Back to Journals » Infection and Drug Resistance » Volume 11
Current eradication rate of Helicobacter pylori with clarithromycin-based triple therapy in a gastroenterology practice in the New York metropolitan area
Authors Nayar DS
Received 25 October 2017
Accepted for publication 13 December 2017
Published 31 January 2018 Volume 2018:11 Pages 205—211
DOI https://doi.org/10.2147/IDR.S153617
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sahil Khanna
Devjit S Nayar
Gastroenterology Associates of Central Jersey, Edison, NJ, USA
Background: In order to mitigate potential issues with antibiotic resistance in the treatment of patients with Helicobacter pylori infection, the selection of a therapeutic regimen is optimized by being aware of local eradication rates as well as the patient’s medication history and previous diagnoses.
Purpose: This study primarily aimed to calculate the eradication rate of H. pylori infection in the New York Metropolitan area when using clarithromycin-based triple therapy per the dosing instructions for Omeclamox®-Pak. A secondary objective was to determine risk factors for therapeutic failure.
Patients and methods: A retrospective analysis was performed on 156 patients treated with clarithromycin-based triple therapy between 2011 and 2017 at a gastroenterology practice in Edison, New Jersey.
Results: The cumulative eradication rate for the intent-to-treat population was 84%, while the per-protocol rate was 86%. No differences were seen in the rates of subgroups defined by demographics or medication history.
Conclusion: Despite evidence and predictions from other sources in the last decade that clarithromycin-based treatments for H. pylori are becoming less effective, the results of this study support the use of clarithromycin-based triple therapy as a first-line treatment in the New York Metropolitan region.
Keywords: H. pylori, dyspepsia, antibiotic resistance, bismuth quadruple therapy, Omeclamox
Introduction
Helicobacter pylori (H. pylori) has long been known as a common, globally distributed pathogen that is associated with gastric ulcers or related dyspeptic symptoms in many infected patients. Although most of the people do not develop symptoms, it is estimated that 50% of the world’s population carry the bacteria. Eradication of H. pylori has been a common subject of research for decades as optimal treatments are sought. Drug therapies composed of both antibiotics and gastric acid-reducing agents are the standard choice, but challenges arise from bacterial antibiotic resistance, lack of follow-up to confirm treatment success, and poor patient compliance due, in part, to high pill burdens. Complicating the issue with antibiotic resistance, H. pylori exists in multiple strains, and patients may be infected simultaneously by multiple strains, further varying the response rate to individual therapies.1 Therefore, standard practice strongly recommends that if H. pylori is not eradicated with an initial treatment, the antibiotic component of the therapy should be changed during subsequent treatment so as not to potentiate the resistance.2
In 2007, the American College of Gastroenterology (ACG) recommended two first-line options for treating H. pylori: clarithromycin-based triple therapy and bismuth quadruple therapy. Both regimens yielded similar eradication rates and similar side effects, with the clarithromycin-based triple therapies requiring less total pills to be consumed.3Eradication rates at the time were documented by numerous studies and meta-analyses. In one summary, rates for per-protocol (PP) treatment with clarithromycin-based triple therapy and bismuth quadruple therapy were 85% and 87%, respectively, while intent-to-treat (ITT) values that incorporate lapses in treatment compliance were calculated as 79% and 80%.4 A few years later, a similar success rate of 70–85% for clarithromycin-based triple therapy was published in ACG guidelines. Shortly after, in 2011, a clarithromycin-based triple therapy was released in the US as a commercially available combination product called Omeclamox®-Pak, designed and packaged to promote patient compliance. This offered practitioners a readily available product to treat H. pylori according to current first-line recommendations.5
Antibiotic resistance, specifically to clarithromycin rather than to the second antibiotic in the regimen (amoxicillin or metronidazole), accounts for the main reason for treatment failure in a clarithromycin-based triple therapy. Although culture and sensitivity testing is typically not performed in practice, when H. pylori strains confirmed as resistant to clarithromycin were treated, eradication rates as low as 44% and 55% have been seen.6,7 Indeed, there is also a risk of promoting drug resistance any time an antibiotic is used, but fails to clear the infection due to poor patient compliance or other factors. Resistance to clarithromycin was shown to be able to increase as much as 12-fold in cases where the antibiotic treatment failed.8
Although there may be a lack of epidemiological data relative to the high incidence of H. pylori infection, resistance rates had been reported to be increasing in the US from 6% in 1993 to 18% in 2013.1 In parallel, there are reports in Europe of the efficacy of clarithromycin-based triple therapy decreasing from 80% in the 2000–2005 era to 62% between 2006 and 2011.9 During these decades, numerous other regimens and treatment strategies have been devised and studied, including concomitant, sequential, hybrid, or levofloxacin-based therapies, as examples. Recently, the status of a clarithromycin-based triple therapy as a first-line recommendation has been challenged. Other antibiotics and treatment regimens for H. pylori, however, also encounter resistance problems. Resistance rates as high as 65% for metronidazole and 50% for levofloxacin have been reported recently.1
Currently, the use of clarithromycin-based triple therapy has the support of organizational bodies in gastroenterology, however with some limitations. The Toronto Consensus in 2016 recommends the use of clarithromycin-based triple therapy in regions with known low clarithromycin resistance or high eradication success rates.10 Similarly, the recent guidelines from the ACG in 2017 support the use of clarithromycin-based triple therapy when there is no previous exposure of the patient to clarithromycin and the resistance to this antibiotic in this geographical area is expected to be low.
To evaluate the potential incidence of resistance in the New York region, the clinical success rate of clarithromycin-based triple therapy was evaluated by retrospectively reviewing patient data in a large gastroenterology practice in Central New Jersey.
The primary objective of this study was to calculate the eradication rate of H. pylori when treated with clarithromycin-based triple therapy. Medication history was collected from each patient toward exploratory objectives to suggest whether previous exposure to clarithromycin, other antibiotics, or proton pump inhibitors (PPIs) had an effect on treatment efficacy.
Methods
Medical records for patients seen at Gastroenterology Associates of Central Jersey, Edison, NJ, from 2011 to 2017 were reviewed against written eligibility criteria for inclusion in a retrospective data analysis. The investigator accessed patient data previously collected at his clinic during the course of standard medical practice. Data devoid of identifiers capable of being linked to an individual were extracted by the investigator and copied to an independent study database. Therefore, no patient consent or institutional review board were required in this study as defined by Title 45 of the Code of Federal Regulations, Part 46.101.
The electronic medical record system at the practice was queried to identify all patients diagnosed with H. pylori, and they were then prescribed clarithromycin-based triple therapy. A positive diagnosis of H. pylori was confirmed if made from any one or more of the following tests: histopathology of gastric mucosa, blood antibody test with supportive history, stool antigen test, or urea breath test. Only adult patients, 18 years and older, who were prescribed clarithromycin triple therapy and no other concurrent treatment for H. pylori were included. Any patient with a surgical resection of the upper gastrointestinal (GI) tract or other current or historical structural abnormalities of the GI tract was not included. Additionally, all subjects who did not perform a follow-up test to confirm eradication were removed from the study.
Domains captured into this study database included baseline characteristics such as demographics, relevant medical history including significant medication history, and diagnosis, treatment, and outcome of the H. pylori infection. Table 1 lists the data parameters captured in this study.
  | Table 1 Data domains captured in the study Abbreviations: GI, gastrointestinal; PPI, proton pump inhibitor; H. pylori, Helicobacter pylori. |
The majority of patients included at this practice were referred from primary care facilities for dyspepsia symptoms. Almost all patients had esophagogastroduodenoscopy (EGD) as part of their diagnostic workup at the specialty clinic because first rounds of treatment for their dyspepsia symptoms had failed. During the period from 2011 to 2017, the main first-line treatment for H. pylori at Gastroenterology Associates of Central Jersey was clarithromycin-based triple therapy. The investigator prescribed Omeclamox®-Pak according to the labeled dose: omeprazole 20 mg plus clarithromycin 500 mg plus amoxicillin 1000 mg were each given twice daily for 10 days in the morning and evening before eating a meal.5
Simultaneously, orders for a urea breath test were given to all patients with instructions to have the test performed at a local laboratory within 1–3 months of the completion of their therapy. Additionally, as per the clinic’s standard procedures and testing requirements, all patients were directed not to take any antibiotics within 1 month prior to test and no histamine 2 (H2) blockers or PPIs or products containing bismuth within 2 weeks prior to test.
Results of the urea breath test were reported by the respective laboratory to the investigator. All patients who received a positive result from the test, indicating that the H. pylori infection was still present, completed a questionnaire given by the investigator. Information collected on the questionnaire determined if the patient received Omeclamox®-Pak from their pharmacy or if the individual components (separate prescriptions of amoxicillin, clarithromycin, and omeprazole) were dispensed. Additionally, patients reported details of their compliance with the oral therapy at home with respect to the prescribed instructions. Ultimately, an eradication rate for the treatment was calculated as the number of patients with a confirmed negative test following treatment divided by the total number of patients treated.
Results
Baseline characteristics
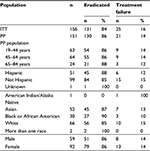
The cases of 156 patients, ranging in age from 21–76 years and receiving prescriptions for clarithromycin-based triple therapy between December 2011 and May 2017, met the eligibility criteria for inclusion into the retrospective analysis. Baseline characteristics of the included patients are listed in Table 2.
  | Table 2 Baseline characteristics of the patient population Abbreviation: PPI, proton pump inhibitor. |
The adult patients in the analysis were distributed over three sequential age groups with 67 out of 156 (43%) patients between the ages of 19 and 44 years, 65 (42%) patients between 45 and 64 years and 24 (15%) patients 65 years and older. Females (62%) were represented in a higher proportion than males (38%). The ethnic distribution of the population reflects the demographics of the region.
Recent exposure to various drugs or drug classes is also listed in Table 2.
Populations
In all, 156 patient records were found that met the eligibility criteria for analysis; data from these patients compile the ITT population. A review of concomitant medications and patient questionnaire responses identified five patients who did not adhere to the physician’s orders for H. pylori treatment with clarithromycin-based triple therapy or otherwise were censored from the efficacy analyses. Three patients had reported that they were not compliant with their oral medication at home and did not finish the prescribed treatment. Another patient was taking nitazoxanide concurrently with clarithromycin-based triple therapy. Nitazoxanide, as a single antimicrobial agent, has been shown to have eradication efficacy against H. pylori when given with a PPI.11 Additionally, another subject was taking nitrofurantoin, which has similar effects against H. pylori. The concomitant medications for both patients interfere with an accurate assessment of efficacy. Therefore, a total of five cases from the ITT were removed to yield the PP analysis population of 151 patients.
The posttreatment H. pylori status for five subjects was ultimately evaluated by methods other than the urea breath test. Two patients requested and received a stool antigen test as they did not want to ingest the oral compound required for the breath test. Three other subjects were scheduled for EGD; hence, biopsies for histopathologic visualization of the organism were evaluated rather than performing a urea breath test.
Primary efficacy evaluation
The results of H. pylori eradication testing following completion of clarithromycin-based triple therapy revealed that 130 of the 151 patients treated according to the standard protocol were negative for H. pylori. Therefore, the eradication rate for the PP population was calculated as 86%. In the ITT population, 131 of 156 patients had their H. pylori eradicated, yielding an ITT rate of 84%.
Table 3 lists the summary of the treatment outcomes for both the PP and the ITT populations. Subanalyses of the PP analysis, based on the demographic traits, are listed as well.
Secondary efficacy evaluations
Eradication rates were compared between patients with and without previous recent exposure to certain drugs that may affect efficacy of clarithromycin-based triple therapy. As shown in Table 4, no difference was detected between patients who had PPIs in the 2 months prior to H. pylori treatment and those who did not. Both groups achieved an 86% treatment success rate. Likewise, exposure to antibiotics other than clarithromycin in the 6 months before clarithromycin-based triple therapy did not reveal a difference, although the population taking antibiotics was small. In all, 86% of the 146 patients without antibiotic exposure achieved eradication, while four of the five (80%) patients who did receive antibiotics had successful treatment results. While sample sizes were too small to make any conclusions about prior clarithromycin exposure, no negative trends were observed. All 10 of the patients with previous exposure had H. pylori eradicated with the current treatment, while 85% (120 of 141) patients with no previous 2-year clarithromycin history were negative for H. pylori following the current treatment.
  | Table 4 H. pylori eradication rates by past drug exposure Abbreviations: H. pylori, Helicobacter pylori; PPI, proton pump inhibitor. |
Discussion
The 2017 guidelines of the ACG promote informed treatment decisions for H. pylori based on local resistance and eradication data for the various antibiotics and treatment combinations. However, it is also noted that such data are scarce and that organized efforts are needed to document this locally.2 This retrospective data analysis intends to support these efforts by evaluating eradication rates of H. pylori to clarithromycin-based triple therapy from 2011 to 2017 in the New York metropolitan region.
Previous guidelines from 2007 concluded that eradication rates seen with the two main first-line treatments for H. pylori, clarithromycin-based triple therapy and bismuth-based quadruple therapy, were <85% and were likely decreasing. Projections based on this summary would suggest modern day eradication rates to be notably lower in many areas; however, the PP eradication rate seen in this study remained high at 86% and the ITT rate was 84%. Although no previous local surveys are known to be available for comparison, there is no decrease observed in this study from the previously published eradication rate of H. pylori with clarithromycin-based triple therapy.
The baseline characteristics of the analysis populations plus a review of efficacy of the various subgroups did not reveal any variation in treatment success based on age, sex, race, and ethnic groups. The gastroenterology practice is located in a busy metropolitan area with a culturally diverse population. Patient feedback from treatment failures due to noncompliance was explained by “busy lifestyles”, “intolerance”, and “forgetfulness”. Additionally, with a high level of primary care accessibility in the area and a lack of global electronic medical records, many patients presenting with H. pylori could have received previous courses of antibiotics for various conditions. This may suggest that this population is less responsive to H. pylori eradication. Better outcomes might be seen in areas without these factors.
Other research has confirmed that antimicrobial resistance is significantly associated with previous antimicrobial treatment;6 therefore, it was unexpected to find that 100% of the 10 patients with previous exposure to clarithromycin were successfully treated for H. pylori with the current treatment. However, population numbers in this group are very small. There are expected inaccuracies in capturing medication history from previous years: patient-reported drug history is subject to recall bias, and patients are often seen by various primary care providers such that the treating physician’s medical record is not comprehensive. Therefore, no conclusions are suggested from the results of this study for patients having previous exposure to clarithromycin. Acknowledging the limitations in collecting a full medication history, there are still recommendations to ask patients about previous antibiotic history and to avoid multiple exposures to the same drug when determining the best first-line therapy for a current infection with H. pylori.2 In this study, patients with or without previous exposure to PPIs or antibiotics other than clarithromycin showed similar eradication rates; no change in efficacy was seen to the current treatment regimen due to previous drug exposure.
Since there is extensive discussion in the literature about H. pylori treatments and also an expanding list of antimicrobial agents and combination therapies being studied, many of the comprehensive surveys or meta-analyses that summarize treatment options define the attributes of each treatment with respect to the statistical quality of the evidence, the strength of the clinical recommendation, and the relative level of consensus among clinicians as to the conclusion. Despite evidence that the efficacy of clarithromycin-based triple therapy is decreasing globally, the author notes that the current ACG guidelines assign a moderate quality of evidence for the treatment.2 This is the highest level seen in the many treatments evaluated; most other treatments have a low quality of evidence.
There is a strong recommendation, a high level of evidence and 100% consensus among a global panel that eradication regimens should be based on the best locally effective regimen, ideally using individual susceptibility testing or community antibiotic susceptibility, or antibiotic consumption data and clinical outcome data.12 Based on the results of this study, any patient with a positive diagnosis of H. pylori from this geographical area is expected to have no less than an 85% eradication rate when treated with clarithromycin-based triple therapy according to the labeled instructions for Omeclamox®-Pak. At this high rate of treatment success, the study results could suggest that determining antibiotic resistance rates to this regimen in this area may not add to the practitioner’s knowledge base on which to select treatment options. Similar eradication studies in other geographical areas and with other treatments would be helpful to further establish wider treatment recommendations, especially at lower rates of success.
The author has incorporated clarithromycin triple therapy as the first-line therapy and reserves non-clarithromycin-based quadruple therapy for treatment failures. Contributing support to this practice is the lower pill burden required with the clarithromycin-based triple therapy, which can be expected to increase compliance for some patients. The author additionally noted that a few compliant patients failed in both clarithromycin-based triple therapy and a subsequent non-clarithromycin-based quadruple therapy. Following results of this study, one could investigate these patients for virulence factors contributing to ineffective host immune response.
Conclusion
The results of this retrospective data analysis provide support that clarithromycin-based triple therapy should remain as a first-line treatment option for H. pylori in the New York metropolitan area. This approach aligns with multiple consensuses that support the use of clarithromycin-based triple therapy in geographical areas where resistance is shown to be low and in patients without previous treatment with a clarithromycin-based therapy.
Acknowledgment
Cumberland Pharmaceuticals Inc. provided the author with funding for the data collection and analysis required for this report.
Disclosure
The author reports no conflicts of interest in this work.
References
Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514–533. | ||
Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment for Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–238. | ||
Chey WD, Wong BCY. American college of gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. | ||
Gené E, Calvet X, Azagra R, Gisbert JP. Triple vs. quadruple therapy for treating Helicobacter pylori infection; a meta-analysis. Aliment Pharmacol Ther. 2003;17(9):1137–1143. | ||
Omeclamox®-Pak (Omeprazole capsules, Clarithromycin Tablets, and Amoxicillin capsules) [package insert]. Nashville TN: Cumberland Pharmaceuticals Inc.; 2016. | ||
Duck WM, Sobel J, Pruckler JM, et al. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004;10:1088–1094. | ||
De Francesco V, Margiotta M, Zullo A, et al. Clarithromycin resistance and Helicobacter pylori genotypes in Italy. J Microbiol. 2006;44:660–664. | ||
Dore MP, Leandro G, Realdi G, Sepulveda AR, Graham DY. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta-analytical approach. Dig Dis Sci. 2000;45(1):68–76. | ||
Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88(1):33–45. | ||
Fallone CA, Chiba N, van Zantan S, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151(1):51–69. | ||
Megraud F, Occhialini A, Rossignol JF. Nitazoxanide, a potential drug for eradication of Helicobacter pylori with no cross-resistance to metronidazole. Animicrob Agents Chemother. 1998;42(11):2836–2840. | ||
Sugano K, Tack J, Kuipers EJ, et al; Faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353–1367. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.