Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Current Progress of COPD Early Detection: Key Points and Novel Strategies
Authors Lin CH, Cheng SL, Chen CZ , Chen CH , Lin SH, Wang HC
Received 24 March 2023
Accepted for publication 9 July 2023
Published 19 July 2023 Volume 2023:18 Pages 1511—1524
DOI https://doi.org/10.2147/COPD.S413969
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Ching-Hsiung Lin,1– 4,* Shih-Lung Cheng,5,6 Chiung-Zuei Chen,7 Chia-Hung Chen,8 Sheng-Hao Lin,1 Hao-Chien Wang9,*
1Division of Chest Medicine, Changhua Christian Hospital, Changhua, 500, Taiwan; 2Institute of Genomics and Bioinformatics, National Chung Hsing University, Taichung, 402, Taiwan; 3Ph.D. Program in Translational Medicine, National Chung Hsing University, Taichung, Taiwan; 4Department of Recreation and Holistic Wellness, MingDao University, Changhua, Taiwan; 5Department of Internal Medicine, Far Eastern Memorial Hospital, Taipei, 220, Taiwan; 6Department of Chemical Engineering and Materials Science, Yuan Ze University, Taoyuan, 320, Taiwan; 7Division of Pulmonary Medicine, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan; 8Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, China Medical University Hospital, Taichung, 404, Taiwan; 9Department of Internal Medicine, National Taiwan University Hospital, Taipei, 100, Taiwan
*These authors contributed equally to this work
Correspondence: Hao-Chien Wang, Department of Internal Medicine, National Taiwan University Hospital, Taipei, 100, Taiwan, Tel + 886 2 2322-0322, Fax + 886 2 2322-0322, Email [email protected] Ching-Hsiung Lin, Department of Internal Medicine, Division of Chest Medicine, Changhua Christian Hospital, 135 Nanhsiao Street, Changhua, 50006, Taiwan, Tel +886-4-7238595, Fax +886-4-7232942, Email [email protected]
Abstract: Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality worldwide, with approximately 70% to 80% of adults with COPD being undiagnosed. Patients with undiagnosed COPD are at increased risk of poor outcomes and a worsened quality of life, making early detection a crucial strategy to mitigate the impact of COPD and reduce the burden on healthcare systems. In the past decade, increased interest has been focused on the development of effective strategies and instrument for COPD early detection. However, identifying undiagnosed cases of COPD is still challenging. Both screening and case-finding approaches have been adopted to identify undiagnosed COPD, with case-finding being recommended by the 2023 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline and the updated United States Preventive Services Task Force (USPTF) recommendation. Nonetheless, the approaches, criteria, and instruments used for early detection of COPD are varied. However, advances in the taxonomy and risk factors of COPD are continuously being investigated. It is important to continuously assess the current state of knowledge on COPD early detection, given the challenges associated with identifying undiagnosed COPD. This review aims to highlight recent advances in early detection of COPD. To discuss the current challenge and opportunity in COPD early detection, providing an overview of existing literature on COPD case-finding strategies, including the approaches, criteria for subjects, and instruments. The review also summarizes the current progress in COPD case-findings and proposes a COPD case-finding flowchart as an efficient method for identifying at risk COPD patients.
Keywords: COPD, undiagnosed, at-risk, case-finding, early detection
Introduction
Chronic obstructive pulmonary disease (COPD) is the leading cause of morbidity and mortality worldwide and its prevalence is increasing. Unfortunately, COPD frequently goes undiagnosed, highlighting its prominence as a pressing healthcare issue.1,2 Underdiagnosis of COPD has significant policy implications because managing COPD appropriately is known to reduce the risk of future exacerbations and hospitalizations, alleviate symptoms, and increase exercise tolerance. Early detection of COPD is therefore a crucial strategy for mitigating its impact and reducing the burden on healthcare systems.
In the past decade, increased interest has been focused on the development of effective strategies for early detection of COPD. Various strategies and instrument for COPD early detection have been developed. Despite these advancements, identifying undiagnosed cases of COPD remains a challenge due to underutilization of spirometry, the failure to identify the initial indications of COPD, and the tendency to overlook risk factors beyond tobacco smoking and advanced age.3,4 For the early detection of COPD, both screening and case-finding approaches have been adopted to identify undiagnosed COPD. A crucial difference exists between screening and case-finding approaches. Screening is performed in the general population to identify individuals with COPD, whereas case-finding is conducted in adults with suspected COPD who have respiratory symptoms and risk for disease.5,6 According to 2023 GOLD guideline and the updated USPTF recommendation, COPD case-finding is advocated strategy for COPD early detection.7,8 However, the approaches, criteria for subjects with COPD, and instruments for early detection are varied. Additionally, advances in the taxonomy and risk factors of COPD are continuously being investigated. It is crucial to continuously assess the current state of knowledge on COPD early detection and explore new approaches and tools, given the challenges associated with identifying undiagnosed COPD.
This review aims to highlight recent advances in early detection of COPD, with a focus on current progress and novel strategies. Firstly, we discussed the current challenge and opportunity in COPD early detection. As case-finding is the advocated strategy for early detection of COPD, we provide an overview of the existing literature on COPD case-finding strategies, including the criteria for subjects, instruments, and approaches. Finally, we summarize the current progress in COPD case-finding and propose a COPD case-finding flowchart as an efficient method for identifying at-risk COPD patients.
Key Points of COPD Early Detection
The Importance of COPD Early Detection
In the past three decades, COPD cases, deaths, and disability-adjusted life years have increased significantly worldwide.9 However, approximately 70% to 80% of adults with COPD are undiagnosed.1,3,10–13 Patients with undiagnosed COPD are at an increased risk of poor outcomes have been identified. Compared with individuals without COPD, adjusted hazard ratio (HR) was 15.5 (95% confidence interval (CI): 11.0–21.8) for exacerbations, 2.8 (95% CI: 2.4–3.3) for pneumonia, 4.3 (95% CI: 2.8–6.7) for death from respiratory causes, and 2.0 (95% CI: 1.8–2.3) for death from all causes in individuals with undiagnosed, symptomatic COPD.14 Individuals with undiagnosed mild COPD had slightly higher rates of ambulatory care visits (adjusted relative risk (aRR), 1.06, 95% CI: 1.03–1.10) than those without COPD.12 Compared with individuals without COPD, patients with moderate to severe undiagnosed COPD were significantly more often visited the hospital (aRR, 1.61, 95% CI, 1.07–2.43).12 Moreover, individuals with undiagnosed COPD had worse health-related quality of life compared to those without airflow obstruction.15 Furthermore, compared to early diagnosis, late detection of COPD is related to higher exacerbation rate and increased comorbidities (such as cardiovascular disease, depression, osteoporosis and diabetes) and costs.16,17 Overall, undiagnosed COPD imposes a substantial burden on both patients and the healthcare system as it results in a worsened quality of life for patients, increased likelihood of comorbidities and poor prognoses, and strains on healthcare resources. Since a greater likelihood of the undiagnosed COPD is associated with milder airway obstruction and fewer respiratory symptoms in contrast to those with a confirmed COPD diagnosis,18,19 early detection and early intervention in patients with mild and moderate COPD may have beneficial effects on disease progression and clinical outcomes. Over the past decade, evidence has increased on the effectiveness of early intervention in COPD, including pharmacological treatment, smoking cessation, or pulmonary rehabilitation programs. Zhou et al showed in a recent trial that tiotropium improved forced expiratory volume in 1 second (FEV1) and reduced the annual decline in the post-bronchodilator FEV1 compared to placebo in COPD patients of GOLD stages 1 or 2 over 24 months. Tiotropium also reduced exacerbations and improved life quality.20 In the Lung Health Study, smokers with mild-to-moderate COPD who quit smoking had a slower decline in FEV1 (31 mL/yr) over 4 years compared to those who continued smoking (62 mL/yr).21 Moreover, a systematic review found that mild COPD patients who underwent pulmonary rehabilitation programs (PR) experienced significant improvements in health-related quality of life and exercise capacity.22 Furthermore, a meta-analysis reported that a significant improvement in health-related quality of life (HRQOL) following PR in COPD with mild symptoms.23 The above findings have demonstrated that patients with mild and moderate COPD benefit from early intervention, and it is noteworthy that the majority of patients with undiagnosed COPD have mild and moderate airflow limitation. Therefore, early detection of COPD could provide an opportunity to implement interventions in the early stages of the disease, which can potentially slow down disease progression and minimize the overall burden of COPD.
Challenges of COPD Early Detection
Despite the potential benefits of CODP early detection, several challenges remain in this area. These challenges include the lack of spirometry use, the failure to recognize early signs of COPD, and the disregard of risk factors for COPD other than tobacco smoke and old age.
Lack of Spirometry Use
Spirometry is an important tool for accurate diagnosis and effective management of COPD. However, the underuse of spirometry is a major factor relating to COPD underdiagnosis. A recent study by Joo et al involving 93,724 newly diagnosed COPD patients found that only 36.7% had spirometry performed.24 Lamprecht et al found that only 67.6% of participants with a reported diagnosis of COPD had a spirometry in the past in Salzburg and Austria.25 In Italy, only 56.2% with doctor-diagnosed COPD have ever performed a spirometry at any time in the past in primary care setting.26 The reasons for underuse of spirometry were lack of expertise in spirometry interpretation, high costs of the spirometer, and unaffordability of the test by the patients, especially in primary care or resource-limited settings.6,27,28 Alternative tools for settings without spirometry is needed for overcoming this barrier.
Missing Early Signs of COPD
Previous studies demonstrated that undiagnosed patients may experience a lower disease burden, which can significantly delay the diagnosis of COPD. Lamprecht et al reported that individuals with mild or moderate airway limitation are significantly more likely to have undiagnosed COPD compared to those with very severe limitation.1 This indicated that milder airway limitation is significantly positively associated with undiagnosed COPD. Furthermore, individuals with undiagnosed COPD experience fewer symptoms, such as wheezing and chest pain, compared to those who have been diagnosed with COPD.4 Previous meta-analysis study concluded that undiagnosed COPD had less severe airflow obstruction and fewer respiratory symptoms compared to diagnosed COPD.19 These studies have shown that the difference in disease severity and symptom burden between diagnosed and undiagnosed patients lead to a delay in the diagnosis of COPD. This could be due to ignoring symptoms, underreporting symptoms to their physician, or physicians missing subtle early signs. For instance, a retrospective study of a clinical cohort in UK demonstrated that 85% of patients sought primary care for lower respiratory symptoms within a five-year period before being diagnosed with COPD. Thus, it is crucial to enhance awareness of COPD among individuals and physicians alike.
Overlooking Other Risk Factors for COPD
COPD has long been associated with tobacco smoking and old age. However, the burden of COPD is increasingly attributed to non-tobacco risks, and these risk factors are expected to surpass tobacco use in the next two decades. COPD arises from the dynamic, cumulative, and repeated interactions of genes (G) and the environment (E) over the course of an individual’s lifetime (T), which can cause damage to the lungs and/or modify their typical developmental or aging processes (GETomics).7 According to the 2023 Global Initiative for Chronic Obstructive Lung Disease, the risk factors beyond tobacco-smoking included environmental factors, such as indoor and outdoor air pollution and lung development and aging. Previous meta-analysis study of 11 cross-sectional and 4 case–control studies covering a wide range of countries demonstrated household biomass smoke exposure as risk factor for developing COPD in both men (OR 4.30, 95% CI 1.85–10.01) and female (OR 2.73, 95% CI 2.28–3.28).29 The study analyzed data from 30,887 individuals aged 40–69 years using the UK Biobank database. The researchers found that PM2.5, PM10, and NO2 concentrations were significantly associated with COPD prevalence (PM2.5: OR 1.52 [95% CI: 1.42–1.62] per 5 μg/m3; PM10: OR 1.08 [95% CI: 1.00–1.16] per 5 μg/m3; and NO2: OR 1.12 [95% CI: 1.10–1.14] per 10 μg/m3, respectively).30 Impaired lung growth is widely recognized as a contributing factor to the development of COPD.31 Previous research has shown that lower peak lung function in early adulthood is associated with an increased risk of COPD later in life.32–34 Overall, indoor and outdoor exposure is strongly linked to an elevated risk of COPD, emphasizing the need for policymakers to implement measures to reduce air pollution and safeguard public health. Impaired lung growth in childhood and early adulthood may be attributed to several factors, including repeated respiratory infections, socioeconomic and dietary factors, and exposure to environmental tobacco smoke.35–40 Efforts to reduce the exposure of individuals to risk factors for impaired lung growth and COPD in early life are essential for improving lifelong lung health.
Opportunities of COPD Early Detection
Early detection of COPD could improve disease recognition and provide appropriate treatment earlier. In the past decade, there are various strategies for identifying undiagnosed COPD, including screening and case-finding. It is important to differentiate between COPD screening and case-finding. Screening is done in asymptomatic populations based on demographic factors such as age and sex, while case finding utilizes an individual patient’s symptoms and disease risk to determine the need for further testing. In 2022, USPTF recommends against screening for COPD in asymptomatic persons due to no net benefit and large associated opportunity costs.8 Lambe et al demonstrate that regular systematic COPD case-finding using a questionnaire in primary care is likely to be cost-effective in the long-term.41 This study provides an important evidence on benefit of identifying undiagnosed COPD using case-finding approach. Moreover, active case-finding is advocated approach for COPD early detection by 2023 GOLD guideline. Therefore, case-finding approach is a potential strategy for identifying individuals at high risk of COPD who are still undiagnosed than screening.
Here, we have summarized the following key points related to early detection of COPD: (1) COPD is often undiagnosed; (2) there is an urgent need to improve awareness of COPD and its risk factors, including non-smoking related factors, among both individuals and physicians; (3) spirometry should not be used for screening asymptomatic individuals, instead a case-finding approach is recommended; and (4) there is a requirement for an effective and locally administered screening procedure that is efficient.
Strategy for COPD Early Detection: Focus on Case-Finding Approach
The 2023 GOLD report advocates an active case-finding approach for early detection of COPD.7 Over the past decade, there are various studies focused on developing case-finding strategies for early detection of COPD due to advances in the proposed taxonomy and risk factors of COPD and importance of early detection for COPD. In this section, we will provide an insightful overview of the evolution of case-finding strategies by reviewing various studies, including approach subjects’ criteria, and instruments.
Case-Finding Approach
Various case-finding approaches have been reported recently; however, there is extensive heterogeneity among case-finding strategies for COPD.42 Moreover, no one strategy has proved to be superior to another. A cluster-randomised controlled trial conducted by Jordan et al aimed to investigate the effectiveness of active case-finding and opportunistic case-finding of identifying undiagnosed COPD in a UK primary care setting, the result demonstrates that newly detected COPD cases were higher in the active case-finding group than in the opportunistic case-finding group (822/1278 vs 370/1278).43 They provided an evidence on an active targeted approach to case finding that is a cost-effective way to identify undiagnosed patients.
Criteria for COPD Case-Finding Initiation
Case finding is a strategy for identifying individuals at risk for a specific condition. Traditionally, COPD is characterized by persistent respiratory symptoms and common among people aged ≥40 years with smoking history. We review COPD case-finding studies from 2011 to 2021 (Appendix Table 1), most of them included participants that were above 35 years of age with smoking history, or who were symptomatic only, or who were symptomatic with smoking history. Amount of smoking is ranged from 1 to 20 pack-year, 10 pack-year is the most used inclusion criteria to evaluate the risk (12 in 20 studies).44–66 Only one studies recruited patients aged >18 years who were former or current smokers or symptomatic smokers, respectively.61 The incidence of COPD is generally above 20% in studies that include people aged 40 or older with a smoking history and/or persistent symptoms. In contrast, studies that include people aged 18 and above have an incidence of COPD that is typically below 20%. It suggests that COPD case-finding may be more effective if targeted towards individuals aged 40 or older with a history of smoking and/or persistent symptoms. However, it also implies that targeting younger individuals may be less fruitful as the incidence of COPD is typically lower in this population. In addition, studies focus on young COPD demonstrated that the mean aged of participant included in these studies range from 31.9 to 47.8,67–71 which supported that the age ≥35 years or ≥40 years is a feasible criterion for COPD case-finding. The identification and management of risk factors is a critical component of effective disease detection strategies, therefore, incorporating risk factors into a case-finding strategy is essential for identifying individuals who are at higher risk of developing COPD. Despite the tobacco-smoking is the dominant risk factor of COPD, non-smoking related risk factors account for over 50% of the burden of COPD worldwide, especially in LMIC, it has been reported.72 Additionally, there is a significant positive association between undiagnosed COPD and never having smoked. This highlights the importance of assessing non-smoking related risk factors for COPD to identify cases that have gone undiagnosed. After 2017, risk factors beyond smoking history, including biomass, outdoor air pollution, and occupational hazards, have been increasingly included as factors in the identification of COPD cases. This suggests that breaking the stereotype and expanding the spectrum of early COPD detection presents an opportunity to more effectively identify undiagnosed COPD cases.
COPD Case-Finding Tools
In the past decade, several case-finding tools have been developed due to the various barriers associated with using spirometry, which is the gold standard for diagnosing COPD. The common tools included hand-held device, questionnaire, and prediction model, which have been validated in different setting. Despite various studies evaluating their performance in identifying undiagnosed COPD, a comprehensive assessment of their advantages and limitations remains scarce. This section presents a summary of the advance in the performance, advantages, and potential limitations of COPD case-finding tools.
Hand-Held Device
Hand-held device includes peak expiratory flow (PEF) device and micro-spirometer. The most evaluated in previous studies are the COPD-6 (Vitalograph Ltd., Ennis, County Clare, Ireland) and the Piko-6 (nSpire Health Inc., Longmont, CO, USA), and the threshold for COPD diagnosis with a micro-spirometer is evaluated using the FEV1/FEV6 ratio. In symptomatic smoker, the highest area under the curve (AUC) of COPD-6 and Piko-6 using FEV1/FEV6 ratio is 0.8 and 0.937, respectively. And the highest AUC is 0.85 and 0.87 in COPD-6 and Piko-6 using FEV1/FEV6 ratio in patients with smoking history only (Table 1). A meta-analysis study demonstrates that the overall AUC of micro-spirometers, including COPD-6 and Piko-6, is 0.84 (95% CI 0.80–0.89).6 Recently, a smart phone based handheld wireless spirometer has been developed which is light, friendly use, and available data transmission in real-time. In previous study, a novel app-based spirometry was validated by Lin et al, the results indicated that FEV1/forced vital capacity (FVC) measured by Spirobank Smart could significantly discriminate COPD from symptomatic smokers, with the corresponding AUC of 0.903 (95% CI = 0.860–0.947).55 There are also two mobile spirometries that have been reported in screening airway obstruction, but these devices have not been validated for COPD case-finding.73–77 Overall, the AUC range for these micro-spirometry devices is between 0.76 and 0.94. Most of them exhibit high sensitivity and specificity, indicating strong discriminative performance.
 |
Table 1 Summarized the Performance (AUC) of Various Alternative Tools Group by Different Criteria for Case-Finding Initiation Using Hand-Held Devices, Questionnaire, or Combination of Tools |
Questionnaire
COPD diagnostic questionnaire (CDQ), Chronic Obstructive Pulmonary Disease-Population Screener (COPD-PS), Lung Function Questionnaire (LFQ) questionnaire are the most widely used for assessing the risk of COPD. Both of them are contained items including age, smoking history and respiratory symptoms (Table 2). In this review, COPD-PS and CDQ are most evaluated. Two meta-analysis studies evaluated the performance of COPD-PS, Gu et al demonstrate that the AUC of the summary ROC curve was 0.78 and similar results were also found by Schnieders et al (summary AUC of COPD-PS=0.77).6,78 The COPD-PS has two cut-off values greater than 4 or 5 points. According to Gu et al, increasing the cut-off value resulted in decreased sensitivity but increased specificity. However, the optimal cut-off value was found to be at 4 points, as it achieved a balance between sensitivity (74.52%) and specificity (70.24%). The AUC of CDQ ranged from 0.67 to 0.81.52,57 There are also two cut-off values of CDQ are 16.5 and 19.5, respectively. The meta-analysis study, which evaluated four different studies, revealed that a cut-off value of ≥19.5 for CDQ resulted in a higher Youden index compared to the threshold of ≥16.5. This indicates that a cut-off value of ≥19.5 is the one that achieves a balance between sensitivity and specificity.6 Based on a meta-analysis, the COPD-PS, LFQ, and CDQ questionnaires were found to have comparable accuracy.6 However, a more recent meta-analysis concluded that the CDQ and COPD-PS questionnaires were equally effective.79 Moreover, three studies that directly compared the CDQ and COPD-PS questionnaire consistently reported a slightly higher AUC for the CDQ.52,57 These discrepancies in results could be attributed to various factors, such as differences in the study population, regional disparities, recall bias, or information bias, and methodology. Environmental exposure is now recognized as a crucial risk factor for COPD beyond tobacco smoking. In recent years, new tools such as CATPURE, COLA, and UCAP-Q have been developed to help identify individuals with COPD. These questionnaires utilize information on occupational and environmental history to assess the risk of developing COPD. The AUC of CATPURE, COLA, and UCAP-Q is 0.80, 0.68, 0.82, respectively.62,66,80 The AUC values of these questionnaires suggest they are moderately to highly effective in identifying individuals who are at risk of developing COPD. These tools consider an individual’s occupational and environmental history, which is important as environmental factors are known to play a critical role in the development of COPD. Continuous research and development of effective tools are crucial to identify undiagnosed cases and better manage COPD.
 |
Table 2 Details of COPD Case-Finding Questionnaire |
Questionnaire and Peak Expiratory Flow (PEF) Device: Combination Method
The combined use of a questionnaire and a peak expiratory flow (PEF) device is an-other validated COPD case-finding strategy. Soriano et al demonstrated that the combination of the COPD-PS and PEF (AUC = 0.761) exhibited higher accuracy than the COPD-PS (AUC = 0.715) only or PEF only (AUC = 0.664). Moreover, the combined use of the COPD-PS questionnaire with a PEF device led to a 90% reduction in the number of spirometry tests performed.65 Recently, two novel combination tools, namely COPD assessment in primary care to identify undiagnosed respiratory disease and exacerbation risk (CAPTURE, COPD assessment in primary care to identify undiagnosed respiratory disease and exacerbation risk) and COPD in low- and middle-income countries assessment (COLA, COPD in low- and middle-income countries assessment) instrument, have been reported. The CAPTURE questionnaire was developed and validated by Martinez et al and used to examine the presence or absence of symptoms (breathing problems and fatigue), smoking or biomass exposure, and recent history of acute respiratory illnesses. The total score of the CAPTURE questionnaire ranges from 0 to 6. Patients with scores ranging from 2 to 4 should undergo PEF testing. Patients with scores of >5 or 2–4 with low PEF (<250 L/min for women and <350 L/min for men) should undergo further evaluation such as spirometry.62 Quezada performed a study in Spain and demonstrated that the use of the combination of the CAPTURE questionnaire along with a PEF device yielded a higher AUC (0.953) than did the use of the CAPTURE questionnaire only (0.928).61 The COLA questionnaire was developed by Siddharthan et al and used in combination with a PEF device for identifying individuals with COPD. The COLA questionnaire was used to evaluate respiratory symptoms, smoking or biomass exposure, and functional status. In terms of scoring, participants were assigned a score of 1 point for each of the seven questions (including those related to respiratory symptoms, smoking or biomass exposure, and functional questions), 1 point if the participant was aged ≥55 years, 1 point if PEF was between 250 and 399 L/min, and 2 points if PEF was <250 L/min. The COLA instrument yielded a cross-validated AUC of 0.83 (95% CI = 0.78–0.89). A COLA score of ≥5 exhibited the highest sensitivity, specificity, and positive predictive value.66 The indirect comparison indicated that the performance of the CAPTURE instrument or COLA instrument was more favorable than that of the combination of the COPD-PS and PEF, indicating that biomass exposure and functional status in the questionnaire play vital roles in the evaluation of the probability of having COPD.
The combination tool begins with a simple questionnaire. Patients can complete it by themselves, at home or in the office, with the result easily scored and interpreted. And PEF increases the accuracy of the case-finding process, but it is performed only as needed. The combination tool exhibited improved precision than either alone.
Taken together, these findings suggest that the combined use of a questionnaire and a PEF device was more effective in identifying individuals with a higher risk of COPD, and a questionnaire can function as a pretest for lung function testing.
Prediction Model
Besides micro-spirometry and questionnaire, prediction model is also developed for identifying undiagnosed COPD using routine data from EHRs. The factors used to develop the prediction model was varied. The model incorporates a variety of factors, with age, medication history, and respiratory disease history being the most commonly used variables (Table 3). We reviewed the existed prediction model for identifying undiagnosed COPD and found that AUC values of these prediction models range from 0.74 to 0.87 (Table 4).81–86 Additionally, the majority of these models demonstrate high NPV, indicating that the occurrence of false negatives is minimized. However, three studies have shown a low positive predictive value (PPV) of less than 25% for prediction model, suggesting a higher rate of false positives.81,83,85 These findings imply that there is a likelihood of misdiagnosis or false alarms. Therefore, before applying these prediction models, it is crucial to adjust the decision threshold or carry out external validation to enhance their accuracy and reduce the likelihood of errors.
 |
Table 3 Details of COPD Case-Finding Prediction Models |
 |
Table 4 Summarized the Performance of COPD Case-Finding Prediction Models |
Comparison of Case-Finding Tools
A meta-analysis comparing various case-finding tools demonstrated that the performance of a micro-spirometer (summary AUC = 0.84) was more favorable than that of a questionnaire. And the CDQ and the COPD-PS questionnaire were similar accurate tools for case-finding (CDQ: summary AUC = 0.72; COPD-PS: summary AUC = 0.77).6 Chen et al provided real-world evidence by comparing Spirobank Smart, a novel app-based spirometer, with the PCOPD and COPD-PS. The results demonstrated that Spirobank Smart (AUC = 0.908) exhibited higher accuracy, sensitivity, and specificity than did the PCOPD (AUC = 0.788) and COPD-PS (AUC = 0.726) in identifying those with COPD;63 this finding is consistent with that of the meta-analysis. This study compares the discriminative accuracy of combination methods, including CATURE, and COLA in 3 low- and middle-income country settings (including Nepal, Peru, and Uganda). The finding demonstrates that performance of two combination tools varied by location. The AUC for COLA-6 is ranged from 75.8 (95% CI: 74.6–77.0) to 84.2 (95% CI: 83.0–85.4). The AUC for CAPTURE is ranged from 77.9 (95% CI: 74.5–79.2) to 88.2 (95% CI: 87.1–89.2). Compared with 3 LMIC settings, the CAPTURE demonstrated a lower AUC than studies in high-income settings, which were ranged from 0.906 to 0.953.87 The possibility is that lack of NCD- and COPD-trained health professionals and awareness of COPD which may hindered the COPD diagnosis. The results demonstrate that the instrument performance varied by LMIC and high-income countries.87
In summary, micro-spirometry is the most accurate tool for identifying undiagnosed COPD with high discrimination performance. However, there are some limitations to using micro-spirometry for COPD early detection. Firstly, it is more expensive than using prediction models or questionnaires. Secondly, appropriate technician training is necessary to operate the equipment accurately. Lastly, micro-spirometry requires calibration to ensure accurate results. In recent years, advancements have been made in micro-spirometry technology through the development of app-based or smart micro-spirometry. These innovations aim to enhance the accuracy and feasibility of micro-spirometry for detecting COPD, with features like calibration-free and automatic error detection. The technology provides real-time feedback on test quality, along with systematic and numeric visualizations of spirometer tests on a smartphone app. Additionally, this technology facilitates effective data communication.
Questionnaire is easy-to-use and cheaper than micro-spirometry and prediction model. The accuracy for discriminating individuals with or without COPD is modest. However, the use of questionnaires in COPD case-findings poses a potential risk of recall bias and/or information bias. Patients may not accurately remember or misunderstand some of the questions, leading to flawed responses. To avoid the recall or information bias, there are some suggestions for applying these validated questionnaires for CODP case-finding. Firstly, physicians should undergo proper training to ensure that patients fully understand the questions being asked. Additionally, standardized instructions and explanations should be provided for each question to minimize confusion. Furthermore, pilot testing of the questionnaire can help identify potential sources of bias, allowing for refinement of the questionnaire to reduce the impact of these biases.
Prediction model for identifying undiagnosed COPD are mostly developed on large sample size and can be integrated with clinical information systems. A larger sample size would have enabled estimation of the parameters with greater precision. Moreover, the prediction model can be seamlessly integrated with clinical information systems by programming it into these digital platforms. This integration enables the model to provide timely assessments of COPD risk for individuals, thereby enhancing the efficiency and accuracy of healthcare delivery. However, the accuracy of the prediction model can be affected by the quality of clinical coding and the absence of predictors that are not routinely recorded. To overcome these challenges, steps such as improving coding practices, capturing additional predictors, validating the model in different settings, and updating the model over time can be taken.
When choosing an optimal tool for COPD case-finding, it is needed to notice the balance of sensitivity and specificity, besides accuracy. Because low sensitivity contributes many subjects with undiagnosed COPD were missed, and low specificity led to unnecessary examinations due to false-positive results. Moreover, cost and feasibility are important questions that warrant consideration. However, if available, post-bronchodilator spirometry should be used for final diagnosis of COPD given its position as the gold standard.
Here, we summarized 3 important viewpoints regarding the strategy for COPD early detection:
- Active case-finding is the best approach to identify individuals with COPD at an early stage of their functional disorder, emphasizing a proactive rather than opportunistic approach in individuals over 35 or 40 years old.
- Hand-held spirometer should be the preferred method for high-risk individuals to ensure high efficiency and minimize the rate of negative results.
- Structured questionnaires, either self-administered or assisted by physicians, should be used to assess personal and environmental risk factors for COPD. These questionnaires, when appropriately designed with suitable cutoff levels, can effectively rule out false-positive cases (high NPV).
The Current Progress of COPD Case Findings and the Novel Strategies
In this section, we highlight three aspects of the current progress of COPD case-finding:
First, there is no consensus regarding the optimal criterion for COPD case-finding. According to Martinez et al, early COPD is defined as smoking histories of at least 10 pack years, FEV1/FVC ratio lower than the lower limit of normal, compatible abnormalities on computed tomography (CT), or accelerated FEV1 decline of at least 60 mL/year in subjects under 50 years of age. In the present review, we found that the recruitment age for case-finding in the literature has been moved from 50 to 35 years of age for early detection of COPD.
Second, biomass exposure was recently considered another crucial risk factor for COPD that needed to be assessed in order to identify undiagnosed COPD besides smoking history. Previous studies have indicated that COPD is commonly seen among nonsmokers exposed to biomass fuel combustion, especially in the low- and middle-income countries. In addition, over 90% of morbidity and mortality associated with COPD occur in these countries (LMICs), where household air pollution secondary to burning biomass is the main risk factor. Thus, assessment of biomass exposure is an important initiation criterion for COPD case-finding, especially among the non-smoker and in LMICs.
Third, micro-spirometry, such as piko-6 and COPD-6, is an accurate case-finding tool for identifying COPD which is characterized by small, ease of use, the requirement of less patient effort, and time-saving features. However, the PiKo-6 device did not report the quality of the test. Recently, the trend of integrating smart devices with micro-spirometry, namely app-based spirometry, has become more widespread and the accuracy of identifying undiagnosed COPD is feasible. The advantage of app-based spirometry is to provide user feedback on test quality in real-time and systematic visualization of spirometer tests and guarantee effective data communication.
The novel strategy of COPD case-finding is combination of questionnaire and PEF device. The advantage of combination tools is that they can improve the cost-accuracy of these tools. However, a head-to-head comparison of questionnaires, spirometry, and combination tools for identifying undiagnosed COPD is needed.
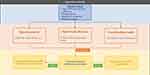
A clear strategy for case-finding is absent in COPD guidelines. We propose a COPD case-finding flowchart to serve as an efficient method of identifying at-risk patients (Figure 1). To identify COPD patients, the initiation criteria include age range of 35–85 years, smoking history, respiratory symptoms, or biomass exposure. Micro-spirometry may be better suited for high-income countries due to its higher technical requirements and cost. Conversely, combination methods could be a more viable option for low- and middle-income countries or resource-limited settings, as they offer a balance between accurately identifying COPD patients and reducing healthcare costs. Questionnaires can be utilized in any setting. To ensure appropriate interventions, we recommend the following clinical procedure for triaging individuals at risk of COPD. Individuals who test positive with case-finding tools for COPD should be referred to a pulmonologist for further diagnosis. And patients who receive a new COPD diagnosis should be invited to join the COPD integrated care program. While patients with an alternative diagnosis other than COPD, they should be recommended to receive smoking cessation program or risk management, such as reducing indoor and outdoor air pollution exposure, as well as regular lung function monitoring, should be recommended.
Conclusion
In summary, when choosing an optimal strategy for COPD case-finding, the criteria, accuracy, feasibility, and cost-effectiveness of approaches must be considered in relation to the operating environment, such as a low-versus-high income country or a different healthcare setting.
Abbreviation
ADL, activity of daily living; AUC, Area Under the Curve; CAPTURE, COPD assessment in primary care to identify undiagnosed respiratory disease and exacerbation risk; CAT, The COPD Assessment Test; CDQ, COPD diagnostic questionnaire; COPD, Chronic obstructive pulmonary disease; COPD-PS, Chronic Obstructive Pulmonary Disease-Population Screener; COPD-DQ, COPD-Diagnostic Questionnaire; COLA, COPD in low- and middle-income countries assessment; EGARPOC, COPD screening questionnaire from Terrassa; FVC, Forced vital capacity; FEV1, Forced expiratory volume in 1 second; HIC, high-income country; HRQL, health-related quality of life; IPAG, International Primary Care Airways Group; LFQ, Lung Function Questionnaire; LMIC, low- and middle-income country; NCD, non-communicable disease; PEF, peak expiratory flow; RHSQ, Respiratory Health Screening Questionnaire; UCAP-Q, Undiagnosed COPD and Asthma Population Questionnaire; USPTF, United States Preventive Services Task Force; VAS EQ-5D, EQ-5D visual analog scale.
Funding
This research was funded by Changhua Christian Hospital. Grant no: 111-CCH-MST-136.
Disclosure
The authors declare no conflict of interest.
References
1. Lamprecht B, Soriano JB, Studnicka M, et al; BOLD Collaborative Research Group, the EPI-SCAN Team, the PLATINO Team, and the PREPOCOL Study Group. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148(4):971–985. doi:10.1378/chest.14-2535
2. Soriano JB, Zielinski J, Price D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet. 2009;374(9691):721–732. doi:10.1016/S0140-6736(09)61290-3
3. Martinez CH, Mannino DM, Jaimes FA, et al. Undiagnosed obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc. 2015;12(12):1788–1795. doi:10.1513/AnnalsATS.201506-388OC
4. Hangaard S, Kronborg T, Hejlesen OK. Characteristics of subjects with undiagnosed COPD based on post-bronchodilator spirometry data. Respir Care. 2019;64(1):63–70. doi:10.4187/respcare.06296
5. Diab N, Gershon AS, Sin DD, et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(9):1130–1139. doi:10.1164/rccm.201804-0621CI
6. Schnieders E, Ünal E, Winkler V, et al. Performance of alternative COPD case-finding tools: a systematic review and meta-analysis. Eur Respir Rev. 2021;30(160):200350. doi:10.1183/16000617.0350-2020
7. Global strategy for prevention, diagnosis and management oF COPD: 2023 report; 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
8. Mangione CM, Barry MJ, Nicholson WK, et al; US Preventive Services Task Force. Screening for chronic obstructive pulmonary disease: US preventive services task force reaffirmation recommendation statement. JAMA. 2022;327(18):1806–1811. doi:10.1001/jama.2022.5692
9. Li HY, Gao TY, Fang W, et al. Global, regional and national burden of chronic obstructive pulmonary disease over a 30-year period: estimates from the 1990 to 2019 Global Burden of Disease Study. Respirology. 2023;28(1):29–36. doi:10.1111/resp.14349
10. Casas Herrera A, Montes de Oca M, López Varela MV, et al. COPD underdiagnosis and misdiagnosis in a high-risk primary care population in four Latin American countries. A key to enhance disease diagnosis: the PUMA study. PLoS One. 2016;11(4):e0152266. doi:10.1371/journal.pone.0152266
11. Echazarreta AL, Arias SJ, Del Olmo R, et al; Grupo de estudio EPOC.AR. Prevalence of COPD in 6 urban clusters in Argentina: the EPOC.AR study. Arch Bronconeumol. 2018;54(5):260–269. doi:10.1016/j.arbres.2017.09.018
12. Gershon AS, Thiruchelvam D, Chapman KR, et al; Canadian Respiratory Research Network. Health services burden of undiagnosed and overdiagnosed COPD. Chest. 2018;153(6):1336–1346. doi:10.1016/j.chest.2018.01.038
13. Soriano JB, Alfageme I, Miravitlles M, et al. Prevalence and determinants of COPD in Spain: EPISCAN II. Arch Bronconeumol. 2021;57(1):61–69. doi:10.1016/j.arbres.2020.07.024
14. Çolak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5(5):426–434. doi:10.1016/S2213-2600(17)30119-4
15. Alhabeeb FF, Whitmore GA, Vandemheen KL, et al. Disease burden in individuals with symptomatic undiagnosed asthma or COPD. Respir Med. 2022;200:106917. doi:10.1016/j.rmed.2022.106917
16. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the Arctic observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995–1008. doi:10.2147/COPD.S195382
17. Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729–1738. doi:10.2147/COPD.S255414
18. Denguezli M, Daldoul H, Harrabi I, et al. Prevalence and characteristics of undiagnosed COPD in adults 40 years and older - reports from the Tunisian population-based burden of obstructive lung disease study. COPD. 2020;17(5):515–522. doi:10.1080/15412555.2020.1804848
19. Johnson KM, Bryan S, Ghanbarian S, Sin DD, Sadatsafavi M. Characterizing undiagnosed chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res. 2018;19(1):26. doi:10.1186/s12931-018-0731-1
20. Zhou Y, Zhong NS, Li X, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377(10):923–935. doi:10.1056/NEJMoa1700228
21. Scanlon PD, Connett JE, Waller LA, et al; Lung Health Study Research Group. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The lung health study. Am J Respir Crit Care Med. 2000;161(2 Pt 1):381–390. doi:10.1164/ajrccm.161.2.9901044
22. Jácome C, Marques A. Pulmonary rehabilitation for mild COPD: a systematic review. Respir Care. 2014;59(4):588–594. doi:10.4187/respcare.02742
23. Rugbjerg M, Iepsen UW, Jørgensen KJ, Lange P. Effectiveness of pulmonary rehabilitation in COPD with mild symptoms: a systematic review with meta-analyses. Int J Chron Obstruct Pulmon Dis. 2015;10:791–801. doi:10.2147/COPD.S78607
24. Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134(1):38–45. doi:10.1378/chest.08-0013
25. Lamprecht B, Mahringer A, Soriano JB, Kaiser B, Buist AS, Studnicka M. Is spirometry properly used to diagnose COPD? Results from the BOLD study in Salzburg, Austria: a population-based analytical study. Prim Care Respir J. 2013;22(2):195–200. doi:10.4104/pcrj.2013.00032
26. Heffler E, Crimi C, Mancuso S, et al. Misdiagnosis of asthma and COPD and underuse of spirometry in primary care unselected patients. Respir Med. 2018;142:48–52. doi:10.1016/j.rmed.2018.07.015
27. Hangaard S, Helle T, Nielsen C, Hejlesen OK. Causes of misdiagnosis of chronic obstructive pulmonary disease: a systematic scoping review. Respir Med. 2017;129:63–84. doi:10.1016/j.rmed.2017.05.015
28. Vanjare N, Chhowala S, Madas S, Kodgule R, Gogtay J, Salvi S. Use of spirometry among chest physicians and primary care physicians in India. NPJ Prim Care Respir Med. 2016;26:16036. doi:10.1038/npjpcrm.2016.36
29. Hu G, Zhou Y, Tian J, et al. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138(1):20–31. doi:10.1378/chest.08-2114
30. Doiron D, de Hoogh K, Probst-Hensch N, et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J. 2019;54:1802140. doi:10.1183/13993003.02140-2018
31. Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med. 2019;7(4):358–364. doi:10.1016/S2213-2600(18)30529-0
32. Çolak Y, Nordestgaard BG, Vestbo J, Lange P, Afzal S. Relationship between supernormal lung function and long-term risk of hospitalisations and mortality: a population-based cohort study. Eur Respir J. 2021;57:2004055. doi:10.1183/13993003.04055-2020
33. Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi:10.1016/S0140-6736(07)61379-8
34. Regan EA, Lynch DA, Curran-Everett D, et al; Genetic Epidemiology of CI. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175:1539–1549. doi:10.1001/jamainternmed.2015.2735
35. Minas M, Hatzoglou C, Karetsi E, et al. COPD prevalence and the differences between newly and previously diagnosed COPD patients in a spirometry program. Prim Care Respir J. 2010;19(4):363–370. doi:10.4104/pcrj.2010.00034
36. Grigsby M, Siddharthan T, Chowdhury MA, et al. Socioeconomic status and COPD among low- and middle-income countries. Int J Chron Obstruct Pulmon Dis. 2016;11:2497–2507. doi:10.2147/COPD.S111145
37. Keranis E, Makris D, Rodopoulou P, et al. Impact of dietary shift to higher-antioxidant foods in COPD: a randomised trial. Eur Respir J. 2010;36:774–780. doi:10.1183/09031936.00113809
38. Seyedrezazadeh E, Moghaddam MP, Ansarin K, et al. Dietary factors and risk of chronic obstructive pulmonary disease: a systemic review and meta-analysis. Tanaffos. 2019;18(4):294–309.
39. Perret JL, Walters H, Johns D, et al. Mother’s smoking and complex lung function of offspring in middle age: a cohort study from childhood. Respirology. 2016;21:911–919. doi:10.1111/resp.12750
40. He Y, Jiang B, Li LS, et al. Secondhand smoke exposure predicted COPD and other tobacco-related mortality in a 17-year cohort study in China. Chest. 2012;142:909–918. doi:10.1378/chest.11-2884
41. Lambe T, Adab P, Jordan RE, et al. Model-based evaluation of the long-term cost-effectiveness of systematic case-finding for COPD in primary care. Thorax. 2019;74(8):730–739. doi:10.1136/thoraxjnl-2018-212148
42. Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
43. Jordan RE, Adab P, Sitch A, et al. Targeted case finding for chronic obstructive pulmonary disease versus routine practice in primary care (TargetCOPD): a cluster-randomised controlled trial. Lancet Respir Med. 2016;4(9):720–730. doi:10.1016/S2213-2600(16)30149-7
44. Frith P, Crockett A, Beilby J, et al. Simplified COPD screening: validation of the PiKo-6® in primary care. Prim Care Respir J. 2011;20(2):190–198. doi:10.4104/pcrj.2011.00040
45. Thorn J, Tilling B, Lisspers K, Jörgensen L, Stenling A, Stratelis G. Improved prediction of COPD in at-risk patients using lung function pre-screening in primary care: a real-life study and cost-effectiveness analysis. Prim Care Respir J. 2012;21(2):159–166. doi:10.4104/pcrj.2011.00104
46. van den Bemt L, Wouters BC, Grootens J, Denis J, Poels PJ, Schermer TR. Diagnostic accuracy of pre-bronchodilator FEV1/FEV6 from microspirometry to detect airflow obstruction in primary care: a randomised cross-sectional study. NPJ Prim Care Respir Med. 2014;24:14033. doi:10.1038/npjpcrm.2014.33
47. Represas-Represas C, Fernández-Villar A, Ruano-Raviña A, Priegue-Carrera A, Botana-Rial M. study group of “validity of COPD-6 in non-specialized healthcare settings”. Screening for chronic obstructive pulmonary disease: validity and reliability of a portable device in non-specialized healthcare settings. PLoS One. 2016;11(1):e0145571. doi:10.1371/journal.pone.0145571
48. Labor M, Vrbica Ž, Gudelj I, Labor S, Plavec D. Diagnostic accuracy of a pocket screening spirometer in diagnosing chronic obstructive pulmonary disease in general practice: a cross sectional validation study using tertiary care as a reference. BMC Fam Pract. 2016;17(1):112. doi:10.1186/s12875-016-0518-8
49. Kim JK, Lee CM, Park JY, et al. Active case finding strategy for chronic obstructive pulmonary disease with handheld spirometry. Medicine. 2016;95(50):e5683. doi:10.1097/MD.0000000000005683
50. Llordés M, Zurdo E, Jaén Á, Vázquez I, Pastrana L, Miravitlles M. Which is the best screening strategy for COPD among smokers in primary care? COPD. 2017;14(1):43–51. doi:10.1080/15412555.2016.1239703
51. Hidalgo Sierra V, Hernández Mezquita MÁ, Palomo Cobos L, et al. Usefulness of the piko-6 portable device for early COPD detection in primary care. Arch Bronconeumol. 2018;54(9):460–466. doi:10.1016/j.arbres.2018.04.015
52. Ronaldson SJ, Dyson L, Clark L, et al; DOC study team. Determining the optimal approach to identifying individuals with chronic obstructive pulmonary disease: the DOC study. J Eval Clin Pract. 2018;24(3):487–495. doi:10.1111/jep.12896
53. Dickens AP, Fitzmaurice DA, Adab P, et al. Accuracy of Vitalograph lung monitor as a screening test for COPD in primary care. NPJ Prim Care Respir Med. 2020;30(1):2. doi:10.1038/s41533-019-0158-2
54. Fujita M, Nagashima K, Takahashi S, Suzuki K, Fujisawa T, Hata A. Handheld flow meter improves COPD detectability regardless of using a conventional questionnaire: a split-sample validation study. Respirology. 2020;25(2):191–197. doi:10.1111/resp.13602
55. Lin CH, Cheng SL, Wang HC, et al. Novel app-based portable spirometer for the early detection of COPD. Diagnostics. 2021;11(5):785. doi:10.3390/diagnostics11050785
56. Lopez Varela MV, Montes de Oca M, Wehrmeister FC, Rodriguez C, Ramirez L, Menezes A. External validation of the PUMA COPD diagnostic questionnaire in a general practice sample and the PLATINO study population. Int J Chron Obstruct Pulmon Dis. 2019;14:1901–1911. doi:10.2147/COPD.S206250
57. Spyratos D, Haidich AB, Chloros D, Michalopoulou D, Sichletidis L. Comparison of three screening questionnaires for chronic obstructive pulmonary disease in the primary care. Respiration. 2017;93(2):83–89. doi:10.1159/000453586
58. López Varela MV, Montes de Oca M, Rey A, Casas A, Stirbulov R, Di Boscio V; PUMA Team. Development of a simple screening tool for opportunistic COPD case finding in primary care in Latin America: the PUMA study. Respirology. 2016;21(7):1227–1234. doi:10.1111/resp.12834
59. Stanley AJ, Hasan I, Crockett AJ, van Schayck OC, Zwar NA. COPD Diagnostic Questionnaire (CDQ) for selecting at-risk patients for spirometry: a cross-sectional study in Australian general practice. NPJ Prim Care Respir Med. 2014;24:14024. doi:10.1038/npjpcrm.2014.24
60. Preteroti M, Whitmore GA, Vandemheen KL, et al. Population-based case-finding to identify subjects with undiagnosed asthma or COPD. Eur Respir J. 2020;55(6):2000024. doi:10.1183/13993003.00024-2020
61. Quezada WA, Whippo BA, Jellen PA, et al; High-Risk-COPD Screening Study Group. How well does CAPTURE translate?: an exploratory analysis of a COPD case-finding method for Spanish-speaking patients. Chest. 2017;152(4):761–770. doi:10.1016/j.chest.2017.03.047
62. Martinez FJ, Mannino D, Leidy NK, et al; High-Risk-COPD Screening Study Group. A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(6):748–756. doi:10.1164/rccm.201603-0622OC
63. Chen CZ, Sheu CC, Cheng SL, et al. Performance and clinical utility of various chronic obstructive pulmonary disease case-finding tools. Int J Chron Obstruct Pulmon Dis. 2021;16:3405–3415. doi:10.2147/COPD.S339340
64. Tanino A, Kawamura T, Hamaguchi M, et al. A novel model-based questionnaire based on low-dose CT screening data for chronic obstructive pulmonary disease diagnosis in Shimane, Japan. Int J Chron Obstruct Pulmon Dis. 2021;16:1823–1833. doi:10.2147/COPD.S308922
65. Soriano JB, Molina J, Miravitlles M. Combining case-finding methods for COPD in primary care: a large, two-stage design study. Int J Tuberc Lung Dis. 2018;22(1):106–111. doi:10.5588/ijtld.17.0334
66. Siddharthan T, Wosu AC, Pollard SL, et al; LiNK Cohort Study Investigators. A novel case-finding instrument for chronic obstructive pulmonary disease in low- and middle-income country settings. Int J Chron Obstruct Pulmon Dis. 2020;15:2769–2777. doi:10.2147/COPD.S268076
67. Divo MJ, Marin JM, Casanova C, et al; BODE Collaborative Group. Comorbidities and mortality risk in adults younger than 50 years of age with chronic obstructive pulmonary disease. Respir Res. 2022;23(1):267. doi:10.1186/s12931-022-02191-7
68. Morice AH, Celli B, Kesten S, Lystig T, Tashkin D, Decramer M. COPD in young patients: a pre-specified analysis of the four-year trial of tiotropium (UPLIFT). Respir Med. 2010;104(11):1659–1667. doi:10.1016/j.rmed.2010.07.016
69. Çolak Y, Afzal S, Nordestgaard BG, Lange P, Vestbo J. Importance of early COPD in young adults for development of clinical COPD: findings from the Copenhagen general population study. Am J Respir Crit Care Med. 2021;203(10):1245–1256. doi:10.1164/rccm.202003-0532OC
70. Cosío BG, Pascual-Guardia S, Borras-Santos A, et al. Phenotypic characterisation of early COPD: a prospective case-control study. ERJ Open Res. 2020;6(4):00047–2020. doi:10.1183/23120541.00047-2020
71. Khalid F, Wang W, Mannino D, Diaz AA. Prevalence and population attributable risk for early chronic obstructive pulmonary disease in U.S. Hispanic/Latino individuals. Ann Am Thorac Soc. 2022;19(3):363–371. doi:10.1513/AnnalsATS.202103-253OC
72. Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022;400(10356):921–972. doi:10.1016/S0140-6736(22)01273-9
73. Du Plessis E, Swart F, Maree D, et al. The utility of hand-held mobile spirometer technology in a resource-constrained setting. S Afr Med J. 2019;109(4):219–222. doi:10.7196/SAMJ.2019.v109i4.13845
74. Jankowski P, Górska K, Mycroft K, et al. The use of a mobile spirometry with a feedback quality assessment in primary care setting - A nationwide cross-sectional feasibility study. Respir Med. 2021;184:106472. doi:10.1016/j.rmed.2021.106472
75. Ramos Hernández C, Núñez Fernández M, Pallares Sanmartín A, et al. Validation of the portable air-smart spirometer. PLoS One. 2018;13(2):e0192789. doi:10.1371/journal.pone.0192789
76. Boros PW, Maciejewski A, Nowicki MM, Wesołowski S. Comparability of portable and desktop spirometry: a randomized, parallel assignment, open-label clinical trial. Adv Respir Med. 2022;90:60–67. doi:10.5603/ARM.a2022.0013
77. Wasilewska E, Sobierajska-Rek A, Małgorzewicz S, Soliński M, Szalewska D, Jassem E. Is it possible to have home E-monitoring of pulmonary function in our patients with Duchenne muscular dystrophy in the COVID-19 pandemic?-A one center pilot study. Int J Environ Res Public Health. 2021;18(17):8967. doi:10.3390/ijerph18178967
78. Gu Y, Zhang Y, Wen Q, et al. Performance of COPD population screener questionnaire in COPD screening: a validation study and meta-analysis. Ann Med. 2021;53(1):1198–1206. doi:10.1080/07853890.2021.1949486
79. Bastidas-Goyes AR, Cardozo-Niñoz AO, Quintero-Muñoz E, López-Gómez KA, Suárez-Escobar LP, Hernández-Santos LE. Clinical questionnaires for chronic obstructive pulmonary disease diagnosis: a systematic review and meta-analysis. Revista de la Facultad de Medicina. 2021;69(1):e204.
80. Huynh C, Whitmore GA, Vandemheen KL, et al. Derivation and validation of the UCAP-Q case-finding questionnaire to detect undiagnosed asthma and COPD. Eur Respir J. 2022;60(3):2103243. doi:10.1183/13993003.03243-2021
81. Mapel DW, Petersen H, Roberts MH, et al. Can outpatient pharmacy data identify persons with undiagnosed COPD? Am J Manag Care. 2010;16:505–512.
82. Kotz D, Simpson CR, Viechtbauer W, et al. Development and validation of a model to predict the 10-year risk of general practitioner-recorded COPD. NPJ Prim Care Respir Med. 2014;24:14011. doi:10.1038/npjpcrm.2014.11
83. Haroon S, Adab P, Riley RD, et al. Predicting risk of COPD in primary care: development and validation of a clinical risk score. BMJ Open Respir Res. 2015;2:e000060. doi:10.1136/bmjresp-2014-000060
84. Smidth M, Sokolowski I, Kaersvang L, et al. Developing an algorithm to identify people with Chronic Obstructive Pulmonary Disease (COPD) using administrative data. BMC Med Inform Decis. 2012;12. doi:10.1186/1472-6947-12-38
85. Haroon S, Adab P, Riley RD, Fitzmaurice D, Jordan RE. Predicting risk of undiagnosed COPD: development and validation of the TargetCOPD score. Eur Respir J. 2017;49(6):1602191. doi:10.1183/13993003.02191-2016
86. Su KC, Ko HK, Chou KT, et al. An accurate prediction model to identify undiagnosed at-risk patients with COPD: a cross-sectional case-finding study. NPJ Prim Care Respir Med. 2019;29(1):22. doi:10.1038/s41533-019-0135-9
87. Siddharthan T, Pollard SL, Quaderi SA, et al; GECo Study Investigators. Discriminative accuracy of chronic obstructive pulmonary disease screening instruments in 3 low- and middle-income country settings. JAMA. 2022;327(2):151–160. doi:10.1001/jama.2021.23065
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.