Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Current Opinions on the Relationship Between CMTM Family and Hepatocellular Carcinoma
Authors Pei Y , Zhang Z, Tan S
Received 16 April 2023
Accepted for publication 12 August 2023
Published 25 August 2023 Volume 2023:10 Pages 1411—1422
DOI https://doi.org/10.2147/JHC.S417202
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmed Kaseb
Yulin Pei,1,2 Zhengbao Zhang,1,2 Shengkui Tan3
1Guangxi Key Laboratory of Environmental Exposomics and Entire Lifecycle Health, Guilin Medical University, Guilin, Guangxi, People’s Republic of China; 2Public Health Department of Guilin Medical University, Guilin, Guangxi, People’s Republic of China; 3Public Health Department of Youjiang Medical University For Nationalities, Baise, Guangxi People’s Republic of China
Correspondence: Zhengbao Zhang; Shengkui Tan, Email [email protected]; [email protected]
Abstract: Hepatocellular carcinoma (HCC) is a typically malignant tumor in the digestive system. The mortality of HCC ranks third place in the world, second only to lung cancer and colorectal cancer. For the characteristics of high invasiveness, high metastasis, high recurrence rate as well as short survival time, HCC treatment has always been difficult in clinical practice. Many causes have contributed to the appearance of these features, including insidious onset, high degree of malignancy, lack of effective early molecular diagnostic markers, and disease prediction models. The human chemokine-like factor superfamily (CMTMs) is a new gene family consisting of CKLF and CMTM1-CMTM8. CMTMs have a marvel domain which can activate and chemotaxis immune cells. Many studies have reported that CMTMs are involved in the regulation of cell growth and development, and play an important role in the malignant progression of the immune system and reproductive system, especially in the development of tumors. In this review, we summarized the structure and function of the human CMTMs, the relationship between its family members and HCC, the prognostic value, potential functions, and mechanisms in HCC. CMTMs could provide a new diagnostic and therapeutic target in clinical practice for patients with HCC.
Keywords: CMTM family, hepatocellular carcinoma, diagnosis, treatment
Introduction
Hepatocellular carcinoma (HCC) is a prevalent malignant tumor of the digestive system with a high incidence in sub-Saharan Africa and coastal zones of Asia. According to the recent announcements on “Global Cancer Statistics 2020” of the International Agency for Research on Cancer (IARC), the cases and deaths of HCC in China account for 45.3% and 47.1% of the global totals, respectively. In addition, the mortality rate of HCC in China ranks third among all cancers, behind lung cancer and colorectal cancer.1 There are three areas with high-incidence of HCC in China, two areas in Guangxi province (Fusui and Long’an) and one area in Jiangsu province (Qidong). In Guangxi province, HCC is the main cause of tumor-related deaths.2 Although with the development of surgical resection, tumor-targeted therapy and immunotherapy, patients with HCC still suffered unfavorable prognosis and low five-year overall survival rate.3 One of the most important reasons is that a great majority of patients were already in the middle to late stages of HCC when they were diagnosed. In comparison to stages C and D, the five-year survival ratio is about 70% for patients with HCC at stages 0 and A, according to Barcelona Clinic Liver Cancer (BCLC).4 Nowadays, it is believed that the occurrence of HCC is the result of gene-environmental interaction. Hepatic cirrhosis and fibrosis caused by chronic hepatitis virus infection are the most important risk factors to induce HCC.1,5,6 However, the exact mechanism of HCC has not been fully clarified. Moreover, due to the high occult nature of early symptoms, the lack of effective molecular markers for early diagnosis, the absence of an effective predictive model, and the high degree of malignancy, HCC suffered from high invasiveness, high metastatic recurrence and low survival rates. These problems have always been difficult to be solved in HCC clinical practice.
Chemokine-like factor superfamily (CMTM, formerly known as CKLFSF) is a new gene family which was first discovered and reported by Professor Wenling Han from Peking University in 2001.7 In homo species, CMTM consists of nine genes: CKLF (chemokine-like factor) and CMTM1-8 (chemokine-like factor-like MARVEL transmembrane domain-containing family member 1–8).7 CMTM1-8 are a new protein family with the structure of chemokine and transmembrane 4 superfamily (TM4SF) (Figure 1). The MARVEL domains have been important functions in membrane molecular transport.7 With the advancement of structural and functional study, CMTM has been found to participate in the regulation of cell proliferation and migration by activation and chemotaxis of immune cells. In particular, CMTM proteins play a critical role in the progression of tumors such as HCC, glioma cancer, non-small cell lung carcinoma, and breast cancer (Figure 1).8–12 In this review, we summarized the current developments between HCC and the CMTM family.
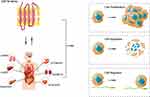 |
Figure 1 CMTM family and human tumors. CMTM family is a new protein family with the structure of chemokine and transmembrane 4 superfamily.7 And it is closely related with various tumors by regulating cancer cell proliferation, apoptosis and migration.8–12 The figure is created by authors through using software Adobe Illustrator 2023. Abbreviation: CMTM, Chemokine-like factor superfamily. |
The Structure and Function of the CMTM Gene Family
CKLF, located on chromosome 16q21, is the first member of the CMTM family discovered by Han.7 There are at least four RNA splice variants in CKLF: CKLF1, CKLF2, CKLF3, CKLF4, of which CKLF2 is the full-length cDNA product.13 CMTM1 is located on chromosome 16q21, composed of 23 subtypes (CMTM1 v1-v23), and the protein of CMTM v1-16 and CMTM v17-23 are encoded by open reading frame 1 and open reading frame 2, respectively.7 On chromosome 16q22, separated by only 311 base pairs (bp), CMTM2 gene is tightly linked with CMTM1 gene. Their amino acid sequences are extremely similar.14 The CMTM3 gene and CMTM4 gene are closely located on tumor suppressor locus 16q22.1. The CMTM3 promoter has a classic CpG island. Aberrant CpG methylation may be the source of CMTM3 silence. Different from other members of the CMTM family, alternatively spliced transcript variants of CMTM3 contain a different 5’UTR but encode the same protein.15 CMTM4 is the most conserved member of the CMTM family, including three transcriptional variants: CMTM4-V1, CMTM4-V2, and CMTM4-V3.7 CMTM5, independently located on chromosome 14q11.2, contained at least six alternative splice variants spanning from CMTM5-V1 to CMTM5-V6.7 The primary form of human CMTM5 is CMTM5-v1, an evolutionarily conserved protein, shares 42% homology with CMTM3 and can secrete related proteins via the vesicle-mediated secretory route.16 CMTM6 is located on chromosome 3p22.3. It shares 55% amino acid identity with CMTM4 and extensively expressed in the plasma membrane of many normal tissues.17 CMTM7 has a typical CpG island in the promoter and has been identified on the oncogene-rich chromosome 3p22. This gene is naturally conserved and encode two alternative splice variants.7 CMTM8 is an evolutionarily conserved product of cDNA. It shares 39.3% homology with TM4SF and have 95.4% amino acid similarity between homo species and Mus musculus specie.7 In summary, CMTM family members have different spliced variants. Their encoded products have MARVEL structural domains which can attract and trigger immune cells as well as playing significant roles in membrane molecular protein transport.10,18,19 Additionally, the CMTM family also contributes significantly to the pathogenesis of various cancers such as HCC, gliomas, non-small cell lung carcinoma, breast cancer.8–12
CMTM Family and HCC
CKLF
CKLF is located on the chromosome 16q21, including four subtypes: CKLF1, CKLF2, CKLF3, CKLF4.13 CKLF1 and CKLF3 are secreted isoforms while CKLF2 and CKLF4 are transmembrane proteins. Nowadays, numerous studies have confirmed that CKLF plays an important role in inflammation, auto-immune diseases and tumors.20,21 Liu et al found that CKLF1 was overexpressed in HCC tissues.22 Further analyses have shown that CKLF1 was associated with tumor stages, vascular invasion, and patient survival. It could promote the growth and metastasis of HCC in biological function analysis. Further experiments demonstrated that CKLF1 could prevent Adriamycin-induced apoptosis and promote the malignant development of HCC by activating the IL6/STAT3 pathway. Based on these findings, CKLF1 might inhibit apoptosis via the IL6/STAT3 pathway, encourage malignant cell transformation, and eventually promote hepato-carcinogenesis and metastasis. For this, we speculate that CKLF1 is an important prognosis factor of HCC, expected to become a potential target for advanced diagnosis and clinical prognosis. Chen et al examined the potential synergistic effect of Kanglaite (KLT) and cisplatin (CDDP) on HepG2 cells.23 It was found that CKLF1 participated in NF-κB-mediated inflammatory response and chemotherapy resistance, as well as drug efflux transporters. Furthermore, they discovered that KLT preconditioning may have a synergistic effect on HepG2 cells, enhancing CDDP’s impact on HepG2 cells. It is suggested that inflammation of the tumor microenvironment and chemotherapy resistance of CDDP in HepG2 cells may be affected by inhibition of the CKLF1-mediated NF-κB pathway. Sadly, up to now, there are few pieces of researches on the relationship between CKLF and HCC, and more studies and discussions are still needed to understand its impact and function mechanism.
CMTM1
CMTM1 is located on chromosome 16q21 with 23 isoforms (CMTM1 v1-v23).24 Current studies have revealed that CMTM1 abnormal expression was closely correlated with HCC,25 non-small cell lung carcinoma,26 lymphoma,27 breast cancer,28 stomach adenocarcinoma,29 and other tumors. CMTM1 over expression in HCC was detected by Song using immunohistochemistry (IHC) and the Cancer Genome Atlas (TCGA) database.25 According to an IHC assay, CMTM1 protein was extensively expressed in both paraneoplastic non-tumor tissues and HCC tissues. TCGA analysis indicated that CMTM1 mRNA expression was upregulated in HCC tissues and that low CMTM1 expression was associated with longer patients’ disease-free survival. HCC patients with CMTM1 over expression usually had a family history of HCC. Cox survival analysis showed that CMTM1 could be used as an independent prognostic factor in HCC.
CMTM2
The CMTM2 gene is closely linked to CMTM1 on chromosome 16q22.14 In recent years, a large number of studies have proven that CMTM2 is a mutated gene with an inhibitory effect on tumor cell migration and invasion, especially in stomach cancer.30–32 More importantly, CMTM2 can be used as a prognostic biological indicator of stomach cancer, playing a key role in the progression of the disease, particularly in diffuse gastric cancer.30–32 By utilizing ELISA, some researchers realized that CMTM2 values were considerably lower in chronic hepatitis B patients than in healthy controls. As well, serum CMTM2 levels were inversely associated with HBV DNA levels, but not related to serum ALT and AST levels. Further ROC curve analysis displayed a clear connection between CMTM2 levels and the diagnostic usefulness of HBV-related diseases. It was also found that CMTM2 expression was decreased by HBV infection through stimulation of the ubiquitin-proteasome system. Consequently, serum CMTM2 levels may serve as a reliable predictor of the pathogenesis of HBV-related illness.30 Guo et al found that the expression of CMTM2 in HCC tissues was notably lower than that in para-cancerous tissues.33 They also noticed that it was profoundly associated with tumor grade in HCC patients. Low expression of CMTM2 was generally associated with poor prognosis. These results were also consistent with bioinformatics analysis. Through using knockdown of CMTM2 in Huh-7 and SMMC7721 cells, Zhang et al verified that low expression of CMTM2 could improve the proliferation ability of HCC cells.34 Further experiments had shown that the knockdown of CMTM2 significantly down-regulated the expression of E-cadherin and β-catenin while up-regulated the expression of Vimentin, N-cadherin, ZEB1, and ZEB2. Taken together, they proposed that down-regulation of CMTM2 expression might induce the epithelial-mesenchymal transformation (EMT) process, promoting the invasion and migration of HCC cells.
CMTM3
CMTM3 is a member of the CMTM family found at 16q22.1.35,36 Recent data have suggested that CMTM3 was closely associated with malignant progression in a variety of tumors. For instance, it was discovered that the expression of CMTM3 was downregulated in various tumors including prostate cancer,37 testicular cancer,38 gastric cancer,39 esophageal cancer,40 and other tumors. On the one side, CMTM3 was regarded as a tumor suppressor gene which was crucial for the development and spread of tumors.37–40 On the contrary, it was also believed that in some tumors, CMTM3 could promote tumor growth and be proposed as an oncogene. In one case, inhibition of the expression of CMTM3 can be a new therapeutic target for prostate cancer (PC).41–46 In HCC, the relationship between CMTM3 and HCC has also been investigated. Li et al reported that CMTM3 was low-expressed in HCC.47 In vitro experiments have shown that overexpression of CMTM3 greatly inhibited the proliferation, invasion and EMT process of HCC. Besides, the phosphorylation of JAK2 and STAT3 in HepG2 cells was drastically lowered by overexpression of CMTM3 in vivo. It was also confirmed that tumor growth in Balb/c nude mice was reduced by CMTM3 overexpression. Yu et al found that CMTM3 was tightly related to m6A-lncRNAs, when elucidating the interactions of N6 methyladenosine (m6A) long non-coding RNAs (lncRNAs) and immune cell infiltration in HCC.48 It is possible to use m6A-lncRNAs in tumor tissues as an HCC prognostic independent biomarker due to its partially high expression. Moreover, a new therapeutic target for HCC may be provided by m6A-lncRNAs and associated immune cell infiltration in the tumor microenvironment,49 but more examination is necessary.
CMTM4
CMTM4, located on chromosome 16q22.1, is one of the most conserved members of the CMTM family.7 There is the MARVEL transmembrane domain found in CMTM4, whose loci contains many tumor suppressor genes.50 CMTM4 was successfully identified by Kittler et al as one of the 37 genes required for HeLa cell division through short interfering RNA technology prepared by endonuclease.51 This indicates that CMTM4 is involved in the process of cell cycle and affects the occurrence and development of disease, especially in the progression of tumors.52–55
In order to better understand the part played by CMTM4 in the progression of HCC, Bei et al examined tissue samples from HCC patients through IHC staining.50 When compared to matched neighboring non-tumor tissues, they noticed that the expression of CMTM4 was down-regulated in HCC tissues. Depending on a Kaplan–Meier survival analysis, HCC patients with CMTM4 negative expression had a lower overall survival rate. All of this implies that CMTM4 plays a tumor-suppressive role in HCC, and its negative expression is a risk factor for bad prognosis. In HCC tissues, CMTM4 expression was downregulated, according to Juan et al.56,57 They also found out that CMTM4 expression was largely linked to clinical stage, distant tumor metastasis, tumor size, and TNM stage in HCC patients. They concluded that patients with HCC had an abnormal expression of CMTM4 and had a poor clinical prognosis. On this basis, further in vitro cell function experiments have shown that the proliferation, invasion, migration, and metastasis ability of HCC cell line Huh-7 were decreased after CMTM4 overexpression. Similarly, the proliferation, migration, invasion, and metastasis of Hep3B cells were enhanced after the CMTM4 knockdown. It was suggested that CMTM4 was a liver cancer suppressor gene. Meanwhile, the EMT-related proteins were also verified, and it was found that CMTM4 knockdown promoted the protein expression levels of epithelial factors β-catenin and E-Cadherins in hepatocellular carcinoma cells. Interstitial factors such as N-Cadherins, Vimentin, and zinc finger E box binding homeobox protein 1 (ZEB1) were reduced. But after CMTM4 overexpression, the opposite occurred. All in all, these findings suggest that the CMTM4 gene can inhibit the EMT process of HCC cells, and then affect the occurrence and development of liver cancer.
CMTM5
The first description of CMTM5 was published in 2003, and its genomic location is 14q11.2.7 Similar to TM4SF in structure, CMTM5 can create a network of four transmembrane proteins by assembling a range of other molecules (such as AKT and EGFR). Therefore, CMTM5 mediates cell proliferation, apoptosis, migration, invasion, and differentiation, and is closely related to malignant progression.16,58–62 Gene polymorphisms may affect CMTM5 expression and alter an individual’s susceptibility to cancer. Bei et al investigated the relationship between CMTM family gene polymorphism and HCC in Southern China.63 They genotyped 10 selected single nucleotide polymorphisms (SNPs) from the CMTM family of genes in 315 patients with HCC and 315 controls without HCC, and they evaluated the association of selected SNPs with HCC risk. According to the findings, individuals with the rs164207 AA genotype had a noticeably higher risk of HCC than people with the CC genotype. Those with the rs3811178 GG genotype were significantly associated with a higher chance of HCC than those with the AA genotype. An increased risk of HCC was observed by gene interactions. Accordingly, it is suggested that rs3811178 in CMTM5 and rs164207 in CMTM6 may be related to genetic susceptibility to HCC in southern China. It was demonstrated that proliferation, migration, and invasion of HCC were restricted by overexpression of CMTM5. By targeting CMTM5, the malignant progression of HCC cells was evaluated by miR-10b-3p upregulation. Nonetheless, many studies have concluded that CMTM5 expression was decreased greatly in HCC tissues and cell lines, which was significantly correlated with poor overall survival of patients. The growth of transplanted tumors in mice was also delayed by CMTM5 overexpression. Furthermore, CMTM5 was found to inhibit the tumor growth of hepatoma cells by down-regulating the PI3K-AKT signal.64 Therefore, the interaction among CMTM5, tumor microenvironment and immune infiltration in HCC need to be well elucidated which will provide new strategies and ideas for the immunotarget therapy of CMTM5 in HCC.
CMTM6
CMTM6 is located on human chromosome 3p22.3, and its encoded protein product contains MARVEL domain and potential quaternary transmembrane structure which is essential for the transport of transmembrane proteins and secreted proteins.17 Recent studies have shown that CMTM6 was contributed to the occurrence and development of diseases, especially the malignant progression of tumors.8–11,65–69 In 2017, two articles were published in Nature in the same day. They both considered CMTM6 as a key protein controlling the stability of PD-L1, which attracted much attention from many scientists. Among them, Burr et al found out that CMTM6 kept PD-L1 stable in a variety of cancer cells.8 They also discovered that by co-localizing with PD-L1 in the plasma membrane and the cyclostome, the important regulator CMTM6 prevented PD-L1 from becoming a target for lysosomal-mediated breakdown. Besides that, they figured out that CMTM6 was particular to PD-L1 by quantitatively examining the complete plasma membrane proteome. These findings serve as a standard for a thorough understanding of PD-L1’s immunobiological function. Since CMTM6 has been a noteworthy immune checkpoint regulator, we propose that CMTM6 as a possible therapeutic target could help combat tumor cells’ immune evasion. At the same time, Mezzadra et al reported that CMTM6 could increase the content of PD-L1 protein in cells, and strengthen the ability of PD-L1 to inhibit T cells in the tumor microenvironment through binding with PD-L1 protein.11 In addition, in glioma studies, the abnormal expression of CMTM6 was closely linked to its prognostic function. Among them, CMTM6 upregulated the signal transduction function of T cells and promoted antigen presentation, further activating macrophages and decreasing T cell function.11 According to the previously mentioned studies, CMTM6 plays an important role in immune system diseases by regulating T cell activation and maintaining the stability of PD-L1. It is expected to become a new target for immunotherapy.
According to Zhu et al, the expression of CMTM6 in HCC was different in various studies.70 Depending on the multivariate Logistic regression analysis, tumor staging, metastasis, and α-fetoprotein (AFP) were significantly correlated with CMTM6 expression. By Kaplan–Meier survival analysis, CMTM6 expression was linked to HCC metastasis and other clinicopathologic features, as well as poor clinical prognosis in HCC patients. Huang et al noted that the expression of CMTM6 in HCC was significantly reduced, which predicted a good prognosis for patients with HCC.71 Through in vitro and in vivo tests, CMTM6 down-regulated HCC cell proliferation by blocking the G1/S phase transition. Other experiments showed that CMTM6 stabilized P21 protein by interacting with P21 and blocking its ubiquitination mediated by SCFSKP2, CRL4CDT2, and APC/CCDC20 in a non-cell cycle-dependent manner. This resulted in the pRB/E2F pathway being inactivated. In addition, CMTM6 sensitized HCC cells to adriamycin and cisplatin, which was positively correlated with the clinical outcome of postoperative recurrence after trans-arterial chemoembolization (TACE). It is speculated that CMTM6 can stabilize P21, confirming that the CMTM6-P21 axis plays a key role in inhibiting the progression of HCC and sensitization of patients with postoperative recurrence to TACE therapy. In some other studies, CMTM6 was decreased in HCC. Ryo Muranushi et al found that the expression of CMTM6 was favorably correlated with the expression of membrane B7 family ligand and CTL infiltration in HCC samples.72 High expression of CMTM6 in HCC was associated with the expression of proliferative marker Ki-67 and shorter relapse-free survival. A decrease in the membrane B7 family ligand and proliferation potential in human hepatoma cell lines with CMTM6 knockout was shown in vitro analysis. Nevertheless, elevated expression of CMTM6 was linked to tumor proliferation and recurrence by controlling the expression of membrane B7 family ligands. Therefore, CMTM6 may be a biomarker to predict the risk of liver cancer recurrence and a therapeutic target to inhibit tumor growth. Huang et al found that compared with neighboring non-cancerous tissues, CMTM6 levels in HCC tissue samples were higher expressed.73 Further experiments showed that silencing CMTM6 stopped HCC cells from proliferation, migration, and invasion. On the contrary, CMTM6 overexpression enhanced the invasion, migration, and proliferation of HCC cells. In addition, CMTM6 interacted with Vimentin and stabilizes vimentin, thereby inducing epithelial-mesenchymal transformation (EMT) and promoting the proliferation, migration, and invasion of HCC cells. CMTM6 overexpression was significantly associated with a poor prognosis in HCC. Because of this, we indicated that CMTM6 expression played an important role in the malignant progression of HCC.
CMTM4 and CMTM6 with PD-L1
In recent years, new hope is offered by Immune checkpoint inhibitors for the treatment of liver cancer patients. Programmed cell death protein 1 (PD-1) is an immunosuppressive receptor present on T cells that interacts with programmed death ligand 1 (PD-L1), a ligand on cancer cells. T-cell survival, proliferation, and cytotoxicity are enhanced by blocking PD-1/PD-L1 binding, thereby increasing their anti-tumor activity. Many studies have found that CMTM4 and CMTM6 were identified as a regulator of PD-L1 protein stability in tumor tissues, which can successfully protect PD-L1 from lysosomal-mediated degradation.8,11,54,74–77 The inhibition ability of T lymphocytes on tumor cells was attenuated by inhibition of CMTM4 and CMTM6 expression. It was suggested that CMTM4 and CMTM6 may contribute to the stability of PD-L1 and are expected to become an exciting new target for tumor immunotherapy. In HCC, CMTM4 and CMTM6 were known to be key PD-L1 regulators and patient survival outcomes were positively associated with CMTM4 and CMTM6 expression. This might be a result of CMTM4’s and CMTM6’s capacity to regulate PD-L1 expression, helping tumor cells in escaping T cell-mediated killing (Figure 2).78 Further experiments showed that the expression of CMTM4 was decreased with the treatment of CMTM4 inhibitors, the growth of liver cancer tumors in mice with normal immune function was also inhibited, and the infiltration level of CD8 T cells was increased. Furthermore, CMTM6 could also improve PD-L1, EMT, and stemness phenotype prognostic value in various tumors.79 However, silencing of CMTM6 results in inhibition of IFNγ-induced PD-L1 expression. This is further increased by concurrent targeting of CMTM4. CMTM4 can, in a nutshell, serve as a backup regulator of PD-L1 expression, and ectopic expression of CMTM4 completely recovered IFN-induced PD-L1 expression in CMTM6-knockout cells.11 Considering these, it is possible that the expression level of CMTM4 and CMTM6 can be used as a predictor of patients’ response to PD-L1 treatment. Additionally, for patients with HCC, CMTM4 and CMTM6 deficiency may be used to increase the clinical effectiveness of anti-PD-L1 immunotherapy.
 |
Figure 2 How CMTM4/6 perform in Hepatocellular carcinoma. CMTM4/CMTM6, and PD-L1 interact to make PD-L1 ubiquitinated and non-degradable, upregulating T-cell signaling and promoting antigen presentation, further activating macrophages and reducing T-cell function.11 Furthermore, CMTM6 interacts with P21 to block P21 ubiquitination, block the G1/S phase transition, and inhibit tumor cell proliferation;75 Knockdown of CMTM6 results in decreased B7 ligand expression and inhibits tumor cell proliferation;76 However, when CMTM6 interacts with Vimentin, inducing EMT and promoting the proliferation of HCC cells.77 Abbreviations: CMTM, Chemokine-like factor superfamily; PD-L1, programmed cell death-ligand 1; Mø, macrophages; P21, aioncogene p21 protein; EMT, epithelial-mesenchymal transformation; HCC, Hepatocellular carcinoma. |
At present, the mechanism of CMTM4 and CMTM6 in tumor immunity still needs to be further studied to provide more safe and effective treatment for HCC patients. In addition, the expression and molecular regulatory mechanism of CMTM4 and CMTM6 in HCC have not been fully clarified. It was speculated that CMTM4 and CMTM6 may participate in the regulation of the immune cell cycle and it is related to the co-expression of PD-L1 protein. Hence, it is necessary to further explore the role and regulatory mechanism of CMTM4 and CMTM6 in HCC immunotherapy, providing the theoretical basis for whether it can be a new target of tumor immunotherapy.
CMTM7
CMTM7, located on chromosome 3p22.3, is a tumor suppressor gene with a MARVEL domain.7 CMTM7 is involved in a variety of cell biological processes, such as tumorigenesis and transporter vesicles.15,19 In addition, CMTM7 decreased the growth of tumor cells by stifling epidermal growth factor receptor protein kinase B (EGFR-AKT) signaling due to promoter hypermethylation and deletion.80 As a result, it was suggested that CMTM7 played an important anticancer role in the development of tumors. In HCC tissues, CMTM7 expression was significantly decreased, and its low expression was negatively correlated with TNM staging and tumor metastasis.81–84 In vitro studies have shown that the cell growth and migration of live cancer cells were inhibited by CMTM7 expression. Further analysis revealed that AKT signaling and G0/G1 cell cycle arrest in HCC cells were also influenced by CMTM7, to act as a tumor suppressor. Li et al found that CMTM7 was downregulated or absent in tumor tissues of the esophagus, stomach, pancreas, liver, lung, and cervix by immunostaining of protein tissue microarray.85 When the loss of promoter CpG methylation and heterozygous contributed to the downregulation of CMTM7, it was necessary to indicate that CMTM7 had tumor-inhibitory effects. Other studies demonstrated that CMTM7’s tumor suppressor function was connected to its role in G1/S cell cycle arrest. It could promote epidermal growth factor receptor (EGFR) internalization and further inhibit AKT signaling by up-regulating p27 and down-regulating cycle-dependent kinases 2 and 6 (CDK2). G1/S transition and EGFR/AKT signaling were modulated during tumor pathogenesis.80 Therefore, CMTM7 could be used as a potential biomarker to predict the possibilities of HCC invasion and metastasis. Larger samples from multiple centers are necessary for pertinent confirmation of the function and regulatory mechanism of CMTM7 in HCC.
CMTM8
As an essential CMTM family member, CMTM8 is initially isolated and cloned from PHA-stimulated lymphoma cells.13 CMTM8 with MAL protein was associated with vesicle transport and membrane binding domain MARVEL. This implied that CMTM8 may play a vital role in membrane protein transport and binding events.19 Previous studies have shown that overexpression of CMTM8 and its variants can not only inhibit cell growth but also induce apoptosis of various tumor cells,86–88 playing a significant part in the occurrence and development of lung cancer, gastric cancer, breast cancer, and other tumors.89–92 Furthermore, in HepG2 cells, epithelium-mesenchymal (EMT) transition was achieved by CMTM8 knockout cells. But EMT-like changes and cell motility were limited by CMTM8 overexpression.93 The real-time quantitative polymerase chain reaction was used to further explore the expression of CMTM8 in multiple solid tumors. It was found that CMTM8 was widely expressed in many normal human tissues and was down-regulated or absent in multiple solid tumors (such as liver, lung, colon, rectum, esophagus, and stomach). It was suggested that CMTM8 may have a potential role as a novel tumor inhibitor.94 Unfortunately, studies on CMTM8 in HCC are relatively few, so the functional mechanism of CMTM8 needs to be studied by scholars to further explore the potential and role of CMTM8 in the diagnosis and treatment of liver cancer.
Discussion
HCC has always been a difficult issue and front research field in clinical practice. With the deepening of basic and clinical research on HCC, there are a lot of good results have been achieved in early diagnosis and treatment, as well as clinical prognosis. However, the clinical cure rate and long-term survival rate of patients with HCC are still very low, but the metastasis and recurrence rate within 5 years is high. In recent years, immunotherapy has become a new hotspot after molecular targeted therapy. In particular, after extensive clinical practice, PD1/PD-L1 immunosuppressant has found its good application prospect in the treatment of various tumors, which is expected to bring a new era for the treatment of tumor patients, especially those with advanced tumors. The CMTM family is a new gene family first discovered and reported by Professor Han Wenling from Peking University in 2001. The protein products encoded by the CMTM family can activate and chemotaxis immune cells, and then participate in the regulation of cell growth and development. This is crucial in the immune system and reproductive system, especially in the occurrence and development of tumors. CMTM family proteins are differently expressed in hepatocellular carcinoma, but all of them are associated with the prognosis of HCC patients as prognostic factors. For example, CMTM1 is high expression in HCC, and its low expression is associated with longer disease-free survival in HCC patients. However, CMTM2, CMTM3, CMTM4, CMTM5, and CMTM7 are down-regulated, and the overall survival rate for HCC patients is lower. It is suggested that tumor growth is suppressed by these CMTM families, resulting in poor prognosis. In addition, numerous studies have verified that CMTM6, CMTM4, and PD-L1 have a synergistic relationship. In fact, CMTM6 plays an important role in immune system diseases by regulating T cell activation and maintaining the stability of PD-L1. It is predicted to become a new target for immunotherapy. A number of results have shown that compared with CMTM6 expression was significantly associated with shorter overall survival (OS) and progression-free survival (DFS) in both the overall HCC and MTM-HCC patient populations. In addition, co-expression of CMTM6/PD-L1 was associated with a high density of inflammatory cells (Figure 2). Patients with CMTM6/PD-L1 co-expression of macro trabecular massive (MTM-HCC) have a higher risk of HCC progression and death, which provides strong evidence for the combination of immune status assessment and anti-CMTM6 and anti-PD-L1 therapy in patients with MTM-HCC.77,95 The above results also indicate the role of the co-expression of CMTM6 and PD-L1 in tumor immunotherapy. But the specific molecular regulation mechanism is still not fully understood. At present, the research on the CMTM family in HCC is not in-depth enough. Most of the studies only reported the expression and prognostic value of CMTM family members while not investigating the in-depth mechanism in HCC. Thus, there is still a long way to applying CMTMs in tumor immunotherapy. Moreover, not all clinical studies on HCC have yielded consistent results due to the different enrolled sample sizes, different stages of HCC patients, different races, and different detection methods. Therefore, a series of in-depth studies are required to explore the molecular mechanism of CMTMs in the malignant progression of HCC. This will provide a new clue for seeking potential diagnosis and therapeutic targets for HCC. It is expected that CMTMs could serve as a novel and effective target for immunotherapy and improve the overall survival of HCC patients.
Abbreviations
HCC, Hepatocellular carcinoma; IARC, International Agency for Research on Cancer; BCLC, Barcelona Clinic Liver Cancer; CMTM/CKLFSF, Chemokine-like factor superfamily; CKLF, chemokine-like factor; CMTM1-8, chemokine-like factor-like MARVEL transmembrane domain-containing family member 1-8; TM4SF, transmembrane 4 superfamily; IL6, Interleukin-6; STAT3, signal transducer and activator of transcription 3; KLT, Kanglaite; CDDP, cisplatin; HepG2, human hepatocellular carcinomas, NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; IHC, immunohistochemistry; TCGA, the Cancer Genome Atlas; ELISA; HBV, hepatitis B virus; ALT, Alanine transaminase; AST, aspartate amino transferase; ROC, receiver operating characteristic curve; ZEB1/ZEB2, zinc finger E box binding homeobox protein 1/2; EMT, epithelial-mesenchymal transformation; PC, prostate cancer; JAK2, Janus Kinase; m6A, N6 methyladenosine; lncRNAs, long non-coding RNAs; TNM stage, tumor node metastasis stage; PD-L1, programmed cell death-ligand 1; AKT, the serine/threonine kinase; EGFR, epidermal growth factor receptor; SNPs, single nucleotide polymorphisms; AFP, α-fetoprotein; TACE, trans-arterial chemoembolization; CTL, cytotoxic T-Lymphocyte; OS, overall survival; MTM, macro trabecular massive; DFS, progression-free survival; CDK2, cycle-dependent kinases 2.
Acknowledgments
All authors contributed significantly to the work reported, participated in drafting, revising or critically commenting on the article; gave final approval for the published version of the article; agreed to the journal in which the work was delivered and agreed to be responsible for all aspects of the work.
Funding
This research was funded by National Natural Science Foundation of China, grant number 82060621.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Lin H, van den Esschert J, Liu C, van Gulik TM. Systematic review of hepatocellular adenoma in China and other regions: hepatocellular adenoma review. J Gastroenterol Hepatol. 2011;26(1):28–35. doi:10.1111/j.1440-1746.2010.06502.x
3. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
4. Tsilimigras DI, Bagante F, Sahara K, et al. Prognosis after resection of Barcelona Clinic Liver Cancer (BCLC) Stage 0, A, and B Hepatocellular Carcinoma: a comprehensive assessment of the current BCLC classification. Ann Surg Oncol. 2019;26(11):3693–3700. doi:10.1245/s10434-019-07580-9
5. Ligat G, Schuster C, Baumert TF. Hepatitis B virus core variants, liver fibrosis, and Hepatocellular Carcinoma. Hepatology. 2019;69(1):5–8. doi:10.1002/hep.30231
6. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi:10.1002/hep.31288
7. Han W, Ding P, Xu M, et al. Identification of eight genes encoding chemokine-like factor superfamily members 1–8 (CKLFSF1–8) by in silico cloning and experimental validation. Genomics. 2003;81(6):609–617. doi:10.1016/S0888-7543(03)00095-8
8. Burr ML, Sparbier CE, Chan YC, et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature. 2017;549(7670):101–105. doi:10.1038/nature23643
9. Guan X, Zhang C, Zhao J, Sun G, Song Q, Jia W. CMTM6 overexpression is associated with molecular and clinical characteristics of malignancy and predicts poor prognosis in gliomas. EBioMedicine. 2018;35:233–243. doi:10.1016/j.ebiom.2018.08.012
10. Cancer research advance in CKLF-like MARVEL transmembrane domain containing member family (review). Available from: https://pubmed.ncbi.nlm.nih.gov/27356683/.
11. Mezzadra R, Sun C, Jae LT, et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature. 2017;549(7670):106–110. doi:10.1038/nature23669
12. Li J, Wang X, Wang X, et al. CMTM family and gastrointestinal tract cancers: a comprehensive review. Cancer Manag Res. 2022;14:1551–1563. doi:10.2147/CMAR.S358963
13. W HAN, Y LOU, Tang J, et al. Molecular cloning and characterization of chemokine-like factor 1 (CKLF1), a novel human cytokine with unique structure and potential chemotactic activity. Biochem J. 2001;357(1):127–135. doi:10.1042/bj3570127
14. Shi S, Rui M, Han W, et al. CKLFSF2 is highly expressed in testis and can be secreted into the seminiferous tubules. Int J Biochem Cell Biol. 2005;37(8):1633–1640. doi:10.1016/j.biocel.2004.04.028
15. Liu B, Li H, Fu W, et al. CMTM3 presents a secreted form released via exosomes. ABBS. 2016;48(6):584–586. doi:10.1093/abbs/gmw029
16. Li H, Guo X, Shao L, et al. CMTM5-v1, a four-transmembrane protein, presents a secreted form released via a vesicle-mediated secretory pathway. BMB Rep. 2010;43(3):182–187. doi:10.5483/BMBRep.2010.43.3.182
17. Barbieri I, Tzelepis K, Pandolfini L, et al. Promoter-bound METTL3 maintains myeloid leukaemia by m6A-dependent translation control. Nature. 2017;552(7683):126–131. doi:10.1038/nature24678
18. Delic S, Thuy A, Schulze M, et al. Systematic investigation of CMTM family genes suggests relevance to glioblastoma pathogenesis and CMTM1 and CMTM3 as priority targets. Genes Chromosomes Cancer. 2015;54(7):433–443. doi:10.1002/gcc.22255
19. Sánchez-Pulido L, Martín-Belmonte F, Valencia A, Alonso MA. MARVEL: a conserved domain involved in membrane apposition events. Trends Biochem Sci. 2002;27(12):599–601. doi:10.1016/s0968-0004(02)02229-6
20. Cai X, Deng J, Ming Q, Cai H, Chen Z. Chemokine-like factor 1: a promising therapeutic target in human diseases. Exp Biol Med. 2020;245(16):1518–1528. doi:10.1177/1535370220945225
21. Liu DD, Song XY, Yang PF, et al. Progress in pharmacological research of chemokine like factor 1 (CKLF1). Cytokine. 2018;102:41–50. doi:10.1016/j.cyto.2017.12.002
22. Liu Y, Liu L, Zhou Y, et al. CKLF1 enhances inflammation-mediated carcinogenesis and prevents doxorubicin-induced apoptosis via IL6/STAT3 signaling in HCC. Clin Cancer Res. 2019;25(13):4141–4154. doi:10.1158/1078-0432.CCR-18-3510
23. Chen C, Ai Q-D, Wei Y-H, et al. Kanglaite enhances the efficacy of cisplatin in suppression of hepatocellular carcinoma via inhibiting CKLF1 mediated NF-κB pathway and regulating transporter mediated drug efflux. J Ethnopharmacol. 2021;264:113388. doi:10.1016/j.jep.2020.113388
24. Wu K, Li X, Gu H, Yang Q, Liu Y, Wang L. Research advances in CKLF-like MARVEL transmembrane domain-containing family in non-small cell lung cancer. Int J Biol Sci. 2019;15(12):2576–2583. doi:10.7150/ijbs.33733
25. Song X, Zhang S, Tian R, et al. Expression and clinical significance of CMTM1 in hepatocellular carcinoma. Open Med. 2021;16(1):217–223. doi:10.1515/med-2021-0221
26. Si J, Zhang P, Tian D, et al. CMTM1_v17 is associated with chemotherapy resistance and poor prognosis in non-small cell lung cancer. World J Surg Oncol. 2017;15(1):34. doi:10.1186/s12957-016-1094-z
27. Cao L, Yang C, Zhu B, et al. A novel protein CMTM1-v5 specifically induced human lymphoma cells apoptosis in vitro and in vivo. Exp Cell Res. 2019;385(1):111623. doi:10.1016/j.yexcr.2019.111623
28. Wang J, Zhang G, Zhang Y, et al. CMTM1_v17 is a novel potential therapeutic target in breast cancer. Oncol Rep. 2014;32(5):1829–1836. doi:10.3892/or.2014.3429
29. Liang Z, Xie J, Huang L, et al. Comprehensive analysis of the prognostic value of the chemokine-like factor-like MARVEL transmembrane domain-containing family in gastric cancer. J Gastrointest Oncol. 2021;12(2):388–406. doi:10.21037/jgo-21-78
30. Chen S, Hu Q, Chen H, et al. Identification of serum CMTM2 as a potential biomarker for HBV-related disorders. Dis Markers. 2020;2020:2032056. doi:10.1155/2020/2032056
31. Choi JH, Kim YB, Ahn JM, et al. Identification of genomic aberrations associated with lymph node metastasis in diffuse-type gastric cancer. Exp Mol Med. 2018;50(4):1–11. doi:10.1038/s12276-017-0009-6
32. van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136(9):1876–1884. doi:10.1016/j.jid.2016.03.042
33. Guo X, Zhang S, Tan S, et al. Downregulated CMTM2 poses potential clinical significance in Hepatocellular Carcinoma. DNA Cell Biol. 2020;39(4):683–689. doi:10.1089/dna.2019.5237
34. Zhang S, Tian R, Bei C, et al. Down-regulated CMTM2 promotes epithelial-mesenchymal transition in Hepatocellular Carcinoma. OTT. 2020;13:5731–5741. doi:10.2147/OTT.S250370
35. Wang Y, Li J, Cui Y, et al. CMTM3, located at the critical tumor suppressor locus 16q22.1, is silenced by CpG methylation in carcinomas and inhibits tumor cell growth through inducing apoptosis. Cancer Res. 2009;69(12):5194–5201. doi:10.1158/0008-5472.CAN-08-3694
36. Wu J, Li L, Wu S, Xu B. CMTM family proteins 1–8: roles in cancer biological processes and potential clinical value. Cancer Biol Med. 2020;17(3):528–542. doi:10.20892/j.issn.2095-3941.2020.0032
37. Hu F, Yuan W, Wang X, et al. CMTM3 is reduced in prostate cancer and inhibits migration, invasion and growth of LNCaP cells. Clin Transl Oncol. 2015;17(8):632–639. doi:10.1007/s12094-015-1288-9
38. Li Z, Xie J, Wu J, et al. CMTM3 inhibits human testicular cancer cell growth through inducing cell-cycle arrest and apoptosis. PLoS One. 2014;9(2):e88965. doi:10.1371/journal.pone.0088965
39. Su Y, Lin Y, Zhang L, et al. CMTM3 inhibits cell migration and invasion and correlates with favorable prognosis in gastric cancer. Cancer Sci. 2014;105(1):26–34. doi:10.1111/cas.12304
40. Han T, Shu T, Dong S, et al. Chemokine-like factor-like MARVEL transmembrane domain-containing 3 expression is associated with a favorable prognosis in esophageal squamous cell carcinoma. Oncol Lett. 2017;13(5):2982–2988. doi:10.3892/ol.2017.5837
41. Zhou Z, Ma Z, Li Z, et al. CMTM3 overexpression predicts poor survival and promotes proliferation and migration in pancreatic cancer. J Cancer. 2021;12(19):5797–5806. doi:10.7150/jca.57082
42. Lu M, Huang Y, Sun W, Li P, Li L, Li L. miR-135b-5p promotes gastric cancer progression by targeting CMTM3. Int J Oncol. 2018;52(2):589–598. doi:10.3892/ijo.2017.4222
43. Yuan W, Li T, Mo X, et al. Knockdown of CMTM3 promotes metastasis of gastric cancer via the STAT3/Twist1/EMT signaling pathway. Oncotarget. 2016;7(20):29507–29519. doi:10.18632/oncotarget.8789
44. Yuan W, Wei F, Ouyang H, et al. CMTM3 suppresses chordoma progress through EGFR/STAT3 regulated EMT and TP53 signaling pathway. Cancer Cell Int. 2021;21(1):510. doi:10.1186/s12935-021-02159-5
45. Yuan W, Liu B, Wang X, et al. CMTM3 decreases EGFR expression and EGF-mediated tumorigenicity by promoting Rab5 activity in gastric cancer. Cancer Lett. 2017;386:77–86. doi:10.1016/j.canlet.2016.11.015
46. Li S, Gao P, Dai X, Ye L, Wang Z, Cheng H. New prognostic biomarker CMTM3 in low grade glioma and its immune infiltration. Ann Transl Med. 2022;10(4):206. doi:10.21037/atm-22-526
47. Li W, Zhang S. CKLF-like MARVEL transmembrane domain-containing member 3 (CMTM3) inhibits the proliferation and tumorigenisis in Hepatocellular Carcinoma cells. Oncol Res. 2017;25(2):285–293. doi:10.3727/096504016X14732523471442
48. Yu ZL, Zhu ZM. Comprehensive analysis of N6-methyladenosine -related long non-coding RNAs and immune cell infiltration in hepatocellular carcinoma. Bioengineered. 2021;12(1):1708–1724. doi:10.1080/21655979.2021.1923381
49. Xu RH, Wei W, Krawczyk M, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16(11):1155–1161. doi:10.1038/nmat4997
50. Bei C, Zhang Y, Wei R, et al. Clinical significance of CMTM4 expression in hepatocellular carcinoma. Onco Targets Ther. 2017;10:5439–5443. doi:10.2147/OTT.S149786
51. Kittler R, Putz G, Pelletier L, et al. An endoribonuclease-prepared siRNA screen in human cells identifies genes essential for cell division. Nature. 2004;432(7020):1036–1040. doi:10.1038/nature03159
52. Li M, Guo H, Wang Q, et al. Pancreatic stellate cells derived exosomal miR-5703 promotes pancreatic cancer by downregulating CMTM4 and activating PI3K/Akt pathway. Cancer Lett. 2020;490:20–30. doi:10.1016/j.canlet.2020.06.009
53. Zhu X, Zhang S, Tan S, et al. Expression of CMTM4 shows clinical significance in lung cancer. Transl Cancer Res. 2020;9(10):6214–6220. doi:10.21037/tcr-20-1254
54. Dai X, Bu X, Gao Y, et al. Energy status dictates PD-L1 protein abundance and anti-tumor immunity to enable checkpoint blockade. Mol Cell. 2021;81(11):2317–2331.e6. doi:10.1016/j.molcel.2021.03.037
55. Zhou L, Lu H, Zeng F, et al. Constructing a new prognostic signature of gastric cancer based on multiple data sets. Bioengineered. 2021;12(1):2820–2835. doi:10.1080/21655979.2021.1940030
56. Kong J,Tan S. CMTM4与肿瘤的研究进展 [Advances in the study of CMTM4 and tumors]. Chinese Journal of Oncology Prevention and Treatment. 2019;11(1):85–88. Chinese.
57. Kong J. CMTM4在肝细胞癌发生发展中的作用及机制研究 [The Role and Mechanism of CMTM4 in the Development of Hepatocellular Carcinoma]. China: Guilin Medical University; 2020. Chinese.
58. Shao L, Cui Y, Li H, et al. CMTM5 exhibits tumor suppressor activities and is frequently silenced by methylation in Carcinoma cell lines. Clin Cancer Res. 2007;13(19):5756–5762. doi:10.1158/1078-0432.CCR-06-3082
59. Chen Z, Cui N, Zhao J-S, et al. Expressions of ZNF436, β-catenin, EGFR, and CMTM5 in breast cancer and their clinical significances. Eur J Histochem. 2021;65(1). doi:10.4081/ejh.2021.3173
60. Yuan Y, Sheng Z, Liu Z, et al. CMTM5-v1 inhibits cell proliferation and migration by downregulating oncogenic EGFR signaling in prostate cancer cells. J Cancer. 2020;11(13):3762–3770. doi:10.7150/jca.42314
61. Li L, Hu Y, Chen D, et al. CMTM5 inhibits the development of prostate cancer via the EGFR/PI3K/AKT signaling pathway. Mol Med Rep. 2022;25(1):1–10. doi:10.3892/mmr.2021.12533
62. Chen F, Li Y, Qin N, et al. RNA-seq analysis identified hormone-related genes associated with prognosis of triple negative breast cancer. J Biomed Res. 2020;34(2):129–138. doi:10.7555/JBR.34.20190111
63. Bei C, Tan C, Zhu X, Wang Z, Tan S. Association between polymorphisms in CMTM family genes and Hepatocellular Carcinoma in Guangxi of China. DNA Cell Biol. 2018;37(8):691–696. doi:10.1089/dna.2018.4274
64. Xu G, Dang C. CMTM5 is downregulated and suppresses tumour growth in hepatocellular carcinoma through regulating PI3K-AKT signalling. Cancer Cell Int. 2017;17(1):113. doi:10.1186/s12935-017-0485-8
65. Zhao Y, Zhang M, Pu H, Guo S, Zhang S, Wang Y. Prognostic implications of pan-cancer CMTM6 expression and its relationship with the immune microenvironment. Front Oncol. 2020;10:585961. doi:10.3389/fonc.2020.585961
66. Liu Y, Li X, Zhang H, Zhang M, Wei Y. HuR up-regulates cell surface PD-L1 via stabilizing CMTM6 transcript in cancer. Oncogene. 2021;40(12):2230–2242. doi:10.1038/s41388-021-01689-6
67. Zhang C, Zhao S, Wang X. Co-expression of CMTM6 and PD-L1: a novel prognostic indicator of gastric cancer. Cancer Cell Int. 2021;21(1):78. doi:10.1186/s12935-020-01734-6
68. Nishi M, Shimada M, Yoshikawa K, et al. Impact of CKLF-like MARVEL transmembrane domain containing 6 (CMTM6) expression in gastric cancer. J Med Invest. 2021;68(3.4):362–367. doi:10.2152/jmi.68.362
69. Martinez-Morilla S, Zugazagoitia J, Wong PF, Kluger HM, Rimm DL. Quantitative analysis of CMTM6 expression in tumor microenvironment in metastatic melanoma and association with outcome on immunotherapy. Oncoimmunology. 2020;10(1):1864909. doi:10.1080/2162402X.2020.1864909
70. Zhu X, Qi G, Li C, et al. Expression and clinical significance of CMTM6 in Hepatocellular Carcinoma. DNA Cell Biol. 2019;38(2):193–197. doi:10.1089/dna.2018.4513
71. Huang Y, Zhu Y, Yang J, et al. CMTM6 inhibits tumor growth and reverses chemoresistance by preventing ubiquitination of p21 in hepatocellular carcinoma. Cell Death Dis. 2022;13(3):251. doi:10.1038/s41419-022-04676-1
72. Muranushi R, Araki K, Yokobori T, et al. High membrane expression of CMTM6 in hepatocellular carcinoma is associated with tumor recurrence. Cancer Sci. 2021;112(8):3314–3323. doi:10.1111/cas.15004
73. Huang X, Xiang L, Wang B, et al. CMTM6 promotes migration, invasion, and EMT by interacting with and stabilizing vimentin in hepatocellular carcinoma cells. J Transl Med. 2021;19(1):120. doi:10.1186/s12967-021-02787-5
74. Imamovic D, Vranic S. Novel regulators of PD-L1 expression in cancer: CMTM6 and CMTM4-a new avenue to enhance the therapeutic benefits of immune checkpoint inhibitors. Ann Transl Med. 2017;5(23):467. doi:10.21037/atm.2017.09.32
75. Li H, Liu YT, Chen L, et al. CMTM4 regulates epithelial-mesenchymal transition and PD-L1 expression in head and neck squamous cell carcinoma. Mol Carcinog. 2021;60(8):556–566. doi:10.1002/mc.23323
76. Wang Z, Peng Z, Liu Q, et al. Co-expression with membrane CMTM6/4 on tumor epithelium enhances the prediction value of PD-L1 on anti-PD-1/L1 therapeutic efficacy in gastric adenocarcinoma. Cancers. 2021;13(20):5175. doi:10.3390/cancers13205175
77. Yugawa K, Itoh S, Yoshizumi T, et al. CMTM6 stabilizes PD-L1 expression and is a new prognostic impact factor in Hepatocellular Carcinoma. Hepatol Commun. 2021;5(2):334–348. doi:10.1002/hep4.1643
78. Chui NNQ, Cheu JWS, Yuen VWH, et al. Inhibition of CMTM4 sensitizes cholangiocarcinoma and Hepatocellular Carcinoma to T cell–mediated antitumor immunity through PD‐L1. Hepatol Commun. 2022;6(1):178. doi:10.1002/hep4.1682
79. Mamessier E, Birnbaum DJ, Finetti P, Birnbaum D, Bertucci F. CMTM6 stabilizes PD-L1 expression and refines its prognostic value in tumors. Ann Transl Med. 2018;6(3):54. doi:10.21037/atm.2017.11.26
80. Rodriguez-Bravo V, Maciejowski J, Corona J, et al. Nuclear pores protect genome integrity by assembling a premitotic and Mad1-dependent anaphase inhibitor. Cell. 2014;156(5):1017–1031. doi:10.1016/j.cell.2014.01.010
81. Lu C, Zhao Y, Wang J, et al. Breast cancer cell-derived extracellular vesicles transfer miR-182-5p and promote breast carcinogenesis via the CMTM7/EGFR/AKT axis. Mol Med. 2021;27(1):78. doi:10.1186/s10020-021-00338-8
82. Xiao M, Hasmim M, Lequeux A, et al. Epithelial to mesenchymal transition regulates surface PD-L1 via CMTM6 and CMTM7 induction in breast cancer. Cancers. 2021;13(5):1165. doi:10.3390/cancers13051165
83. Liu Q, Su Y, Jiang GC, et al. Change of CMTM7 expression, a potential tumor suppressor, is associated with poor clinical outcome in human non-small cell lung cancer. Chin Med J. 2013;126(16):3006–3012.
84. Liu B, Lu Y, Zhang T, et al. CMTM7 as a novel molecule of ATG14L-Beclin1-VPS34 complex enhances autophagy by Rab5 to regulate tumorigenicity. Cell Commun Signal. 2021;19(1):77. doi:10.1186/s12964-021-00720-3
85. Zm H, Pl L, Y P, et al. Overexpression of CMTM7 inhibits cell growth and migration in liver cancer. Kaohsiung J Med Sci. 2019;35(6). doi:10.1002/kjm2.12058
86. Both J, Krijgsman O, Bras J, et al. Focal chromosomal copy number aberrations identify CMTM8 and GPR177 as new candidate driver genes in osteosarcoma. PLoS One. 2014;9(12):e115835. doi:10.1371/journal.pone.0115835
87. Jin C, Wang Y, Han W, et al. CMTM8 induces caspase-dependent and -independent apoptosis through a mitochondria-mediated pathway. J Cell Physiol. 2007;211(1):112–120. doi:10.1002/jcp.20914
88. Neal DE, Marsh C, Bennett MK, et al. Epidermal-growth-factor receptors in human bladder cancer: comparison of invasive and superficial tumours. Lancet. 1985;325(8425):366–368. doi:10.1016/s0140-6736(85)91386-8
89. Wu J, Sun Y, Xiong Z, et al. Influence of CMTM8 polymorphisms on lung cancer susceptibility in the Chinese Han population. Pharmacogenet Genomics. 2021;31(4):89–95. doi:10.1097/FPC.0000000000000426
90. Shi W, Zhang C, Ning Z, et al. CMTM8 as an LPA1-associated partner mediates lysophosphatidic acid-induced pancreatic cancer metastasis. Ann Transl Med. 2021;9(1):42. doi:10.21037/atm-20-1013
91. Yan M, Zhu X, Qiao H, Zhang H, Xie W, Cai J. Downregulated CMTM8 correlates with poor prognosis in gastric cancer patients. DNA Cell Biol. 2021;40(6):791–797. doi:10.1089/dna.2021.0110
92. Zeng X, Ma X, Guo H, et al. MicroRNA-582-5p promotes triple-negative breast cancer invasion and metastasis by antagonizing CMTM8. Bioengineered. 2021;12(2):10126–10135. doi:10.1080/21655979.2021.2000741
93. Zhang W, Mendoza MC, Pei X, et al. Down-regulation of CMTM8 induces epithelial-to-mesenchymal transition-like changes via c-MET/extracellular signal-regulated kinase (ERK) signaling. J Biol Chem. 2012;287(15):11850–11858. doi:10.1074/jbc.M111.258236
94. Zhang W, Qi H, Mo X, et al. CMTM8 is frequently downregulated in multiple solid tumors. Appl Immunohistochem Mol Morphol. 2017;25(2):122–128. doi:10.1097/PAI.0000000000000274
95. Liu LL, Zhang SW, Chao X, et al. Coexpression of CMTM6 and PD-L1 as a predictor of poor prognosis in macrotrabecular-massive hepatocellular carcinoma. Cancer Immunol Immunother. 2021;70(2):417–429. doi:10.1007/s00262-020-02691-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
