation of diabetic nephropathy by zinc oxide nanoparticles in streptozotocin-induced type 1 diabetes in rats. IET Nanobiotechnol. 2021;15(5):473–483. doi:10.1049/nbt2.12026
86. Rahman HS, Othman HH, Abdullah R, et al. Beneficial and toxicological aspects of zinc oxide nanoparticles in animals. Vet Med Sci. 2022;8(4):1769–1779. doi:10.1002/vms3.814
87. Turan O, Bielecki P, Perera V, et al. Delivery of drugs into brain tumors using multicomponent silica nanoparticles. Nanoscale. 2019;11(24):11910–11921. doi:10.1039/C9NR02876E
88. Nuthalapati S, Shirhatti V, Kedambaimoole V, et al. Highly sensitive, scalable reduced graphene oxide with palladium nano-composite as strain sensor. Nanotechnology. 2020;31(3):035501. doi:10.1088/1361-6528/ab4855
89. Guo H, Wu B, Cui H, et al. NiCl2-down-regulated antioxidant enzyme mRNA expression causes oxidative damage in the broiler(‘)s kidney. Biol Trace Elem Res. 2014;162(1–3):288–295. doi:10.1007/s12011-014-0132-3
90. Ceriello A, Testa R. Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S232–6. doi:10.2337/dc09-S316
91. Tong Y, Zhang L, Gong R, et al. A ROS-scavenging multifunctional nanoparticle for combinational therapy of diabetic nephropathy. Nanoscale. 2020;12(46):23607–23619. doi:10.1039/D0NR06098D
92. Mohammadpour R, Cheney DL, Grunberger JW, et al. One-year chronic toxicity evaluation of single dose intravenously administered silica nanoparticles in mice and their Ex vivo human hemocompatibility. J Control Release. 2020;324:471–481. doi:10.1016/j.jconrel.2020.05.027
93. Schoneborn M, Harmening T, Giménez-Mañogil J, et al. Improved NOx storage/release properties of ceria-based lean NOx trap compositions with MnOx modification. Materials. 2019;12(13):2127. doi:10.3390/ma12132127
94. Alkilany AM, Murphy CJ. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J Nanopart Res. 2010;12(7):2313–2333. doi:10.1007/s11051-010-9911-8
95. Manna K, Mishra S, Saha M, et al. Amelioration of diabetic nephropathy using pomegranate peel extract-stabilized gold nanoparticles: assessment of NF-κB and Nrf2 signaling system. Int J Nanomedicine. 2019;14:1753–1777. doi:10.2147/IJN.S176013
96. Gandhi S, Srinivasan BP, Akarte AS. An experimental assessment of toxic potential of nanoparticle preparation of heavy metals in streptozotocin induced diabetes. Exp Toxicol Pathol. 2013;65(7–8):1127–1135. doi:10.1016/j.etp.2013.05.004
97. Hamzawy MA, Salem HF, Mohammed SA, et al. Antitumor activity of intratracheal inhalation of temozolomide (TMZ) loaded into gold nanoparticles and/or liposomes against urethane-induced lung cancer in BALB/c mice. Drug Deliv. 2017;24(1):599–607. doi:10.1080/10717544.2016.1247924
98. Connor EE, Mwamuka J, Gole A, et al. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–327. doi:10.1002/smll.200400093
99. Alomari G, Al-Trad B, Hamdan S, et al. Gold nanoparticles attenuate albuminuria by inhibiting podocyte injury in a rat model of diabetic nephropathy. Drug Deliv Transl Res. 2020;10(1):216–226. doi:10.1007/s13346-019-00675-6
100. Al-Trad B, Aljabali A, Al-Zoubi M, et al. Effect of gold nanoparticles treatment on the testosterone-induced benign prostatic hyperplasia in rats. Int J Nanomedicine. 2019;14:3145–3154. doi:10.2147/IJN.S202645
101. Xia Q, Huang J, Feng Q, et al. Size- and cell type-dependent cellular uptake, cytotoxicity and in vivo distribution of gold nanoparticles. Int J Nanomedicine. 2019;14:6957–6970. doi:10.2147/IJN.S214008
102. Wang YY, Tang LQ, Wei W. Berberine attenuates podocytes injury caused by exosomes derived from high glucose-induced mesangial cells through TGFbeta1-PI3K/AKT pathway. Eur J Pharmacol. 2018;824:185–192. doi:10.1016/j.ejphar.2018.01.034
103. Chen X, Sun L, Li D, et al. Green tea peptides ameliorate diabetic nephropathy by inhibiting the TGF-beta/Smad signaling pathway in mice. Food Funct. 2022;13(6):3258–3270. doi:10.1039/D1FO03615G
104. Wu D, Peng F, Zhang B, et al. PKC-beta1 mediates glucose-induced Akt activation and TGF-beta1 upregulation in mesangial cells. J Am Soc Nephrol. 2009;20(3):554–566. doi:10.1681/ASN.2008040445
105. Eissa S, Matboli M, Aboushahba R, et al. Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. J Diabetes Complications. 2016;30(8):1585–1592. doi:10.1016/j.jdiacomp.2016.07.012
106. Jiang Y, Shi M, Liu Y, et al. Aptamer/AuNP biosensor for colorimetric profiling of exosomal proteins. Angew Chem Int Ed Engl. 2017;56(39):11916–11920. doi:10.1002/anie.201703807
107. Varkonyi-Gasic E, Wu R, Wood M, et al. Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods. 2007;3(1):12. doi:10.1186/1746-4811-3-12
108. Kheybari S, Samadi N, Hosseini SV, et al. Synthesis and antimicrobial effects of silver nanoparticles produced by chemical reduction method. Daru. 2010;18(3):168–172.
109. Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7(6):454–465. doi:10.1038/nri2093
110. Elekofehinti OO, Oyedokun VO, Iwaloye O, et al. Momordica charantia silver nanoparticles modulate SOCS/JAK/STAT and P13K/Akt/PTEN signalling pathways in the kidney of streptozotocin-induced diabetic rats. J Diabetes Metab Disord. 2021;20(1):245–260. doi:10.1007/s40200-021-00739-w
111. Zinchenko OA, Marchenko SV, Sergeyeva TA, et al. Application of creatinine-sensitive biosensor for hemodialysis control. Biosens Bioelectron. 2012;35(1):466–469. doi:10.1016/j.bios.2012.02.062
112. Narimani R, Azizi M, Esmaeili M, et al. An optimal method for measuring biomarkers: colorimetric optical image processing for determination of creatinine concentration using silver nanoparticles. Biotech. 2020;10(10):416. doi:10.1007/s13205-020-02405-z
113. Xu YM, Tan HW, Zheng W, et al. Cadmium telluride quantum dot-exposed human bronchial epithelial cells: a further study of the cellular response by proteomics. Toxicol Res. 2019;8(6):994–1001. doi:10.1039/c9tx00126c
114. Pollinger K, Hennig R, Bauer S, et al. Biodistribution of quantum dots in the kidney after intravenous injection. J Nanosci Nanotechnol. 2014;14(5):3313–3319. doi:10.1166/jnn.2014.8716
115. Smith AM, Duan H, Mohs A, et al. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60(11):1226–1240. doi:10.1016/j.addr.2008.03.015
116. Barutta F, Bellini S, Gruden G. Mechanisms of podocyte injury and implications for diabetic nephropathy. Clin Sci. 2022;136(7):493–520.
117. Liu X, Hu R, Lian H, et al. Dual-color immunofluorescent labeling with quantum dots of the diabetes-associated proteins aldose reductase and Toll-like receptor 4 in the kidneys of diabetic rats. Int J Nanomedicine. 2015;10:3651–3662. doi:10.2147/IJN.S81395
118. Wang M, Abbineni G, Clevenger A, et al. Upconversion nanoparticles: synthesis, surface modification and biological applications. Nanomedicine. 2011;7(6):710–729. doi:10.1016/j.nano.2011.02.013
119. Mirnajafizadeh F, Ramsey D, McAlpine S, et al. Nanoparticles for bioapplications: study of the cytotoxicity of water dispersible CdSe(S) and CdSe(S)/ZnO quantum dots. Nanomaterials. 2019;9(3):465. doi:10.3390/nano9030465
120. Yadav D, Kwak M, Chauhan PS, et al. Cancer immunotherapy by immune checkpoint blockade and its advanced application using bio-nanomaterials. Semin Cancer Biol. 2022. doi:10.1016/j.semcancer.2022.02.016
121. Maslanka Figueroa S, Veser A, Abstiens K, et al. Influenza A virus mimetic nanoparticles trigger selective cell uptake. Proc Natl Acad Sci U S A. 2019;116(20):9831–9836. doi:10.1073/pnas.1902563116
122. Boulant S, Stanifer M, Lozach PY. Dynamics of virus-receptor interactions in virus binding, signaling, and endocytosis. Viruses. 2015;7(6):2794–2815. doi:10.3390/v7062747
123. Maslanka Figueroa S, Fleischmann D, Beck S, et al. Nanoparticles mimicking viral cell recognition strategies are superior transporters into mesangial cells. Adv Sci. 2020;7(11):1903204. doi:10.1002/advs.201903204
124. Fleischmann D, Harloff M, Maslanka Figueroa S, et al. Targeted delivery of Soluble Guanylate Cyclase (sGC) activator cinaciguat to renal mesangial cells via virus-mimetic nanoparticles potentiates anti-fibrotic effects by cGMP-mediated suppression of the TGF-beta pathway. Int J Mol Sci. 2021;22(5):2557. doi:10.3390/ijms22052557
125. Schinner E, Wetzl V, Schlossmann J. Cyclic nucleotide signalling in kidney fibrosis. Int J Mol Sci. 2015;16(2):2320–2351. doi:10.3390/ijms16022320
126. Sawaf H, Thomas G, Taliercio JJ, et al. Therapeutic advances in diabetic nephropathy. J Clin Med. 2022;11(2):378. doi:10.3390/jcm11020378
127. Pereira PR, Carrageta DF, Oliveira PF, et al. Metabolomics as a tool for the early diagnosis and prognosis of diabetic kidney disease. Med Res Rev. 2022;42(4):1518–1544. doi:10.1002/med.21883
128. Wu L, Wen W, Wang X, et al. Ultrasmall iron oxide nanoparticles cause significant toxicity by specifically inducing acute oxidative stress to multiple organs. Part Fibre Toxicol. 2022;19(1):24. doi:10.1186/s12989-022-00465-y
129. Almanaa TN, Aref M, Kakakhel MA, et al. Silica nanoparticle acute toxicity on male rattus norvegicus domestica: ethological behavior, hematological disorders, biochemical analyses, hepato-renal function, and antioxidant-immune response. Front Bioeng Biotechnol. 2022;10:868111. doi:10.3389/fbioe.2022.868111
130. Zhang Y, Hu B, Wang M, et al. Selenium protects against zearalenone-induced oxidative stress and apoptosis in the mouse kidney by inhibiting endoplasmic reticulum stress. Oxid Med Cell Longev. 2020;2020:6059058. doi:10.1155/2020/6059058
131. Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–146. doi:10.1016/j.jconrel.2010.08.027
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
