Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Current Status of the Treatment of COPD in China: A Multicenter Prospective Observational Study
Authors Zeng Y , Cai S, Chen Y , Duan J, Zhao Y , Li X , Ma L, Liu Q, Zhu Y, Chen M, Zhou M, Chen P
Received 31 July 2020
Accepted for publication 16 November 2020
Published 7 December 2020 Volume 2020:15 Pages 3227—3237
DOI https://doi.org/10.2147/COPD.S274024
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Yuqin Zeng,1– 3 Shan Cai,1– 3 Yan Chen,1– 3 Jiaxi Duan,1– 3 Yiyang Zhao,1– 3 Xin Li,4 Libing Ma,5 Qimi Liu,6 Yingqun Zhu,7 Ming Chen,8 Meiling Zhou,9 Ping Chen1– 3
1Department of Pulmonary and Critical Care Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 2Research Unit of Respiratory Disease, Central South University, Changsha, Hunan, People’s Republic of China; 3Hunan Centre for Evidence-Based Medicine, Changsha, Hunan, People’s Republic of China; 4Division 4 of Occupational Disease, Hunan Occupational Disease Prevention and Treatment Hospital, Changsha, Hunan, People’s Republic of China; 5Department of Respiratory and Critical Care Medicine, Affiliated Hospital of Guilin Medical University, Guilin, Guangxi, People’s Republic of China; 6Department of Respiratory and Critical Care Medicine, The Second People’s Hospital of Guilin, Guilin, Guangxi, People’s Republic of China; 7Department of Respiratory and Critical Care Medicine, The Third Hospital of Changsha, Changsha, Hunan, People’s Republic of China; 8Department of Respiratory and Critical Care Medicine, No.1 Traditional Chinese Medicine Hospital of Changde City, Changde, Hunan, People’s Republic of China; 9Department of Respiratory and Critical Care Medicine, The First People’s Hospital of Huaihua City, Huaihua, Hunan, People’s Republic of China
Correspondence: Ping Chen
Department of Pulmonary and Critical Care Medicine, The Second Xiangya Hospital, Central South University, 139 Middle Ren Min Road, Changsha, Hunan 410011, People’s Republic of China
Tel + 86-731-85295047
Fax + 86-731-85295044
Email [email protected]
Background: There is a large gap in the treatments for patients with COPD according to the Global Initiative for COPD (GOLD) recommendations. Determining the situation of therapies in the real world is necessary. This study aimed to characterize the real-world practical therapies of COPD and prognosis of patients after treatment for 1 year.
Methods: This study was a multicenter prospective observational study performed using a database set up by the Second Xiangya Hospital of Center South University. Detailed usage information for pharmacotherapies and nonpharmacotherapies for patients was collected, as well as the consistency of recommendations and patient adherence. Moreover, the effect of therapies after 1 year was calculated by comparing lung function and symptoms.
Results: Ultimately, 4,796 patients with COPD from 12 hospitals in China were eligible. LAMA (39.1%), LAMA + LABA/ICS (39.0%) and LABA/ICS (14.4%) were the top three inhalants. We found that 42.7% of Group A patients, 61.6% of Group B patients and 30% of Group C patients were following inappropriate therapy, especially overuse of ICS. Only 3.9% (95% CI 2.4, 5.4) of patients used oxygen therapy, and 1.8% (95% CI 1.5, 2.3) used noninvasive positive pressure ventilation at home. Among these patients, 33.2% had poor adherence. A total of 452 patients completed 1 year of follow-up. After 1 year of treatment, the lung function of FEV1/FVC decreased (P=0.001) and the mMRC score increased (P< 0.001). There was no change in CAT scores (P> 0.05).
Conclusion: This study highlights a significant discrepancy between recommendations for managing patients with COPD in GOLD report, and in real-world clinical practice in China. Over-prescription of ICS and under-prescription of nonpharmacologic therapy were common. The adherence to treatment of patients was poor, and the real-life treatment effectiveness was unsatisfactory. More attention should be paid to the implementation of recommendations and standardized administration of therapies.
Keywords: COPD, treatment, discrepancy, observational study
Plain Language Summary
Recommendations developed by Global Initiative for chronic obstructive pulmonary disease (COPD) (GOLD) are updated regularly. It is a reference for clinic physicians to treat patients with COPD all over the world. The compliance of the recommendations in clinic work is differentiated in different countries. We had collected the data of treatments for patients with COPD from 12 hospitals to observe current situation of therapies for COPD, and to investigate the gap between GOLD report and clinical practice in China. We had found that a high rate of discrepancy (40.5%) between GOLD 2017 recommendations and real-world clinical practice existed in China. Over-prescription of ICS (38.6%–55.6%) and under-prescription of nonpharmacologic therapy (0.08%–3.9%) were common. In addition, many researches have reported that kinds of inhalations for patients with COPD can improve the health quality, reduce the exacerbation rate and hospitalization rate. How about the therapeutic effect in the real world? If the inhaled treatments are really effective in the real world? The answer is in our study. We found that the adherence of medicines in patients with COPD was not very good (66.8%) and the real-life treatment effectiveness after 1 year of follow-up was unsatisfactory. We had listed some reasons about this phenomenon, such as the natural progress of disease and inappropriate therapy. Therefore, we recommended that more attention should be paid to the implementation of recommendations and standardized administration of therapies, including pharmacologic and nonpharmacologic treatments.
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease with a prevalence of 13.7% in China that contributes to a large economic and social burden.1,2 Inhaled bronchodilators are the first-line maintenance therapy for patients with COPD to reduce exacerbations and to improve quality of life and lung function.3 The 2017 report developed by the updated Global Initiative for COPD (GOLD) recommends a proposed model for the initiation and subsequent escalation of pharmacologic management of stable COPD, according to the assessment of symptoms and exacerbation risk.1 Although the GOLD recommendations have declared detailed therapies for patients with COPD, a significant dissociation has been reported between recommendations and clinicians’ practices.4,5 Nearly 62.1% of the treatments for patients with COPD are inappropriate, especially overprescription.6 Among the COPD patients treated with triple inhaled therapy, 34.5% of the patients were classified as having mild disease (GOLD 1) and 40.8% as having moderate disease (GOLD 2).7 The use of different inhaled drugs is markedly variable in different countries, similar to compliance with the COPD treatment recommendations.8,9 Oral pharmacologic treatment can also be used as maintenance medication for patients with COPD. A few years ago, a multicenter study including 2,072 patients with COPD showed that theophylline (53.7%, 351/653) was the most commonly used treatment, followed by bronchodilators in China.10
There is a large gap in the treatments for patients with COPD according to the GOLD recommendations. This study aimed to observe the current status of treatments including pharmacotherapy and nonpharmacotherapy for stable COPD patients in China and to investigate the discrepancies between GOLD recommendations and real-world clinical practice. In addition, adherence to medicines in patients and effectiveness of treatments 1 year later were calculated.
Methods
Study Design and Data Source
This study was a prospective observational study performed in the real world from December 1, 2016 to October 31, 2019 using the database set up by the Second Xiangya Hospital of Center South University (ChiCTR-POC-17010431). We gathered data from 12 hospitals (10 hospitals in Hunan Province and 2 in Guangxi Province) in China (Website: http://218.4.234.74:9007/a/login). The study was approved by an institutional review board from the Second Xiangya Hospital of Central South University (2016076) and conducted in accordance with the Declaration of Helsinki. All data were compliant with Health Insurance Portability and Accountability Act to protect patient privacy. All participants provided written informed consent.
Patients
Patients were eligible for this study if they had a diagnosis of emphysema (identified by the International Classification of Diseases, Clinical Modification Tenth Revision [ICD-10] codes for emphysema: XJ43.xx) with the forced expiratory volume in 1 second (FEV1)/forced volume vital capacity (FVC) ratio <0.7 or COPD (ICD-10 codes for COPD: XJ44.0, 44.8, 44.9) from the medical record.11 Patients were at least 40 years of age and visited a pneumological outpatient clinic for the first time. Among these patients who had finished at least 1 year of follow-up were enrolled in our study to calculate the therapeutic effect. Patients with at least one medical claim with a diagnosis of asthma (ICD-10: XJ45.xx), bronchiectasis (ICD-10: XJ47), or lung cancer (ICD-10: C34.xx) during the study period were excluded.11
Basic Variables
Basic variables, including demographics, diagnosis, exacerbation history, lung function test, modified Medical Research Council (mMRC) score, COPD Assessment Test (CAT) score, and current therapy, were extracted from a disease-specific questionnaire (available as supplementary materials). Three technical assistants reconfirmed all of the data to eliminate typing omission and errors. Smoking history was recorded in the study. Participants who had never smoked a cigarette or smoked less than 100 cigarettes in their life were defined as “Never smoker”. Participants who had smoked ≥100 cigarettes in their life and currently smoked cigarettes were defined as “Current smoker”. Participants who had smoked ≥100 cigarettes in their life but did not currently smoke and had quit smoking longer than 6 months, were defined as “Former smoker”. At the follow-up visit, smoking cessation was considered equal to or longer than 6 months of abstinence.12
Pulmonary function was measured using a MasterScreen™ pulmonary function test system spirometer (Germany), and spirometric measurements met the standards of the American Thoracic Society and the European Respiratory Society.13 According to the airway limitation severity of FEV1, patients were divided into GOLD 1: FEV1 >80% predicted, GOLD 2: 50% ≤ FEV1 <80% predicted, GOLD 3: 30% ≤ FEV1<50% predicted, and GOLD 4: FEV1<30% predicted.
Exacerbations were defined as an acute worsening of respiratory symptoms that results in additional therapy.14 Exacerbations can be classified into three levels: mild (treated with short-acting bronchodilators only, SABDs), moderate (treated with SABDs plus antibiotics and/or oral corticosteroids) and severe (patient requires hospitalization or visits the emergency room). In our research, patient estimates of moderate and severe exacerbation numbers in the past year before they visited were recorded.15 According to the history of exacerbations and severity of symptoms by the CAT and mMRC questionnaires, patients were classified into Groups A/B/C/D.1
Treatments
Detailed treatments, including pharmacotherapy (inhaled and oral) and nonpharmacotherapy, were extracted from the study. Inhaled COPD treatment was recorded at the moment of every visit. All COPD treatments were classified as short-acting bronchodilators (SABDs): short-acting β2-agonists (SABAs) and short-acting muscarinic agonists (SAMAs); long-acting bronchodilators (LABDs): long-acting β2-agonists (LABAs) and long-acting muscarinic agonists (LAMAs); and other inhalations: LAMA combined with LABA (LAMA/LABA) in an inhaler, LABA combined with inhaled corticosteroid (LABA/ICS) in an inhaler and ICS monotherapy. Oral therapy with theophylline was recorded. In addition, nonpharmacologic therapies such as home oxygen therapy, noninvasive positive pressure ventilation (NIPPV) at home, vaccination, pulmonary rehabilitation and lung transplantation were calculated in our study. In this study, LABA/ICS means that LABA and ICS were administered in an inhaler. LAMA/LABA also means they are in an inhaler. “+” means “and”, which was defined to indicate inhalations with different inhalers used simultaneously by patients.
Consistency with GOLD Recommendations
On the basis of GOLD 2017, the appropriateness of the inhaled pharmacological treatment of COPD patients was established for Groups A/B/C/D.1 The first choice of inhaled therapy for different groups is recommended as follows, if not defined as inappropriate therapy or as a discrepancy with the GOLD report.
Group A: A short- or a long-acting bronchodilator.
Group B: LAMA or LABA, or LAMA + LABA.
Group C: LAMA, or LABA + ICS or LAMA + LABA.
Group D: LAMA, or LAMA + LABA or LABA + ICS, or LAMA + LABA+ICS.
Patient Adherence
During the 1-year of follow-up, telephone contact follow-ups were arranged every 6 months following the first visit at the outpatient clinic through a follow-up questionnaire, including numbers of exacerbations, smoking history, changes in therapies and reasons for drug withdrawal (available as supplementary materials). Patients were considered fully adherent if they reported having taken their medication every day within the last 30 days; otherwise, they were recording as having poor adherence.16 The reasons for poor adherence were recorded, including feeling better and not needing to continue treatments, ineffective, worried about adverse effects of drugs, cumbersome inhalation techniques, economic burden and other reasons such as accessibility of drugs.
Effect of Therapy
Patients with COPD who had been followed for at least 1 year were enrolled. The demographic data, pharmacotherapy, exacerbations, lung function, CAT and mMRC were recorded. Changes in lung function and symptoms (CAT and mMRC scores) were used to calculate the effect of therapy.
Statistical Analysis
Descriptive statistics included the absolute and relative frequencies for categorical variables and the mean ± SD for numerical variables. Continuous variables were statistically compared using t-tests, and categorical variables were compared using the nonparametric test. To test for differences in proportion in some variables according to the type of bronchodilators taken, a Chi-square test or Fisher’s test was used. We considered a p-value less than 5% as statistically significant. We used the approximate normal distribution method to compute binomial proportion 95 confidence intervals. When we calculated the pharmacotherapy in different groups and grades, and effect of therapy in the follow-up, we had removed some missing data to avoid data bias. All analyses were performed using PASW version 18.0 software (PASW, Inc., Chicago, Illinois).
Results
Patient Selection and Demographics
In total, data from 8,058 patients with COPD were enrolled in our study. Among these patients, 4,796 (59.5%) met the inclusion criteria. In this population, 452 patients with COPD who finished 1 year of follow-up were recorded (Figure 1). The demographic characteristics of all the patients with COPD are shown in Table 1. The mean age was 64 years and the majority were male (87.7%). A total of 41.5% of the patients were current smokers. The mean of FEV1% predicted and the FEV1/FVC% were 47.6% and 48.6%, respectively. The mean CAT score was 16.3. Patients were assigned to Groups A (8.0%), B (55.5%), C (1.5%), and D (35.0%). The classifications were as follows: GOLD 1 (7.6%), 2 (33.9%), 3 (37.9%) and 4 (20.6%).
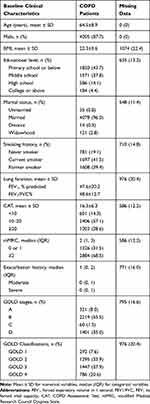 |
Table 1 Baseline Clinical Characteristics of COPD Patients (N=4796) |
Pharmacotherapy
The treatment therapies are shown in Table 2. LAMA (39.1%), LAMA + LABA/ICS (39.0%) and LABA/ICS (14.4%) were the top three inhalants used. It is worth mentioning that for 11.4% (545/4796) of patients, theophylline was also used.
 |
Table 2 Description of Inhalation Therapies Used in the Study Population (N=4796) |
The detailed pharmacotherapies of 4,001 COPD patients in Groups A/B/C/D are shown in Table 3. A total of 795 patients were excluded because of incomplete data. In Group A, LAMA (55.5%) was commonly used. We found that 42.7% (137/321) of Group A patients were following inappropriate therapy. In Group B, LAMA + LABA/ICS was also the most common (39.9%). A total of 61.6% (1367/2219) were following a therapy in contrast with GOLD recommendations. Among Group C patients, 53.3% were using LAMA, while 30% (18/60) were following an inappropriate therapy. In Group D, LAMA + LABA/ICS was also the most prescribed (44.3%). Only 6.9% (97/1401) were following an inappropriate therapy. The overall discrepancy rate between GOLD recommendations and clinical practice was 40.5% (1619/4001) in Groups A/B/C/D. LAMA/LABA was only used in Groups B (0.2%) and D (0.1%). ICS overtreatment occurred in 55.6% (1413/2540) of patients in Groups A and B. Among the different groups, significant differences were found in the proportions of LAMA, LABA/ICS and LAMA + LABA/ICS (P<0.001, P=0.004 and P<0.001, respectively).
 |
Table 3 Description of Inhalation Therapies Used in Different GOLD-Groups (N=4001) |
The pharmacotherapies of 3,820 COPD patients in GOLD 1/2/3/4 grades are presented in Table 4. A total of 976 patients were removed because of a lack of lung function tests. We found that there were significant differences across the LAMA, LABA/ICS and LAMA + LABA/ICS treatments (P<0.001, P=0.044 and P<0.001). When compared to other grades, LAMA was used less as the grade increased, with 70.2% in GOLD 1, 51.9% in GOLD 2, 31.0% in GOLD 3 and 20.0% in GOLD 4. In contrast, LAMA + LABA/ICS was commonly used in GOLD 4 (60.7%), followed by GOLD 3 (47.3%), GOLD 2 (23.1%) and GOLD 1 (6.8%). ICS was overused in 38.6% (612/1587) of cases in GOLD 1/2. When treatments were based on symptoms or airway limitation, there were some inappropriate treatments with SABDs combined with ICS or LABDs (5.6% and 5.6%, respectively).
 |
Table 4 Description of Inhalation Therapies Used in Different GOLD Classifications Based on Airflow Limitation (N=3820) |
Nonpharmacologic Therapy
In this population, nonpharmacologic therapies were used, including 3.9% (95% CI 2.4, 5.4) home oxygen therapy, 1.8% (95% CI 1.5, 2.3) NIPPV at home, 0.3% (95% CI 0.01,1.2) vaccination, 0.1% (95% CI 0.0, 5.6) pulmonary rehabilitation, and 0.08% (95% CI 0.0, 0.2) lung transplantation. Among the patients, 33.2% had poor adherence after 6 months of follow-up. When the COPD patients were asked about the reasons for withdrawal, 30.0% of them thought that they were better and did not need to continue treatments, 18.5% considered the treatment ineffective, 14.5% had trouble with inaccessibility of drugs, 1.9% worried about adverse effects, and 0.7% considered the economic burden, while, 34.4% gave other reasons.
Effect of Therapy
The baseline clinical characteristics of 452 COPD patients treated for at least 1 year are presented in Table 5. The changes in patients after treatment are shown in Table 6. The numbers of moderate and severe exacerbations were obviously increased (1 vs 2, and 0 vs 2, P<0.001 and P<0.001, respectively). The lung function of FEV1/FVC decreased (P=0.001), and the mMRC score increased (P<0.001). There was no change in FEV1 and CAT scores (P=0.732 and P=0.094, respectively). One year later, the poor adherence rate of treatment was 20.4% (79/387). The use of LAMA declined (P <0.001), while the use of LAMA + LABA/ICS increased (P=0.009).
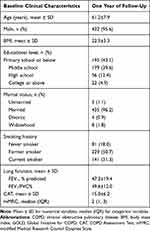 |
Table 5 Baseline Clinical Characteristics of COPD Patients Finished 1 Year of Follow-Up Visit (N=452) |
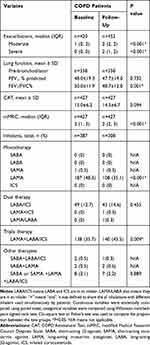 |
Table 6 Lung Function, Symptoms and Inhalation Therapy at Baseline and After 12 Months (N=452) |
Discussion
This real-world prospective observational study demonstrated that the treatment modalities for patients with COPD had major discrepancies compared to GOLD 2017 report, especially overuse of LABA/ICS and LAMA + LABA/ICS. The use of nonpharmacologic therapy including home oxygen therapy, NIPPV, vaccine, pulmonary rehabilitation and lung transplantation, was seriously insufficient in China. Poor adherence to treatment in patients and unsatisfactory therapeutic effect after 1 year of follow-up were identified.
In our study, the majority of COPD patients were divided into Groups B/D and GOLD 2/3 grades. LAMA, LAMA + LABA/ICS and LABA/ICS inhalants were commonly used to treat these patients. Except for Group D, there was a high rate of inconsistency with the GOLD recommendations in Groups A/B/C (42.7% in Group A, 61.6% in Group B, and 30% in Group C). This was consistent with Grewe FA’s study.17 They also found that prescriptions in patients with very severe COPD were most likely to comply with recommendations. LAMA was the most frequently used treatment in Group A according to our study, which is inconsistent with a study in the UK primary care setting.8 In their population, LABA + ICS therapies were the most commonly used. Overtreatment of ICS combined with one or more long-acting bronchodilators was demonstrated as the most common inappropriate prescription in patients with COPD, whether categorized by symptom or airway limitation, and was lower than in other studies.18 One reason for the overuse may be the lack of inhalation formulations of LABA and LAMA/LABA available on the market until 2019 in China. The longer marketing history and better availability of LAMA and LABA/ICS in the public health care system may contribute to their much higher utilization rate. When classified based on airflow limitation, we found that the use of LAMA was lower as the grades increased, which was inverse in triple therapies. Before the GOLD 2017 was issued, many physicians treated COPD patients according to the severity of airway limitation,19 and the influence can be seen currently.
Oral pharmacologic treatment with theophylline is still commonly used in China, although it has been relegated to third-line therapy in the current GOLD recommendations. Ram et al20 found that theophylline has a modest effect on lung function in moderate to severe COPD. Compared with other inhaled bronchodilators, theophylline was much less expensive and its prescription was not restricted by the public health care system in China. Devereux et al21 showed that in people with COPD at high risk of exacerbation, no benefit came from the addition of low-dose theophylline. Studies of the real function of theophylline in patients with different severities of COPD are warranted in the future.
We also investigated the current status of nonpharmacologic treatment. For example, home oxygen therapy, which can improve survival in patients with COPD, was rarely used (3.9%) in this study. In other countries, home oxygen therapy appears to be universal in patients with COPD.22,23 Home NIPPV has been determined to improve outcomes in COPD patients with chronic respiratory failure. It was used at home in only 1.8% of our population because its use was only considered in some selected patients with pronounced daytime hypercapnia.24 Vaccination is recommended for the management of COPD patients, especially those over 50 years of age.25 However, it was rarely used (0.3%) in this study, with considerably lower rates than in the United States population, which has a vaccination rate of 48.5%.26 We need to pay more attention to the implementation of vaccines and the awareness of patients and physicians.27 Moreover, few COPD patients (0.1%) participated in pulmonary rehabilitation in our study, which was lower than that in some developed countries. In Canada, 0.4% of all Canadians with COPD have access to pulmonary rehabilitation, and in Australia, 5–10% of the patients with moderate to severe COPD have access.28,29 Factors including insufficient knowledge, attitudes for physicians and perceived practical/physical concerns for patients are related to influence patients’ participation.30,31 Interventional therapy of lung transplantation was also scarce in our study. It is an alternative treatment modality for patients with end-stage COPD. Further study is still required to assess the overall survival benefit of lung transplantation in the COPD patient population.32
Nonadherence to treatments in patients with COPD is common all over the world. In our study, a total of 33.2% of the patients stopped medication after 6 months of follow-up. In the USA, the proportion of patients with nonadherence to triple therapy at 6 months was 59.0% in a commercially insured population.33 Tøttenborg et al34 showed that a higher risk of poor adherence was present among unemployed and low-income patients. However, we found that patients feeling better and seeing no need to be treated, considering the treatment ineffective and encountering access restrictions contributed to poor adherence. Unwillingness and privacy issues may be the two main reasons why patients avoid their economic problems in China.
Many studies have shown that dual or triple therapy will produce a statistically significant improvement in FEV1 and reduce the exacerbation risk compared among different groups.35–37 Unfortunately, in our study, the lung function of FEV1/FVC, symptoms of dyspnea and exacerbations after 1-year of follow-up were not improved, even worse compared to the baseline. The FEV1 and CAT scores were stable. In other words, the treatment resulted in little improvement in symptoms and lung function over time. One reason for this phenomenon is that lung function will decline nearly 53 mL per year for COPD patients.38 In addition, inhaler misuse has been associated with poor disease control, which is common in not only naive but also experienced users. Schedule education and proper evaluation of inhalation, such as the FSI-10 questionnaire, are important for teaching patients with COPD how to handle the inhaler.39 Moreover, the inappropriate therapies and poor adherence to treatment were the main contributors to poor therapeutic effect in this study.
The present study is subject to several limitations. First, there were missing data in our study. No significant difference in treatments was found compared to the available data group. Moreover, we used statistical methods such as deleting the missing data at random and mean completers to reduce the bias. Therefore, we thought the outcomes were not affected by missing data. Second, our study is a multicenter prospective observational study in the real world at the outpatient clinic to investigate the current status of treatments for patients with COPD. LABA/LAMA was not accessible in each hospital until 2019, which led to a low usage rate. Third, only a few of COPD patients were followed for 1 year. That was because we did not take intrusive measures to improve the rate of follow-up visits in order to restore the real condition in China. In our country, the Universal Health Care will not cover the cost patients have at the outpatient clinic. Most of the patients in our study came from remote rural areas and reluctant to revisit the same hospitals due to transportation costs and medical expenses. Therefore, patients can choose to visit any hospital or doctor they want, which possibly leads to the missing follow-ups in our research centers. Perhaps more studies should be conducted to investigate the prognosis and mortality of patients who are lost to follow-up in the future.
Conclusions
In summary, the findings of this study provide insights into current status of treatment in patients with COPD in the real world in China. There was a high rate of discrepancy between the GOLD recommendation and the real-world clinical practice. Over-prescription of ICS and under-prescription of nonpharmacologic therapy were common. The adherence of treatment in patients is not good, and the real effects of treatment are still unsatisfactory. Our results suggest that more attention should be paid to the implementation of GOLD recommendations and standardized administration of therapies, including inhaled bronchodilators, oral pharmacologic and nonpharmacologic treatments. Different kinds of bronchodilators should be marketed and accessible at different levels of hospitals and areas in China.
Abbreviations
COPD, chronic obstructive pulmonary disease; BMI, body mass index; GOLD, Global Initiative for COPD; CAT, COPD Assessment Test; mMRC, modified Medical Research Council Dyspnea Scale; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic agonist; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2-agonist; ICS, inhaled corticosteroids; SABDs, short-acting bronchodilators; LABDs, long-acting bronchodilators; NIPPV, non-invasive positive pressure ventilation.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available in the Department of Pulmonary and Critical Care Medicine, the Second Xiangya Hospital repository http://218.4.234.74:9007/a/login. The data that support the findings of this study are available upon reasonable request from the author Yuqin Zeng or Pingchen.
Ethical Statement
The study was registered in the Chinese Clinical Trial Registry (Title: The analysis of current status in diagnosis and treatment of COPD. Registration number: ChiCTR-POC-17010431. http://www.chictr.org.cn/)
Acknowledgments
Thanks to Mingyan Jiang from Xiangtan Central Hospital, Lingmei Huang from the First People’s Hospital of Yueyang, Weimin Feng from Hengyang Central Hospital, Ying Xiao from Gui Lin People’s Hospital and Yi Liu from Zhuzhou Central Hospital for providing the data of COPD patients.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; revised the article critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Key Clinical Specialty Construction Projects, the National Natural Science Foundation of China (NSFC, Grants 81770046), NSFC (Grants 1970044) and Xiangya Mingyi grant (2013).
Disclosure
The authors report no financial or non-financial conflicts of interest for this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. 2017. Available from: http://goldcopd.org.
2. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
3. Tashkin DP, Cooper CB. The role of long-acting bronchodilators in the management of stable COPD. Chest. 2004;125(1):249–259. doi:10.1378/chest.125.1.249
4. Miravitlles M. Guidelines versus clinical practice in the treatment of chronic obstructive pulmonary disease. Eur Respir J. 2002;20(1):243–244. doi:10.1183/09031936.02.01302001
5. Riario-Sforza GG, Incorvaia C, Pravettoni C, Dugnani N. Guidelines versus clinical practice in the treatment of COPD: a reappraisal. Eur Respir J. 2006;27(3):656. doi:10.1183/09031936.06.00120105
6. Corrado A, Rossi A. How far is real life from COPD therapy guidelines? An Italian observational study. Respir Med. 2012;106(7):989–997. doi:10.1016/j.rmed.2012.03.008
7. Simeone JC, Luthra R, Kaila S, et al. Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J Chron Obstruct Pulmon Dis. 2017;12:73–83. doi:10.2147/COPD.S122013
8. Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–904. doi:10.2147/COPD.S62750
9. Chan KK, Ko F, Chan HS, et al. Adherence to a COPD treatment guideline among patients in Hong Kong. Int J Chron Obstruct Pulmon Dis. 2017;12:3371–3379. doi:10.2147/COPD.S147070
10. Y H C, W Z Y, Kang J, et al. Attitudes and actions of chronic obstructive pulmonary disease patients on treatment: a national multi-center investigative study. Zhonghua Jie He He Hu Xi Za Zhi. 2010;33(10):750–753.
11. International Classification of Diseases. Clinical modification tenth revision. Available from: http://apps.who.int/classifications/icd10/browse/2016/en#/J43.
12. Thabane M, Group CW. Smoking cessation for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser. 2012;12(4):1–50.
13. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
14. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi:10.1016/S0140-6736(07)61382-8
15. Quint JK, Donaldson GC, Hurst JR, Goldring JJ, Seemungal TR, Wedzicha JA. Predictive accuracy of patient-reported exacerbation frequency in COPD. Eur Respir J. 2011;37(3):501–507. doi:10.1183/09031936.00035909
16. Ding B, Small M, Scheffel G, Holmgren U. Maintenance inhaler preference, attribute importance, and satisfaction in prescribing physicians and patients with asthma, COPD, or asthma-COPD overlap syndrome consulting for routine care. Int J Chron Obstruct Pulmon Dis. 2018;13:927–936. doi:10.2147/COPD.S154525
17. Grewe FA, Sievi NA, Bradicich M, et al. Compliance of pharmacotherapy with GOLD guidelines: a longitudinal study in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:627–635. doi:10.2147/COPD.S240444
18. Turan O, Emre JC, Deniz S, Baysak A, Turan PA, Mirici A. Adherence to current COPD guidelines in Turkey. Expert Opin Pharmacother. 2016;17(2):153–158. doi:10.1517/14656566.2016.1115482
19. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. 2014. Available from: http://goldcopd.org.
20. Ram FS, Jones PW, Castro AA, et al. Oral theophylline for chronic obstructive pulmonary disease (Review). Cochrane Database Syst Rev. 2002;4:CD003902.
21. Devereux G, Cotton S, Fielding S, et al. Effect of theophylline as adjunct to inhaled corticosteroids on exacerbations in patients with COPD: a randomized clinical trial. JAMA. 2018;320(15):1548–1559. doi:10.1001/jama.2018.14432
22. Kida K, Motegi T, Ishii T, Hattori K. Long-term oxygen therapy in Japan: history, present status, and current problems. Pneumonol Alergol Pol. 2013;81(5):468–478.
23. Branson RD. Oxygen therapy in COPD. Respir Care. 2018;63(6):734–748. doi:10.4187/respcare.06312
24. Liao H, Pei W, Li H, et al. Efficacy of long-term noninvasive positive pressure ventilation in stable hypercapnic COPD patients with respiratory failure: a meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2017;12:2977–2985. doi:10.2147/COPD.S148422
25. Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594–3602. doi:10.1016/j.vaccine.2013.04.084
26. Hsu DJ, North CM, Brode SK, Celli BR. Identification of barriers to influenza vaccination in patients with chronic obstructive pulmonary disease: analysis of the 2012 behavioral risk factors surveillance system. Chronic Obstr Pulm Dis. 2016;3(3):620–627.
27. Lall D, Cason E, Pasquel FJ, Ali MK, Narayan KM. Effectiveness of influenza vaccination for individuals with chronic obstructive pulmonary disease (COPD) in low- and middle-income countries. COPD. 2016;13(1):93–99. doi:10.3109/15412555.2015.1043518
28. Camp PG, Hernandez P, Bourbeau J, et al. Pulmonary rehabilitation in Canada: a report from the Canadian Thoracic Society COPD Clinical Assembly. Can Respir J. 2015;22(3):147–152. doi:10.1155/2015/369851
29. Australian Institute of Health and Welfare. Monitoring pulmonary rehabilitation and long-term oxygen therapy for people with chronic obstructive pulmonary disease (COPD) in Australia: a discussion paper. 2013. Available from: https://www.aihw.gov.au/.
30. Chen Y-J, Fan J-Y, Guo S-E, Hwang SL, Yang TM. Factors facilitating and hindering the intention to promote pulmonary rehabilitation for patients with COPD among respiratory therapists. Int J Chron Obstruct Pulmon Dis. 2017;12:2695–2702. doi:10.2147/COPD.S142124
31. Sohanpal R, Steed L, Mars T, Taylor SJ. Understanding patient participation behaviour in studies of COPD support programmes such as pulmonary rehabilitation and self-management: a qualitative synthesis with application of theory. NPJ Prim Care Respir Med. 2015;25:15054. doi:10.1038/npjpcrm.2015.54
32. Siddiqui FM, Diamond JM. Lung transplantation for chronic obstructive pulmonary disease: past, present, and future directions. Curr Opin Pulm Med. 2018;24(2):199–204. doi:10.1097/MCP.0000000000000452
33. Bogart M, Stanford RH, Laliberté F, Germain G, Wu JW, Duh MS. Medication adherence and persistence in chronic obstructive pulmonary disease patients receiving triple therapy in a USA commercially insured population. Int J Chron Obstruct Pulmon Dis. 2019;14:343–352. doi:10.2147/COPD.S184653
34. Tøttenborg SS, Lange P, Johnsen SP, Nielsen H, Ingebrigtsen TS, Thomsen RW. Socioeconomic inequalities in adherence to inhaled maintenance medications and clinical prognosis of COPD. Respir Med. 2016;119:160–167. doi:10.1016/j.rmed.2016.09.007
35. Calzetta L, Cazzola M, Matera MG, Rogliani P. Adding a LAMA to ICS/LABA therapy: a meta-analysis of triple combination therapy in COPD. Chest. 2019;155(4):758–770. doi:10.1016/j.chest.2018.12.016
36. Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness and safety of LABA-LAMA vs LABA-ICS treatment of COPD in real-world clinical practice. Chest. 2019;155(6):1158–1165. doi:10.1016/j.chest.2019.03.005
37. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
38. Lange P, Celli B, Agusti A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–122.
39. Karampitsakos T, Hillas G, Zervas E, et al. Prospective evAluatIon foR inhalation devices in Greek patients with COPD and asthma: the PAIR study. Pulm Pharmacol Ther. 2020;60:101882. doi:10.1016/j.pupt.2019.101882
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

