Back to Journals » Patient Related Outcome Measures » Volume 7
Cultural adaptation: translatability assessment and linguistic validation of the patient-reported outcome instrument for irritable bowel syndrome with diarrhea
Authors Delgado-Herrera L, Lasch K, Popielnicki A, Nishida A, Arbuckle R , Banderas B, Zentner S, Gagainis I, Zeiher B
Received 17 December 2015
Accepted for publication 26 April 2016
Published 22 June 2016 Volume 2016:7 Pages 81—92
DOI https://doi.org/10.2147/PROM.S102647
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Robert Howland
Leticia Delgado-Herrera,1 Kathryn Lasch,2 Ana Popielnicki,3 Akito Nishida,4 Rob Arbuckle,5 Benjamin Banderas,6 Susan Zentner,1 Ingrid Gagainis,1 Bernhardt Zeiher1
1Astellas Pharma Global Development, Northbrook, IL, 2Pharmerit International, Newton, MA, USA; 3TransPerfect, Linguistic Validation Group, Boston, MA, USA; 4Development Project Management, Astellas Pharma Inc, Tokyo, Japan; 5Patient-Centered Outcomes Adelphi Values, Bollington, UK; 6Patient-Centered Outcomes Adelphi Values, Boston, MA, USA
Background and objective: Following a 2009 US Food and Drug Administration guidance, a new patient-reported outcome (PRO) instrument was developed to support end points in multinational clinical trials assessing irritable bowel syndrome with diarrhea (IBS-D) symptom severity. Our objective was to assess the translatability of the IBS-D PRO instrument into ten languages, and subsequently perform a cultural adaptation/linguistic validation of the questionnaire into Japanese and US Spanish.
Materials and methods: Translatability assessments of the US English version of the IBS-D PRO were performed by experienced PRO translators who were native speakers of each target language and currently residing in target-language countries. Languages were Chinese (People’s Republic of China), Dutch (the Netherlands), French (Belgium), German (Germany), Japanese (Japan), Polish (Poland), Portuguese (Brazil), Russian (Russia), Spanish (Mexico), and Spanish (US). The project team assessed the instrument to identify potential linguistic and/or cultural adaptation issues. After the issues identified were resolved, the instrument was translated into Spanish (US) and Japanese through a process of two forward translations, one reconciled translation, and one backward translation. The project team reviewed the translated versions before the instruments were evaluated by cognitive debriefing interviews with samples of five Spanish (US) and five Japanese IBS-D patients.
Results: Linguistic and cultural adaptation concerns identified during the translatability assessment required minor revisions, mainly the presentation of dates/times and word structure. During the cognitive debriefing interviews, two of five Spanish respondents misunderstood the term “bowel movement” to mean only diarrhea in the Spanish version. Consequently, the term was changed from “movimiento intestinal” to “evacuaciones”. None of the Japanese respondents identified issues with the Japanese version.
Conclusion: The translatability of the IBS-D PRO instrument into ten target languages was confirmed, with only minor changes made to the translations of the instrument. The translation and linguistic validation into Spanish (US) and Japanese provide evidence that this instrument can be used in multinational trials and clinical settings.
Keywords: stool consistency, stool-form scale, translatability, IBS-D PRO instrument
Introduction
Irritable bowel syndrome (IBS) is typically diagnosed using symptom-based diagnostic criteria, such as the Rome III criteria for IBS.1,2 Upon diagnosis, IBS is often subtyped into one of four categories, based on the predominant stool pattern: IBS with diarrhea (IBS-D), IBS with constipation, IBS with mixed stool pattern, or unspecified IBS (insufficient stool consistency to be classified according to one of the other three subtypes).2 Data suggest that ~75% of individuals change subtypes within a 1-year period.3
Following a 2009 US Food and Drug Administration guidance, a new patient-reported outcome (PRO) instrument was developed to support end points in multinational clinical trials assessing IBS-D symptom severity. For a new instrument to be used in global trials and clinical settings, it needs to be culturally adapted to the target patient population. Cultural adaptation – including translation and linguistic validation – is important, because it considers the target language and culture, medical culture, and conceptual equivalence of an instrument’s wording, rather than simply the literally translated text.4 Prior to the actual translation of an instrument, a translatability assessment is often performed to help determine potential areas that require further clarification and issues that may occur during the translation process.5 During a formal translation process, various steps are taken to ensure cultural and linguistic equivalence between the source and target languages, including 1) defining the concepts of the instrument, 2) two forward and one backward translations, 3) cognitive debriefing to confirm acceptability and conceptual equivalence in the target language, and 4) documentation of the translation and validation process.4
The aim of this manuscript was to assess the translatability of the IBS-D PRO instrument into ten languages, and subsequently perform a cultural adaptation/linguistic validation of the questionnaire into US Spanish (a generic Spanish dialect spoken by the native speakers living in the US)6 and Japanese.
Objectives
The overall goal of this research was to translate the IBS-D PRO to be used in international clinical trials and clinical settings. To achieve this goal, two stages of work were identified: 1) translatability assessment and 2) linguistic validation.
The objective of the translatability assessment (stage 1) was to determine whether there would be any issues to attaining conceptually, culturally, and linguistically equivalent translations of the IBS-D PRO instrument text across ten different languages. The assessment specifically identified potential linguistic, sociolinguistic, or cultural issues that could emerge during the translation of words, phrases, idioms, and metaphors that are culturally anchored in the source language (ie, US English) and syntax (word order).
The objective of the formal and more comprehensive and rigorous translation and linguistic validation (stage 2) was to translate the instrument and confirm its conceptual equivalence across cultures. Confirmation of equivalence was achieved by testing the translated text with patients within the target patient population and languages (Spanish [US] and Japanese [Japan]) through cognitive debriefing interviews.
Materials and methods
Design of the translatability assessment (stage 1)
The US English version of the PRO instrument (the IBS-D symptom diary and event log) was reviewed by experienced translatability evaluators who were residents of the ten countries and native speakers of the respective languages: Chinese (People’s Republic of China), Dutch (the Netherlands), French (Belgium), German (Germany), Japanese (Japan), Polish (Poland), Portuguese (Brazil), Russian (Russia), Spanish (Mexico), and Spanish (US). This process was overseen by a translatability specialist with 13 years’ experience in translatability assessment and linguistic validation, with a clinical psychology and psychometric background and cognitive debriefing training, as well as familiarity with International Society for Pharmacoeconomics and Outcomes Research (ISPOR) best practices, followed throughout the project.7
Each evaluator examined the source text and flagged any potential translatability issues by completing a translatability checklist. During the assessment, if a word or item would potentially present translation issues (eg, because of ambiguity), the evaluator (or the linguistic validation specialist) then defined in more detail or elaborated on the concept intended, so that the linguist knew which equivalent word to use in his/her translation. This study was performed in accordance with the ethical principles of the Declaration of Helsinki and are consistent with Good Clinical Practice and applicable regulatory requirements. Ethical approval for this study was not sought. All patients provided written informed consent prior to the start of the study.
Translatability assessment (stage 1)
The questionnaire focused on seven different aspects of translatability (ie, there were seven levels of analysis for each language), including concept elaboration, appropriateness of audience design, structure and design of an item, grammatical structure of an item, identification of idiomatic expressions, metaphors, and colloquialisms, evaluation of response choices, and any additional comments.
The questionnaire required each evaluator to flag any words or phrases that required additional concept information. If there were issues with an item, the evaluator was then required to provide further information to clarify the text. The appropriateness of the “audience design”, which is a sociolinguistic concept whereby certain text would be useful only if it were written (“designed”) appropriately for a specific target audience,8 was evaluated next. The evaluator provided information as to whether the text of an item was written appropriately for the target population.
The evaluator assessed the structure and design of each item and determined whether there were any missing response categories multiconcept (double-barreled) questions, double negatives, inconsistent use of words, etc, and whether there were any problems with syntax (ie, word order and/or sentence structure). The evaluator also reviewed the PRO instrument for any phrases anchored in the source language/culture that may be difficult to express in the target language. If issues were identified, the evaluator was to suggest strategies/text that would improve the translation to the target language.
The response choices in the questionnaire were also evaluated to identify any potential difficulties with translation, and the evaluator was to suggest strategies/text to resolve translation problems. The evaluator also provided details of any other issues that were not covered by the assessment strategy described earlier. After all of the linguistic and cultural issues that were identified during the translatability assessments were resolved by appropriate revisions to the text, the translation and linguistic validation/cultural adaptation stage was initiated.
Design of the translation and linguistic validation process (stage 2)
The translation and linguistic validation processes followed the recommendations of the ISPOR Patient-Reported Outcomes Translation and Linguistic Validation Good Research Practices Task Force.7
Following completion of the translatability assessments (ie, stage 1), the instrument was subjected to translation and linguistic validation into Spanish (US) and Japanese. For each language, three native-speaking translators (two forward translators, one backward translator) participated in the process. All of the translators had experience in several areas of the life sciences and worked independently on their respective steps of the translation and validation process. The translation and validation process consisted of two forward translations, one reconciled translation, and one backward translation. Upon completion of the process, clinical experts and project-team members reviewed the translations for accuracy and cultural appropriateness. The instruments were then evaluated by cognitive debriefing interviews with samples of five Spanish (US) and five Japanese IBS-D patients. The respondents for the cognitive interviews were native-speaking Spanish individuals living in the US, or native-speaking Japanese individuals living in Japan, mainly because these were the most relevant languages to the sponsor’s clinical trials performed in Japan and the US.
Steps in the translation and linguistic validation process (stage 2)
The translation and linguistic validation process is shown in Table 1. During the forward translation, the two translators for each language independently translated the IBS-D instrument into the respective target languages. The translators were provided with the original instrument and definitions of concepts, and were asked to focus on ensuring cultural relevance and conceptual equivalence of the item content, not just of literal translation. A third translator, who was also provided with the original instrument and definitions of concepts, examined the translated document item by item and selected the best word, phrase, or sentence between the two translations or provided an alternative option. This reconciliation (or harmonization) process addressed any discrepancies between the source language and the translations, any linguistic limitations, and any cultural differences in conveying the exact source meaning.
  | Table 1 Translation and linguistic validation process Abbreviation: COA, Clinical Outcomes Assessment. |
The translated documents were subsequently backward translated by a fourth translator in order to ensure the forward translation was successful and conceptually equivalent to the source. The backward translator was only provided with the reconciled forward translation, and had no access to the original instrument or the concepts. Following the backward translation, a fifth translator of the target language and subsequently a project manager reviewed the translated version for any discrepancies between the backward translation and the source. Any discrepancies were addressed to ensure conceptual equivalence.
A medical reviewer who was a native speaker of the target language was then consulted to review the translated version, to ensure that appropriate medical terminology was maintained. A project team review followed to ensure further the accuracy and appropriateness of the translated version. Any necessary changes were made to the forward and backward translations.
Five face-to-face cognitive debriefing interviews, each with one interviewer and one respondent, were performed for each of the translated, culturally adapted versions of the PRO instrument in the target language (ie, patients’ native language) in order to confirm their content validity (ie, the extent to which the instrument [in this case, the IBS-D PRO] measured the concept of interest).5 The interviewers were trained by TransPerfect and were provided with appropriate guidelines prior to the interview. The respondents were recruited via physician referrals and patient-association groups and meetings. They had been diagnosed with IBS, but IBS-D was not specifically confirmed. During the cognitive debriefing interviews, the respondents were asked to review and provide feedback on their understanding of the items and relevance of the concepts. Their responses were subsequently evaluated and categorized as stylistic (ie, preferential), objective (ie, a correction of a grammar/spelling mistake), or related to comprehension/cultural appropriateness.
Results
Translatability assessment: overall and country-by-country findings (stage 1)
The overall findings and recommended wording for improvements to the text are shown in Table 2. Among these findings, cultural issues, such as the presentation of dates and times, were identified by evaluators of nine of the ten languages included in the translatability assessment. Of the nine evaluators, all nine suggested that the time should be written as a 24-hour entry, since some countries do not use “am” or “pm”, and that the date should be written in the standard format for each country. The idea of “abdominal pain” and “stomach pain” being separate symptoms/sensations was identified as requiring further clarification during the evaluators’ concept elaboration, to allow for appropriate differentiation and adaptation into each language. The evaluators for the Chinese, Dutch, Portuguese, and Spanish (Mexico) assessments also noted issues with word structure and concept elaboration for the text “irritable bowel syndrome – diarrhea predominant (IBS-D) symptom-event log”: half of these evaluators suggested that a hyphen should be added to the acronym between “diarrhea” and “predominant” for clarity; subsequently, one was added. Lastly, the evaluators of the Chinese, German, Polish, and Spanish (Mexico) languages also identified issues with the word “immediate”, and suggested changing this to “urgent”, as it is more appropriate in the context of IBS-D (eg, “How urgent was your need?”).
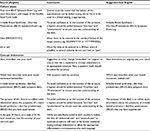  | Table 2 Translatability assessment: summary of overall findings and recommendations |
The country-by-country findings are available in Table S1. The evaluator for the translation into Spanish (US) did not identify any potential translatability issues. With regard to the images and descriptors, all of the evaluators perceived little or no room for misinterpretation or ambiguity. The linguistic and cultural issues highlighted earlier and in the tables were addressed by appropriate revisions to the text prior to initiation of the translation and linguistic validation of the Spanish (US) and Japanese (Japan) versions.
Translation and linguistic validation: reconciliation of forward and backward translations and cognitive debriefing (stage 2)
There were a number of areas during the translation process that were identified in the Spanish (US) and Japanese versions of the instrument that required further revision. A summary of the key findings from the forward and backward translations is shown in Table 3.
  | Table 3 Key findings from the forward and backward translations |
Following the forward and backward translations, the cognitive debriefing interviews were conducted with five respondents for each language; their demographics are shown in Table 4. The sample covered a range of age and educational levels, as well as being as close as possible to a 50:50 sex representation. The five cognitive debriefing interviews conducted for the Spanish (US) version identified additional areas requiring revision for clarity; a summary of the key areas is shown in Table 5.
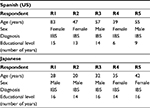  | Table 4 Demographics of the cognitive debriefing-interview respondents Abbreviation: IBS, irritable bowel syndrome. |
  | Table 5 Summary of the cognitive debriefing-interview analysis Abbreviation: IBS-D, irritable bowel syndrome with diarrhea. |
Mainly, in the Spanish (US) version, although “bowel movement” had been translated literally during the forward and backward translations, two of five respondents misunderstood the term to include only diarrhea. As a result, the wording was changed throughout the instrument from “movimiento intestinal” to “evacuaciones”, which was better understood as having the meaning of “bowel movement”. All necessary revisions to the translated Spanish version were related to comprehension/cultural appropriateness. No areas requiring additional revision were identified for the Japanese version.
Discussion
Until recently, no validated PRO instrument existed to measure IBS-D symptoms in clinical trials that examine the efficacy of various IBS-D treatments. An IBS-D PRO instrument, the Astellas Stool Form Scale, was developed to assess clinical symptoms and stool form and consistency in clinical trials.9 This scale captures the continuum of stool consistency experienced by patients with IBS-D.10 In order for this instrument to be used in multinational trials and settings, it underwent translatability assessments for various target languages prior to its translation and linguistic validation into Spanish (US) and Japanese.
A number of cultural issues were identified during the translatability assessments by evaluators of nine of the ten languages. These included the presentation of dates and times, word structure and concept elaboration for the text “irritable bowel syndrome – diarrhea predominant (IBS-D) symptom-event log”, difficulties understanding the word “immediate” to describe urgency, and the difference between sensations of “abdominal pain” and “stomach pain.” These issues, however, varied by country. The translatability assessment for each country led to some important changes in the IBS-D PRO instrument. Translatability assessments included European, South American, and Asian languages, which allowed for the identification of potential issues across a broad spectrum of additional languages.
Following the translatability assessments, the IBS-D PRO instrument underwent a rigorous translation and linguistic validation (cultural adaptation) process with a representative sample of the target patient population. Feedback from these reviews and cognitive debriefing interviews confirmed the validated translation of the instrument into Spanish (US) and Japanese. The respondents of both the languages easily understood the items in the translated instrument, demonstrating conceptual and linguistic equivalence and cultural appropriateness. Only minor changes to the text were made for the term “bowel movement” (revised from “movimiento intestinal” to “evacuaciones”), which was cited as causing some confusion and misunderstanding.
The translatability assessment and cognitive debriefing interview processes were incorporated into the overall translation of the instrument, because literal translations potentially can pose problems with the validity and interpretability of the PRO measure.11,12 No significant modifications after changes made in accordance with the translatability assessments were required to improve the integrity and quality of the IBS-D PRO instrument in any of the languages evaluated. The usefulness of translatability assessments prior to the actual translation has previously been documented and is commonly accepted.13 Although it is often difficult to achieve conceptual and linguistic equivalence, as well as cultural appropriateness via the use of representative languages, similar findings were observed across all ten languages that were evaluated. The final translated versions of the instrument followed the ISPOR guidance for the translation, linguistic validation, and cultural adaptation of a PRO instrument for use in multinational trials and settings.7,14 The methodology used here (ie, the forward and backward translations, followed by cognitive debriefing interviews with native-speaking individuals of the target languages) is also consistent with the previously described methodology for translating other PRO instruments for use in multiple countries and languages.15–19
Psychometric evaluation of the US English version of the PRO instrument had been previously performed and provided evidence that supports its psychometric validity (unpublished observation/manuscript in progress). Psychometric evaluation of the translated versions, however, has not yet been performed, and can be viewed as a limitation to this study; it is necessary to test whether the psychometric properties observed in the US English version are preserved in the Spanish (US) and Japanese versions. Another limitation is that the minimum standard of five respondents for each of the cognitive debriefing interviews was used. Additional testing with a larger sample would further strengthen the validation results.
Conclusion
In summary, the results of the translatability assessment reported here provide evidence that the IBS-D PRO is worded in a manner that is easily translated into numerous other languages. In addition, the linguistic validation results support the conceptual equivalence of the Spanish (US) and Japanese translations of the IBS-D PRO instrument. These linguistically validated versions can be used in future research to assess their content validity and psychometric properties. These versions can also be used in multinational trials and studies to evaluate treatment benefit in IBS-D.
Acknowledgments
The PRO instrument for IBS-D was developed by Astellas. The authors would like to thank Tara N Miller, PhD, CMPP, and Gill Sperrin, CBiol, MRSB, CMPP at Envision Scientific Solutions for providing medical writing support, funded by Astellas, and Angela Stroupe, MS (medical anthropology) at Pharmerit for reviewing the manuscript and providing valuable feedback. This study and the preparation of this paper were funded in full by Astellas.
Author contributions
LD-H, KL, AN, RA, BB, SZ, IG, and BZ contributed to the design of the study; LD-H, KL, BB, RA, and AP performed the research and participated in data acquisition; all authors were involved in the analysis/interpretation of the data and drafting or critically reviewing/revising the manuscript; and all authors approved the final version of the article. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
AN, LD-H, IG, SZ, and BZ are employees of Astellas Pharma Inc. KL was an employee of Adelphi Values (formerly Mapi Values) at the time of the study, which was contracted by Astellas to work on IBS-D PRO instrument development. BB and RA are employed by Adelphi Values, and were contracted by Astellas to work on IBS-D PRO instrument development. BB and RA have also worked with other pharmaceutical companies on PRO development and validation projects over the previous 2-year period. AP is employed by TransPerfect, and was contracted by Astellas to work on the translatability assessments and linguistic validation. A US patent (application 13/274,040) is pending for the IBS-D daily symptom diary and symptom-event log ( Astellas Stool Form Scale). The authors report no other conflicts of interest in this work.
References
Rome Foundation. Rome III diagnostic criteria for functional gastrointestinal disorders. 2006. Available from: http://www.romecriteria.org/assets/pdf/19_RomeIII_apA_885-898.pdf. Accessed May 2, 2016. | ||
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. | ||
Drossman DA, Morris CB, Hu Y, et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology. 2005;128(3):580–589. | ||
Burke L. Harmonising study endpoints for multi-national clinical trials. Poster presented at: Drug Information Association 23rd Annual EuroMeeting; March 28–30, 2011; Geneva, Switzerland. | ||
US Food and Drug Administration. Guidance for industry: patient-reported outcome measures – use in medical product development to support labeling claims. 2009. Available from: http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. Accessed April 18, 2014. | ||
Guardian. US now has more Spanish speakers than Spain – only Mexico has more. 2015. Available from: http://www.theguardian.com/us-news/2015/jun/29/us-second-biggest-spanish-speaking-country. Accessed April 8, 2016. | ||
Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8(2):94–104. | ||
Bell A. Language style as audience design. Lang Soc. 1984;13(2):145–204. | ||
Lasch K, Delgado-Herrera L, Waldman LT, et al. Development of a new instrument to assess stool form and consistency in irritable bowel syndrome with diarrhea. Value Health. 2014;4(1). | ||
Lasch K, Delgado-Herrera L, Waldman LT, et al. Development of a new instrument to assess stool form and consistency in irritable bowel syndrome with diarrhea. Gastroenterol Hepatol Open Access. 2016;4(1):00085. | ||
Arffman I. Unwanted literal translation: an underdiscussed problem in international achievement studies. Educ Res Int. 2012;2012(12):1–13. | ||
Arffman I. Translating International Achievement Tests: Translators’ View. Jyväskylä, Finland: University of Jyväskylä; 2012. | ||
Conway K, Acquadro C, Patrick DL. Usefulness of translatability assessment: results from a retrospective study. Qual Life Res. 2014;23(4):1199–1210. | ||
Wild D, Eremenco S, Mear I, et al. Multinational trials – recommendations on the translations required, approaches to using the same language in different countries, and the approaches to support pooling the data: the ISPOR Patient-Reported Outcomes Translation and Linguistic Validation Good Research Practices Task Force report. Value Health. 2009;12(4):430–440. | ||
Two R, Verjee-Lorenz A, Clayson D, Dalal M, Grotzinger K, Younossi ZM. A methodology for successfully producing global translations of patient reported outcome measures for use in multiple countries. Value Health. 2010;13(1):128–131. | ||
Paradowski PT, Witonski D, Keska R, Roos EM. Cross-cultural translation and measurement properties of the Polish version of the Knee injury and Osteoarthritis Outcome Score (KOOS) following anterior cruciate ligament reconstruction. Health Qual Life Outcomes. 2013;11:107. | ||
Paulsen A, Odgaard A, Overgaard S. Translation, cross-cultural adaptation and validation of the Danish version of the Oxford hip score: assessed against generic and disease-specific questionnaires. Bone Joint Res. 2012;1(9):225–233. | ||
Coyne KS, Tubaro A, Brubaker L, Bavendam T. Development and validation of patient-reported outcomes measures for overactive bladder: a review of concepts. Urology. 2006;68(2 Suppl):9–16. | ||
de la Loge C, Trudeau E, Marquis P, et al. Cross-cultural development and validation of a patient self-administered questionnaire to assess quality of life in upper gastrointestinal disorders: the PAGI-QOL. Qual Life Res. 2004;13(10):1751–1762. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
